Abstract
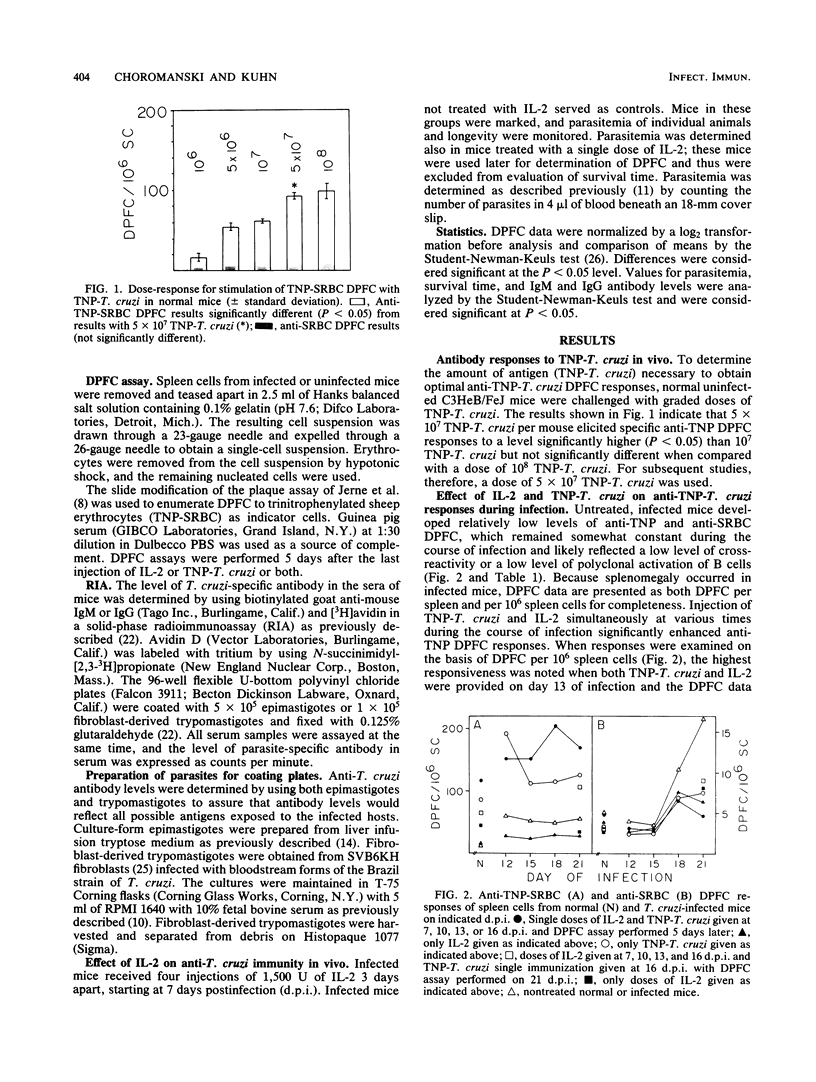
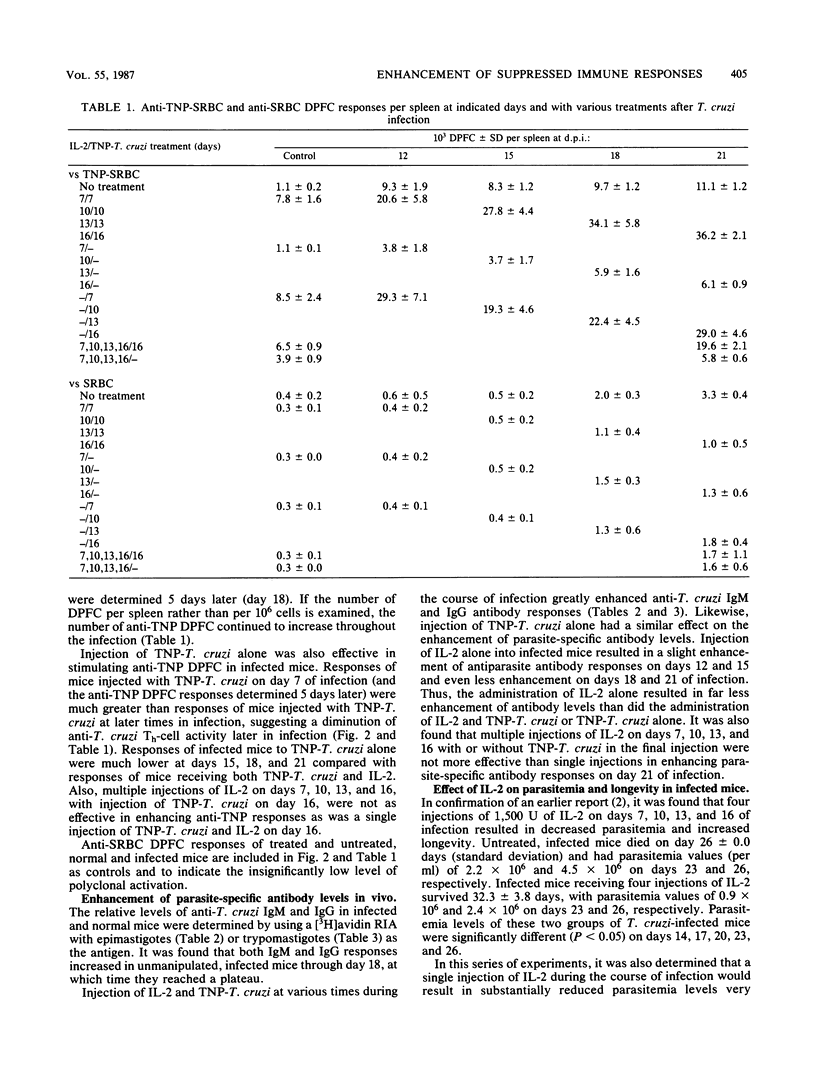
Mice infected with Trypanosoma cruzi exhibit an early and profound suppression of parasite-specific and nonspecific immune responses. Earlier studies have shown that this suppression is due, at least in part, to suppressor macrophages, deficiency in production of interleukin-2 (IL-2), and reduced T helper (Th)-cell activity. In the present study, the effect of exogenously supplied IL-2 on enhancement of parasite-specific Th-cell activity, anti-parasite immunoglobulin G (IgG) and IgM antibody levels, parasitemia, and longevity was examined in infected mice. The results showed that administration of IL-2 with and without antigenic stimulation with trinitrophenylated T. cruzi significantly enhanced parasite-specific IgM and IgG levels. Injection of IL-2 and trinitrophenylated T. cruzi together significantly enhanced parasite-specific Th-cell activity and was more effective in enhancement of parasite-specific antibody levels. In addition, it was found that IL-2 alone had a rapid and lasting effect in reducing parasitemia. These results suggest that deficiency in IL-2 may play a major role in host susceptibility to T. cruzi.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess D. E., Kuhn R. E., Carlson K. S. Induction of parasite-specific helper T lymphocytes during Trypanosoma cruzi infections in mice. J Immunol. 1981 Nov;127(5):2092–2095. [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Interleukin 2 enhances specific and nonspecific immune responses in experimental Chagas' disease. Infect Immun. 1985 Nov;50(2):354–357. doi: 10.1128/iai.50.2.354-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton B. A., Ortiz-Ortiz L., Garcia W., Martinez T., Capin R. Trypanosoma cruzi: early immune responses in infected mice. Exp Parasitol. 1975 Jun;37(3):417–425. doi: 10.1016/0014-4894(75)90012-0. [DOI] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E., Rowland E. C. Suppression of humoral responses during Trypanosoma cruzi infections in mice. Infect Immun. 1978 Oct;22(1):155–160. doi: 10.1128/iai.22.1.155-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppression of the primary immune response in murine cell cultures to T-cell-dependent and -independent antigens. J Parasitol. 1980 Feb;66(1):16–27. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Immunologic deficiency during experimental Chagas' disease (Trypanosoma cruzi infection): role of adherent, nonspecific esterase-positive splenic cells. J Immunol. 1982 Nov;129(5):2202–2205. [PubMed] [Google Scholar]
- Kuhn R. E., Murnane J. E. Trypanosoma cruzi: immune destruction of parasitized mouse fibroblasts in vitro. Exp Parasitol. 1977 Feb;41(1):66–73. doi: 10.1016/0014-4894(77)90130-8. [DOI] [PubMed] [Google Scholar]
- Kuhn R. E., Vaughn R. T., Herbst G. A. The effect of BCG on the course of experimental Chagas' disease in mice. Int J Parasitol. 1975 Oct;5(5):557–560. doi: 10.1016/0020-7519(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- OVARY Z., BENACERRAF B. IMMUNOLOGICAL SPECIFICITY OF THE SECONDARY RESPONSE WITH DINITROPHENYLATED PROTEINS. Proc Soc Exp Biol Med. 1963 Oct;114:72–76. doi: 10.3181/00379727-114-28589. [DOI] [PubMed] [Google Scholar]
- Powell M. R., Kuhn R. E. Measurement of cytolytic antibody in experimental Chagas' disease using a terminal radiolabelling procedure. J Parasitol. 1980 Jun;66(3):399–406. [PubMed] [Google Scholar]
- Reed S. G., Inverso J. A., Roters S. B. Heterologous antibody responses in mice with chronic T. cruzi infection: depressed T helper function restored with supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1558–1563. [PubMed] [Google Scholar]
- Reed S. G., Inverso J. A., Roters S. B. Suppressed antibody responses to sheep erythrocytes in mice with chronic Trypanosoma cruzi infections are restored with interleukin 2. J Immunol. 1984 Dec;133(6):3333–3337. [PubMed] [Google Scholar]
- Reed S. G., Larson C. L., Speer C. A. Suppression of cell-mediated immunity in experimental Chagas' disease. Z Parasitenkd. 1977 Jun 3;52(1):11–17. doi: 10.1007/BF00380553. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Roters S. B., Goidl E. A. Spleen cell-mediated suppression of IgG production to a non-parasite antigen during chronic Trypanosoma cruzi infection in mice. J Immunol. 1983 Oct;131(4):1978–1982. [PubMed] [Google Scholar]
- Rowland E. C., Kuhn R. E. Suppression of cellular responses in mice during Trypanosoma cruzi infections. Infect Immun. 1978 May;20(2):393–397. doi: 10.1128/iai.20.2.393-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snary D., Hudson L. Trypanosoma cruzi cell surface proteins: identification of one major glycoprotein. FEBS Lett. 1979 Apr 1;100(1):166–170. doi: 10.1016/0014-5793(79)81156-4. [DOI] [PubMed] [Google Scholar]
- Snary D. Trypanosoma cruzi: antigenic invariance of the cell surface glycoprotein. Exp Parasitol. 1980 Feb;49(1):68–77. doi: 10.1016/0014-4894(80)90057-0. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Measurement of parasite-specific antibody responses using a tritiated avidin-solid phase radioimmunoassay. J Immunol Methods. 1983 May 27;60(1-2):213–220. doi: 10.1016/0022-1759(83)90349-6. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Measurement of parasite-specific immune responses in vitro: evidence for suppression of the antibody response to Trypanosoma cruzi. Eur J Immunol. 1985 Aug;15(8):845–850. doi: 10.1002/eji.1830150820. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1570–1575. [PubMed] [Google Scholar]
- Tarleton R. L., Schulz C. L., Grögl M., Kuhn R. E. Diagnosis of Chagas' disease in humans using a biotin-3H-avidin radioimmunoassay. Am J Trop Med Hyg. 1984 Jan;33(1):34–40. doi: 10.4269/ajtmh.1984.33.34. [DOI] [PubMed] [Google Scholar]



