Abstract
Two poorly soluble, potent anticancer drugs, paclitaxel and camptothecin, were successfully solubilized by mixed micelles of polyethylene glycol-phosphatidyl ethanolamine (PEG-PE) and vitamin E. Drug containing micelles were additionally modified with anti-nucleosome monoclonal antibody 2C5 (mAb 2C5), which can specifically bring micelles to tumor cells in vitro. The optimized micelles had an average size of about 13-to-22 nm and the immuno-modification of micelles did not significantly change it. The solubilization of both drugs by the mixed micelles was more efficient than by micelles made of PEG-PE alone. Solubilization of camptothecin in micelles prevented also the hydrolysis of active lactone form of the drug to inactive carboxylate form. Drug loaded mixed micelles and mAb 2C5-immunomicelles demonstrated significantly higher in vitro cytotoxicity than free drug against various cancer cell lines.
Keywords: Camptothecin, paclitaxel, immunomicelles, monoclonal antibody, polyethyleneglycol-phosphatidyl ethanolamine, vitamin E, polymeric micelles
1. Introduction
Polymeric micelles are currently widely used as pharmaceutical carriers for poorly soluble drugs, including anti-cancer drugs, providing advantages such as small size (10–100 nm), good solubilization efficiency, and extremely high stability due to very low critical micelles concentration [CMC] (typically in the micromolar range) [1–3]. Thus, after the in vivo administration and strong dilution with the large volume of blood, the micelles with low CMC value will still exist, while micelles with high CMC value will dissociate resulting in precipitation of solubilized drug [2]. The small size of micelles allows for their efficient accumulation in pathological tissues with leaky vasculature, such as tumors and infarct tissues, via the enhanced permeability and retention (EPR) effect [2, 4].
We have recently shown that micelles prepared from polyethylene glycol-phosphatidyl ethanolamine (PEG-PE) conjugates can be efficiently loaded with certain anti-cancer drugs [5–7] and accumulate well in experimental tumors via the EPR effect [8]. Moreover, these micelles can also be made specifically targeted to tumors by attaching the anti-cancer nucleosome-specific monoclonal antibody 2C5 (mAb 2C5) to their outer shell [9–12], which recognizes the surface of a broad variety of tumor cells via tumor cell surface-bound nucleosomes.
Two potent poorly soluble anti-cancer drugs, namely paclitaxel (PCT) and camptothecin (CPT), were used in the present study. Currently, PCT is formulated in a 50:50 mixture of Cremophor EL (polyoxyethylated castor oil) and dehydrated ethanol for clinical administration. Cremophor EL has been reported to elicit various side effects in patients, including hypersensitivity, nephrotoxicity, and neurotoxicity [13]. Various formulations of PCT, such as polymeric micro/nanospheres [14, 15], liposomes [16, 17], microemulsions [18] and polymeric micelles [19–21] have been developed to increase the solubility of PCT, minimize its side effects, and decrease its systemic clearance.
In case of CPT, in addition to low solubility and systemic toxicity, the stability of this drug at physiological pH is also critical for its therapeutic activity. Depending on the pH value, CPT can exist in two different forms (Fig. 1), namely, in biologically active lactone form at pH below 5, and in an inactive carboxylate form at basic pH [22], and at physiological pH values most CPT molecules exist in the inactive carboxylate form. Hertzberg et al. [23] and Hsiang et al. [24] have shown that the lactone form of CPT is essential for passive diffusion into cancer cells and for the interaction with its therapeutic target, topoisomerase I. Because of the inactivation of the free form of CPT and its hydrolysis into the inactive carboxylate form, high amounts of drug are required to be administered to the patient. The severe and unexpected systemic toxicity reported are mostly due to high doses required and fast accumulation of the active lactone form of CPT in the lipid membrane of RBCs leading to hemorrhagic cystitis [25–27]. Various approaches have been reported in literature in order to reduce these severe systemic toxicities and enhance antitumor effects of CPT. These includes conjugation to synthetic polymers [28–31], incorporation into liposomes [32, 33], microspheres [34, 35], nanohybrids [36], and polymeric micelles [37–41].
Figure 1.
Chemical structure of CPT.
It has been shown earlier that both these drugs can be successfully incorporated in the PEG-PE-based micellar system [6, 9]. Here, we made an attempt to further increase the solubilization efficiency of PEG-PE micellar system by preparing mixed micelles of PEG-PE and vitamin E. One can expect that these mixed micelles may allow for the better solubilization of both drugs due to the increased volume of the lipophilic core of mixed micelle created by the spacious lipophilic moiety of the vitamin E. One can expect also that the increased inner lipophilic core of mixed micelles would provide better protection for the active lactone form of CPT. Vitamin E has been already successfully used in the composition of mixed micellar formulations to enhance the solubilization of rapamycin in PEG-polycaprolactone micelles [42].
In present work, we describe the preparation and characterization of PCT- or CPT-loaded PEG-PE/vitamin-E mixed tumor-targeted immunomicelles modified with mAb 2C5 as well as their cytotoxicity against cancer cells in vitro.
2. Materials and methods
2.1 Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG2000–PE) was purchased from Avanti Polar Lipids (Alabaster, AL, USA) and used without further purification. p-Nitrophenylcarbonyl-polyethyleneglycol-phosphatidylethanolamine (pNP-PEG-PE) was synthesized in our lab following the procedure described in [43, 44]. PCT, CPT and vitamin E were purchased from Sigma (St. Louis, MO, USA). Cell culture media and supplements were from CellGro (Kansas City, MO, USA). Cancer-specific antinucleosome mAb 2C5 was prepared by Harlan Bioproducts for Science (Indianapolis, IN, USA) using the cell line provided by our laboratory. 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Promega (Madison, WI, USA). Cancer cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA). All other reagents and buffer solution components were analytical grade preparations. Distilled and deionized water was used in all experiments.
2.2 Methods
2.2.1. Preparation of PCT- or CPT-loaded mixed micelles
PCT or CPT solubilized in mixed micelles were prepared by the following method. PCT (1 mg/mL in methanol) or CPT (1 mg in the mixture of 2 mL chloroform and 2 mL acetonitrile) were added to PEG2000-PE and vitamin E (89:11 molar ratio) solution in chloroform. The organic solvents were removed by the rotary evaporation to form a thin film of drug/micelle material mixture. This film was further dried under high vacuum overnight to remove any traces of remaining solvents. Drug-loaded micelles were formed by resuspending the film obtained in 5 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES)-buffered saline (HBS), pH 7.4 or in 10 mM acetate buffer, pH 5.0 for PCT and CPT, respectively. The mixture was incubated in water bath at 50°C for 10 min. Excess non-incorporated drugs were separated by centrifugation (13,000g) before characterization.
2.2.2. Preparation of PCT- or CPT-loaded 2C5-immunomicelles
To attach the mAb 2C5 to micelles to obtain immunomicelles [9, 12, 43], a chloroform solution of PEG2000-PE/vitamin E (89:11 molar ratio) was supplemented with 5 % mol of the reactive component, pNP-PEG-PE. To this mixture, PCT (in methanol) or CPT (in chloroform and acetonitrile mixture) were added to obtain the mixture containing 10 mg of the micelle-forming material and 0.3 mg or 0.015 mg of PCT or CPT, respectively. Organic solvents were removed by the rotary evaporation. The dry film was dispersed in 5 mM Na-citrate buffered saline to obtain net final concentration of 20 mg/mL of lipid. To 0.5 mL of the resultant mixture 0.3 mL of 2.94 mg/mL mAb 2C5 solution in 100 mM phosphate buffered saline, pH 9.0, was added with vortexing. The final pH of the mixture was adjusted to pH 8.5 with 1M NaOH to allow for the reaction between protein (antibody) amino-groups and pNP groups of pNP-PEG-PE yielding immunomicelles. The mixture was incubated for 3 h at room temperature. For PCT-loaded immunomicelles the above sample was dialyzed against 5 mM HBS, pH 7.4, using cellulose ester membranes with a cut off size of 250 kDa (Spectrum Medical Industries, Rancho Dominguez, CA). For CPT-loaded immunomicelles the pH of the above sample was adjusted to pH 5.0 with 1N HCl and the sample was incubated at 4°C for 24 h. The sample was then dialyzed against 10 mM acetate buffer, pH 5.0, using cellulose ester membranes with a cut off size of 250 kDa.
2.3. Characterization of micelles
2.3.1. Micelle size
The micelle size (hydrodynamic diameter) was measured by the dynamic light scattering (DLS) using a N4 Plus Submicron Particle System (Coulter Corporation, Miami, FL, USA). The micelle suspensions were diluted with the deionized distilled water until the concentration providing light scattering intensity of 5 × 104 to 1 × 106 counts/s was achieved. The particle size distribution of all samples was measured in triplicate.
2.3.2. Critical micelle concentration (CMC) determination
CMC value of the mixed micelles of chosen composition was estimated by the standard pyrene method [45]. Briefly, tubes containing 1 mg crystals of pyrene were prepared. To these crystals, 10−4 to 10−9 M micellar solution of PEG2000–PE/ vitamin E in HBS was added. The mixtures were incubated for 24 h with shaking at room temperature. Free pyrene was removed by filtration through 0.2 µm polycarbonate membranes. The fluorescence of filtered samples was measured at the excitation wavelength of 339 nm and emission wavelength of 390 nm using a F-2000 fluorescence spectrometer (Hitachi, Japan). CMC values correspond to the concentration of the polymer at which the sharp increase in pyrene fluorescence in solution is observed.
2.3.3. Effect of mixed micelles on CPT lactone ring protection
The effect of the mixed micelles on the lactone-carboxylate hydrolysis at physiological pH 7.4 was evaluated as follows. CPT-loaded mixed micelles were incubated in the phosphate buffered saline (PBS), pH 7.4 and 50% fetal bovine serum (FBS) at CPT concentration of 30 µg/mL. At specific time intervals, 10 µL aliquots were withdrawn followed by immediate reverse-phase HPLC analysis (described below) of the lactone and carboxylate forms of CPT. For comparison, a 10 µg/mL CPT solution in a PBS buffer, pH 7.4, and 50% FBS was also investigated by the same method as that for the micelles. (The reason for using low free drug concentration for CPT hydrolysis was low solubility of drug. It was not possible to prepare drug solution with 30µg/mL of CPT since it resulted in precipitation of drug. In case of mixed micelles, due to the higher solubilization efficiency, we have used the concentration of 30 µg/mL for CPT hydrolysis study to make the registration process easier. Importantly, at the concentrations used, there are no concentration-dependent effects on lactone stability.)
The HPLC system consisted of the following: D-7000 HPLC system equipped with a diode array and fluorescence detector (Hitachi, Japan) and Spherisorb ODS2 column, 4.6 mm × 250 mm (Waters, Milford, MA, USA) fitted with a 50 µL sample loop. The mobile phase was composed of 30% vol. acetonitrile and 70% vol. triethylamine acetate buffer (TEAA) (1% v/v triethylamine in water, adjusted to pH 5.5 with glacial acetic acid) and was delivered at a flow rate of 1.0 mL/min in all experiments. The detection was performed using a fluorescence detector with an excitation wavelength of 360 nm and emission wavelength of 430 nm. The retention times of the carboxylate and lactone were around 2.57 and 7.98 min, respectively. All samples were analyzed in triplicate.
2.3.4. Drug solubilization efficiency
The amount of drug in the micellar phase was measured by the reversed phase-HPLC. The clear aqueous dispersion was diluted with the mobile phase prior to applying onto the HPLC column (since the mobile phase contains acetonitrile, micelles are disrupted and free drug is determined). The concentration of CPT in samples was determined by HPLC as described in 2.3.3.
For PCT analysis, the D-7000 HPLC system equipped with a diode array and fluorescence detector (Hitachi, Japan) and Spherisorb ODS2 column, 4.6 mm × 250 mm (Waters, Milford, MA, USA) was used. The column was eluted with acetonitrile/water (65:35, v/v) at 1.0 mL/min. PCT was detected at 227 nm. Injection volume was 50 µL; all samples were analyzed in triplicate.
2.3.5. Specific activity of the micelle-attached mAb 2C5
To verify the immunological activity of micelle-attached mAb 2C5, a standard ELISA was used [12]. Briefly, ELISA plates pretreated with 40 µg/mL polylysine solution in TBS, pH 7.4, were coated with 50 µL of 1 µg/mL nucleosomes (the water-soluble fraction of calf thymus nucleohistone, Worthington Biochemical, Lakewood, NJ, USA) and incubated overnight at 4°C. The plates were rinsed with 0.2% casein, 0.05% Tween 20 in TBS (casein/TBS), pH 7.4. To these plates, serial dilutions of mAb 2C5-containig samples were added and incubated for 4 h at room temperature. The plates were extensively washed with casein/TBS and coated with horseradish peroxidase goat antimouse IgG conjugate (ICN Biomedical, Aurora, OH, USA) diluted according to the manufacturer's recommendation. After 3-h incubation at room temperature, the plates were washed with casein/TBS. Bound peroxidase was quantified by the degradation of its substrate, diaminobenzidine supplied as a ready-for-use solution, Enhanced K-Blue TMB substrate (Neogen, Lexington, KY, USA). The microplate was read at a dual wavelength of 620 nm with the reference filter at 492 nm using a Labsystems Multiskan MCC/340 microplate reader (Labsystems and Life Sciences International, UK) installed with GENESIS-LITE windows based microplate software.
2.3.6. Cell cultures
The B16 (murine melanoma) and 4T1 (murine mammary carcinoma) cells were maintained in DMEM cell culture medium at 37 °C, 5% CO2. DMEM media were supplemented with 10% FBS, 1 mM Na-pyruvate, 50 U/mL penicillin, and 50 µg/mL streptomycin.
2.3.7. Cytotoxicity assay
Cells were plated at 5×103 cells per well density in 96-well plates (Corning, Inc., Corning, NY, USA). After 24 h incubation at 37°C, 5% CO2, the medium was replaced with medium containing free drug dissolved in DMSO or drug loaded PEG-PE/vitamin E micelles or mAb 2C5-PEG-PE/vitamin E-immunomicelles for 48h with PCT concentration ranging from 0 to 62.5 ng/mL, and CPT concentrations ranging from 0 to 80 ng/mL. After incubation, each well was washed twice with the Hank's buffer and the cell survival was then measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method. The absorbance of the degraded MTT at 492 nm (the measure of the cytotoxicity) was measured using a Labsystems Multiskan MCC/340 microplate reader (Labsystems and Life Sciences International, UK).
2.3.8. Statistics
The cytotoxicity data from various formulations were compared using One-Way ANOVA using OriginPro software, version 7.5 (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
In this study, we aimed to prepare a micellar form of two poorly soluble anti-cancer drugs with better level of drug solubilization and higher content of a drug than was achieved earlier in case of the micelles made of PEG-PE alone. Mixed micelles can provide this opportunity. Earlier, we have already reported mixed micelle-based preparation of PCT in PEG-PE/eggPC micelles and PCT and CPT in PEG-PE/ d-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) micelles [9, 46, 47]. Here, we have tried a new combination of the micelle-forming components and prepared mixed micelles from the mixture of PEG-PE and vitamin E. It was repeatedly shown that within the core-shell structure of PEG-PE micelles, the core is formed by the hydrophobic diacyllipid part, while the corona is made of hydrophilic PEG residues. In aqueous systems, nonpolar molecules will be solubilized within the micelle core, polar molecules will be adsorbed on the micelle surface, and substances with intermediate polarity will be distributed along surfactant molecules in certain intermediate positions [2, 11]. The addition of vitamin E was expected to enhance the drug load due to higher content of hydrophobic fragment in its molecules, which should increase a core volume and its ability to solubilize the hydrophobic molecules in mixed micelles compared to the micelles made of PEG-PE alone.
At 10 mM concentration of mixed micelles components (PEG2000-PE: vitamin E molar ratio 89:11), the micelle formulations were produced containing 35±2.25µg and 850±3.45µg of CPT and PCT, respectively, per mL of micelle formulation. These values are significantly higher than for solubilization of these drugs in monocomponent PEG-PE micelles: 25±2.15µg and 291±3.45µg of CPT and PCT, respectively, per mL of micelle formulation.
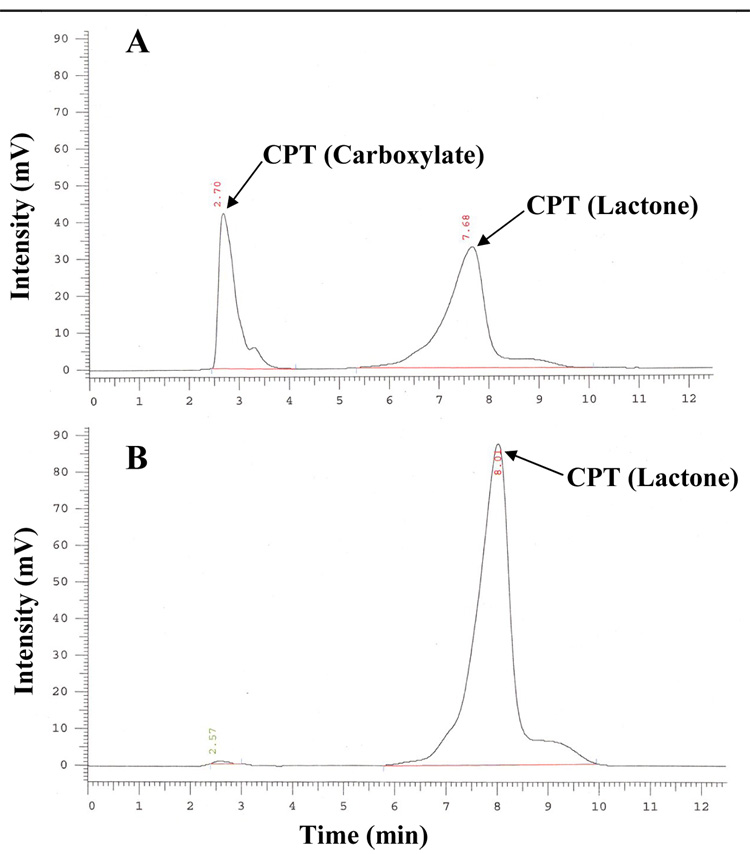
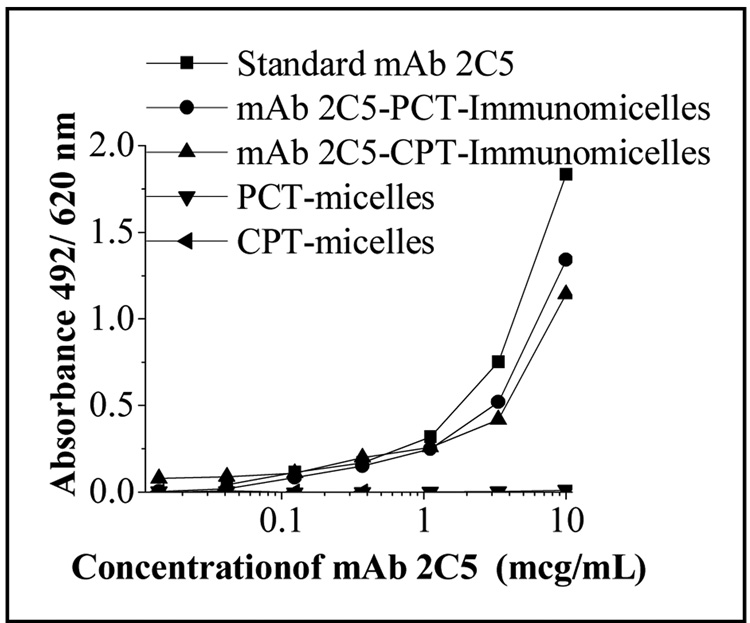
Antibody attachment to drug-loaded mixed micelles was performed by using a simple and reproducible protocol utilizing pNP-PEG-PE reagent earlier developed by us for the attachment of various amino group-containing ligands to long-circulating PEGylated liposomes and micelles [7, 12, 43]. As per our standard protocol, the coupling reaction was done at pH 8.5. However at this basic pH about 60 per cent of CPT was converted to inactive carboxylate form (determined by HPLC, Fig.2A). Since the conversion of lactone to carboxylate form of CPT is a reversible reaction, the pH of the reaction mixture was decreased to 5.0 with 1N HCl after the conjugation with antibody. It was observed that after the overnight incubation at pH 5.0 and 4°C the carboxylate form of CPT was converted back to active lactone form (determined by HPLC, Fig. 2B). After this, the unreacted antibody was removed by dialysis with 10 mM acetate buffer, pH 5.0, using cellulose ester membranes with a cut off size of 250 kDa. The preservation of the specific activity of the micelle-attached mAb 2C5 was studied by ELISA using nucleosomes as a binding substrate. Using a non-modified (standard) mAb 2C5 as a positive control, it was found that the conjugated antibody preserves high level of the specific activity (Fig. 3).
Figure 2.
HPLC chromatograms illustrating the conversion of CPT carboxylate to CPT lactone form in immunomicelles upon the incubation in the reaction buffer at pH 8.5 for 3h (A) and at pH 5.0 after 24h (B).
Figure 3.
Immunoreactivity of 2C5-PEG-PE/Vitamin E mixed micelles by ELISA.
As can be seen in the Table 1, the average size of drug-free micelles, drug-loaded micelles and drug-loaded immunomicelles was in the range of 13-to-22 nm. The modification with antibody did not noticeably change the micelle size.
Table 1.
Particle size in different micelle formulations (Mean diameter ± standard deviation, n = 3).
| Formulations | Size (nm) | |
|---|---|---|
| 1. | ‘Empty’ PEG2000-PE micelles | 14.7 ± 1.6 |
| 2. | mAb2C5 ‘Empty’ PEG2000-PE immunomicelles | 16.8 ± 1.4 |
| 3. | ‘Empty’ mixed (PEG-PE: Vit.E) micelles | 15.8 ± 1.6 |
| 4. | CPT-loaded mixed (PEG-PE: Vit.E) micelles | 15.3 ± 2.7 |
| 5. | PCT-loaded mixed (PEG-PE: Vit.E) micelles | 18.5 ± 1.9 |
| 6. | mAb2C5 CPT-loaded mixed (PEG-PE: Vit.E) immunomicelles | 19.3 ± 3.0 |
| 7. | mAb2C5 PCT-loaded mixed (PEG-PE: Vit.E) immunomicelles | 19.7 ± 3.0 |
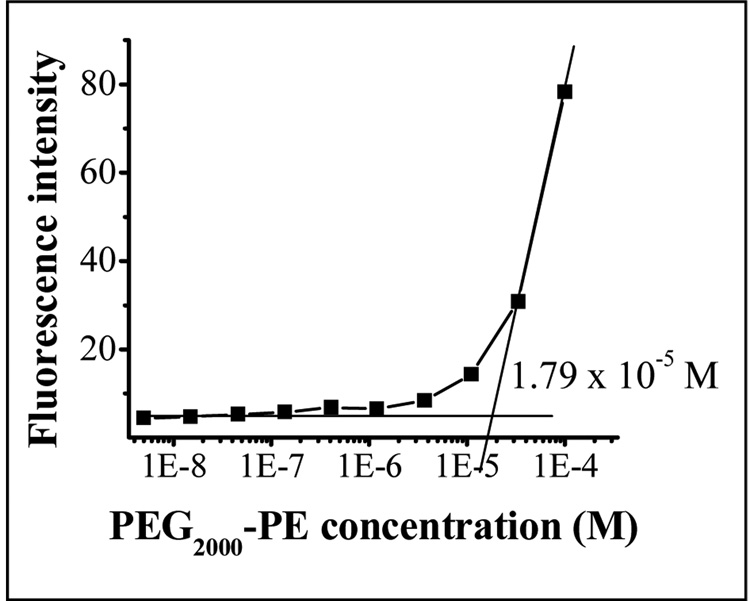
CMC of the micelle-forming compound influences the in vitro and in vivo stability. The low CMC values of PEG-PE underlay high stability of PEG-PE micelles in solutions upon dilution [48]. The CMC of the mixed micelles composed of PEG2000-PE and vitamin E (89:11 molar ratio) was found to be also low − 1.79×10−5 M (Fig. 4). (The CMC for the Vitamin E-free plain PEG2000-PE was found to be 1.1×10−5 M, [46]). This promises their high stability and ability to maintain integrity upon strong dilution in body.
Figure 4.
CMC determination of PEG-PE/Vitamin E mixed micelles.
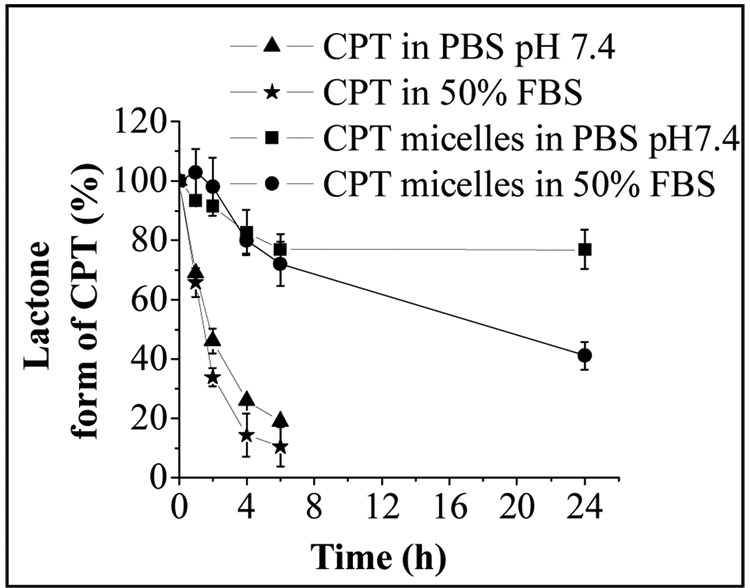
As was said above, apart from the poor solubility of CPT, another major concern is its instability at physiological pH. Several pre-clinical trials conducted worldwide reported the unexpected severe systemic toxicity and poor tumor response of CPT mainly due to rapid formation of the open ring carboxylate form of CPT, which is 10-fold less potent than the CPT lactone form. It has been reported that the carboxylate form of CPT preferentially binds to human serum albumin, which further reduces the equilibrium amount of active lactone and greatly lowers CPT anti-tumor efficacy [49]. Fig. 5 compares the hydrolysis profiles of free CPT and CPT in CPT-loaded micelles in PBS buffer pH 7.4 and in 50% FBS. The free form of CPT exhibited rapid lactone ring opening in both PBS and FBS. Mixed micelles were able to protect the lactone ring after 24h by 80 % in PBS, and 40 % in FBS. This indicated that the incorporation of CPT into the hydrophobic inner core of micelles assisted the preservation of the active lactone form.
Figure 5.
Stability of free CPT (lactone form) and CPT (lactone form) loaded “plain” micelles in PBS pH 7.4 and 50% FBS.
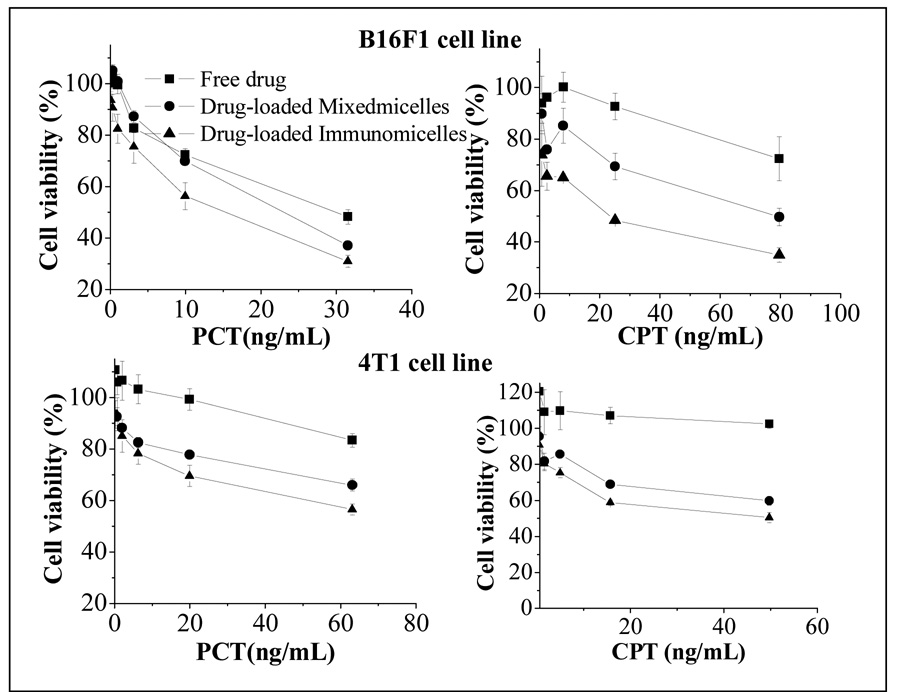
The in vitro cytotoxicity of different micelle formulations was investigated and compared to that of the free drug using B16F1 and 4T1 cell lines. The cells were incubated with free drugs and different drug-loaded micellar formulations for 2 days and analyzed for their survival using the MTT colorimetric assay for the dehydrogenase activity of viable cell. In all studied cell lines, drugs in PEG-PE/vitamin E mixed micelles demonstrated a significantly superior cytotoxicity compared to that of the free drugs (Fig. 6). This increase in cytotoxicity could be explained by the increased solubility of the poorly soluble drugs in micelle solution, enhanced endocytotic uptake of drug-containing micelles by the cells resulting in multiple drug molecules entering cell with each taken micelles, and, in case of CPT, increased stability of its cytotoxic lactone form inside the micelle core. Thus, the enhanced cytotoxicity effect obtained with Vitamin E formulations compared to free drugs (similarly to the drug in plain micelles) is evidently due to better drug solubilization (and protection of the lactone form in case of CPT) inside micelles, while the biological properties of the drugs remain the same.
Figure 6.
In vitro cytotoxicity of various formulations of CPT and PCT against different cancer cell lines.
The attachment of the targeting ligand naturally enhances this cytotoxicity still further and drug-loaded mixed immunomicelles showed significantly higher cytotoxicity than non-targeted mixed micelles (Fig. 6). This is clearly explained by the increased quantity of immunomicelles (and higher drug quantity) brought to the target cells due to attached mAb 2C5. Drug-free plain or antibody-modified micelles, as well as the antibody itself were not toxic to cells at concentrations studied (data not shown).
4. Conclusion
The mixed micelles composed of PEG-PE and vitamin E could efficiently solubilize poorly soluble anticancer drugs. These mixed micelles were also able to stabilize the active lactone form of CPT. Further, the drug-loaded mixed micelles demonstrate increased in vitro cytotoxicity compared to free drug. This cytotoxicity could be further enhanced by attaching the tumor-specific mAb 2C5 antibody to the surface of drug-loaded mixed micelles. This might be especially important for the in vivo application of immunomicelles, which combine the property of both passive tumor targeting via the EPR effect and active tumor targeting via the attached tumor-specific antibody.
Acknowledgement
This work was supported by the NIH grant RO1 EB001961 to Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Current Pharmaceutical Design. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. Journal of Controlled Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharmaceutical Research. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 5.Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyl polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Letters. 2002;2:979–982. [Google Scholar]
- 6.Li M, Chrastina A, Levchenko T, Torchilin VP. Micelles from poly(ethylene glycol)-phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical nanocarriers for poorly soluble drug camptothecin. J. Biomed. Nanotechnol. 2005;1:190–195. [Google Scholar]
- 7.Roby A, Erdogan S, Torchilin VP. Solubilization of poorly soluble PDT agent, meso-tetraphenylporphin, in plain or immunotargeted PEG-PE micelles results in dramatically improved cancer cell killing in vitro. European Journal of Pharmaceutics and Biopharmaceutics. 2006;62:235–240. doi: 10.1016/j.ejpb.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukyanov AN, Gao Z, Torchilin VP. Micelles from polyethylene glycol/phosphatidylethanolamine conjugates for tumor drug delivery. Journal of Controlled Release. 2003;91:97–102. doi: 10.1016/s0168-3659(03)00217-7. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, Lukyanov AN, Chakilam AR, Torchilin VP. PEG-PE/phosphatidylcholine mixed immunomicelles specifically deliver encapsulated taxol to tumor cells of different origin and promote their efficient killing. Journal of Drug Targeting. 2003;11:87–92. doi: 10.1080/1061186031000138623. [DOI] [PubMed] [Google Scholar]
- 10.Roby A, Erdogan S, Torchilin VP. Enhanced In Vivo Antitumor Efficacy of Poorly Soluble PDT Agent, Meso-Tetraphenylporphine, in PEG-PE-Based Tumor-Targeted Immunomicelles. Cancer Biol Ther. 2007;6:1136–1142. doi: 10.4161/cbt.6.7.4345. [DOI] [PubMed] [Google Scholar]
- 11.Torchilin VP. Lipid-core micelles for targeted drug delivery. Current Drug Delivery. 2005;2:319–327. doi: 10.2174/156720105774370221. [DOI] [PubMed] [Google Scholar]
- 12.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from taxol. Journal of Clinical Oncology. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 14.Ruan G, Feng SS. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24:5037–5044. doi: 10.1016/s0142-9612(03)00419-8. [DOI] [PubMed] [Google Scholar]
- 15.Stinchcombe TE. Nanoparticle albumin-bound paclitaxel: a novel Cremphor-EL-free formulation of paclitaxel. Nanomedicine. 2007;2:415–423. doi: 10.2217/17435889.2.4.415. [DOI] [PubMed] [Google Scholar]
- 16.Campbell RB, Balasubramanian SV, Straubinger RM. Influence of cationic lipids on the stability and membrane properties of paclitaxel-containing liposomes. Journal of Pharmaceutical Sciences. 2001;90:1091–1105. doi: 10.1002/jps.1063. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, Kim DD. Antitumor Effect of Paclitaxel-Loaded PEGylated Immunoliposomes Against Human Breast Cancer Cells. Pharmaceutical Research. 2007;24:2402–2411. doi: 10.1007/s11095-007-9425-y. [DOI] [PubMed] [Google Scholar]
- 18.Nornoo AO, Osborne DW, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel I: formulation, cytotoxicity and hemolysis. Int J Pharm. 2008;349:108–116. doi: 10.1016/j.ijpharm.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8:202–208. doi: 10.1021/bm060307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seow WY, Xue JM, Yang YY. Targeted and intracellular delivery of paclitaxel using multi-functional polymeric micelles. Biomaterials. 2007;28:1730–1740. doi: 10.1016/j.biomaterials.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 21.You J, Hu FQ, Du YZ, Yuan H. Polymeric micelles with glycolipid-like structure and multiple hydrophobic domains for mediating molecular target delivery of paclitaxel. Biomacromolecules. 2007;8:2450–2456. doi: 10.1021/bm070365c. [DOI] [PubMed] [Google Scholar]
- 22.Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. Journal of Pharmaceutical Sciences. 1992;81:676–684. doi: 10.1002/jps.2600810718. [DOI] [PubMed] [Google Scholar]
- 23.Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JO, Johnson RK, Kingsbury WD. Modification of the hydroxy lactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. Journal of Medicinal Chemistry. 1989;32:715–720. doi: 10.1021/jm00123a038. [DOI] [PubMed] [Google Scholar]
- 24.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. Journal of Biological Chemistry. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 25.Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–661. [PubMed] [Google Scholar]
- 26.Gottlieb JA, Guarino AM, Call JB, Oliverio VT, Block JB. Preliminary pharmacologic and clinical evaluation of camptothecin sodium (NSC-100880) Cancer chemotherapy reports. 1970;54:461–470. [PubMed] [Google Scholar]
- 27.O'Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34:1500–1508. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 28.Conover CD, Greenwald RB, Pendri A, Shum KL. Camptothecin delivery systems: the utility of amino acid spacers for the conjugation of camptothecin with polyethylene glycol to create prodrugs. Anti-Cancer Drug Design. 1999;14:499–506. [PubMed] [Google Scholar]
- 29.Dharap SS, Qiu B, Williams GC, Sinko P, Stein S, Minko T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. Journal of Controlled Release. 2003;91:61–73. doi: 10.1016/s0168-3659(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 30.Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, Sinko PJ, Stein S, Farmanfarmaian A, Minko T. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12962–12967. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paranjpe PV, Stein S, Sinko PJ. Tumor-targeted and activated bioconjugates for improved camptothecin delivery. Anti-Cancer Drugs. 2005;16:763–775. doi: 10.1097/01.cad.0000172834.78068.7c. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Lynn BC, Zhang J, Song L, Bom D, Du W, Curran DP, Burke TG. A versatile prodrug approach for liposomal core-loading of water-insoluble camptothecin anticancer drugs. Journal of the American Chemical Society. 2002;124:7650–7651. doi: 10.1021/ja0256212. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg BB. Biologically active camptothecin derivatives for incorporation into liposome bilayers and lipid emulsions. Anti-Cancer Drug Design. 1998;13:453–461. [PubMed] [Google Scholar]
- 34.Ertl B, Platzer P, Wirth M, Gabor F. Poly(D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin. Journal of Controlled Release. 1999;61:305–317. doi: 10.1016/s0168-3659(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 35.Tong W, Wang L, D'Souza MJ. Evaluation of PLGA microspheres as delivery system for antitumor agent-camptothecin. Drug Development and Industrial Pharmacy. 2003;29:745–756. doi: 10.1081/ddc-120021774. [DOI] [PubMed] [Google Scholar]
- 36.Tyner KM, Schiffman SR, Giannelis EP. Nanobiohybrids as delivery vehicles for camptothecin. Journal of Controlled Release. 2004;95:501–514. doi: 10.1016/j.jconrel.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Kawano K, Watanabe M, Yamamoto T, Yokoyama M, Opanasopit P, Okano T, Maitani Y. Enhanced antitumor effect of camptothecin loaded in long-circulating polymeric micelles. Journal of Controlled Release. 2006;112:329–332. doi: 10.1016/j.jconrel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Koo OM, Rubinstein I, Onyuksel H. Camptothecin in sterically stabilized phospholipid micelles: a novel nanomedicine. Nanomedicine. 2005;1:77–84. doi: 10.1016/j.nano.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Koo OM, Rubinstein I, Onyuksel H. Camptothecin in sterically stabilized phospholipid nano-micelles: a novel solvent pH change solubilization method. Journal of Nanoscience and Nanotechnology. 2006;6:2996–3000. doi: 10.1166/jnn.2006.460. [DOI] [PubMed] [Google Scholar]
- 40.Opanasopit P, Ngawhirunpat T, Chaidedgumjorn A, Rojanarata T, Apirakaramwong A, Phongying S, Choochottiros C, Chirachanchai S. Incorporation of camptothecin into N-phthaloyl chitosan-g-mPEG self-assembly micellar system. European Journal of Pharmaceutics and Biopharmaceutics. 2006;64:269–276. doi: 10.1016/j.ejpb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama M, Opanasopit P, Okano T, Kawano K, Maitani Y. Polymer design and incorporation methods for polymeric micelle carrier system containing water-insoluble anti-cancer agent camptothecin. Journal of Drug Targeting. 2004;12:373–384. doi: 10.1080/10611860412331285251. [DOI] [PubMed] [Google Scholar]
- 42.Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110:370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochimica et Biophysica Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 44.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La SB, Okano T, Kataoka K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. Journal of Pharmaceutical Sciences. 1996;85:85–90. doi: 10.1021/js950204r. [DOI] [PubMed] [Google Scholar]
- 46.Dabholkar RD, Sawant RM, Mongayt DA, Devarajan PV, Torchilin VP. Polyethylene glycol-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. International Journal of Pharmaceutics. 2006;315:148–157. doi: 10.1016/j.ijpharm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Mu L, Elbayoumi TA, Torchilin VP. Mixed micelles made of poly(ethylene glycol)-phosphatidylethanolamine conjugate and d-alpha-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. International Journal of Pharmaceutics. 2005;306:142–149. doi: 10.1016/j.ijpharm.2005.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Advanced Drug Delivery Reviews. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Mi Z, Burke TG. Differential interactions of camptothecin lactone and carboxylate forms with human blood components. Biochemistry. 1994;33:10325–10336. doi: 10.1021/bi00200a013. [DOI] [PubMed] [Google Scholar]