Abstract
Dorsal interacting protein 3 (Dip3) contains a MADF DNA-binding domain and a BESS protein interaction domain. The Dip3 BESS domain was previously shown to bind to the Dorsal (DL) Rel homology domain. We show here that Dip3 also binds to the Relish Rel homology domain and enhances Rel family transcription factor function in both dorsoventral patterning and the immune response. While Dip3 is not essential, Dip3 mutations enhance the embryonic patterning defects that result from dorsal haplo-insufficiency, indicating that Dip3 may render dorsoventral patterning more robust. Dip3 is also required for optimal resistance to immune challenge since Dip3 mutant adults and larvae infected with bacteria have shortened lifetimes relative to infected wild-type flies. Furthermore, the mutant larvae exhibit significantly reduced expression of antimicrobial defense genes. Chromatin immunoprecipitation experiments in S2 cells indicate the presence of Dip3 at the promoters of these genes, and this binding requires the presence of Rel proteins at these promoters.
Keywords: Rel family, D/V patterning, antimicrobial defense genes, centromere, ChIP, innate immunity
Introduction
The Drosophila melanogaster genome encodes three rel homology domain (RHD) containing proteins, Dorsal (DL), Dorsal-related immunity factor (Dif), and Relish (Rel)[1–6]. The RHD, which is also found in the human NFκB family of transcriptional activators, mediates dimerization and sequence-specific DNA binding. Rel/NFκB family proteins in vertebrates and invertebrates play central roles in the innate immune response by triggering the expression of antimicrobial defense genes in response to signals transduced by Toll and the Immune deficiency (Imd) signal transduction pathways [3, 5]. In Drosophila, DL also directs dorsoventral (D/V) patterning of the embryo. Specifically, the regulated nuclear localization of maternally expressed DL in response to Toll signaling in the embryo leads to the formation of a ventral-to-dorsal nuclear concentration gradient of DL and to the spatially restricted regulation of a large number of genes, including twist (twi), snail (sna), and rhomboid (rho), which are activated by DL, and zerknullt and decapentaplegic, which are repressed by DL. This serves to subdivide the embryo into multiple developmental domains along its D/V axis [7–9].
Unlike DL, Dif and Rel are not required for D/V patterning. Instead, these two rel-family proteins function along with DL in the innate immune response [2, 5, 10, 11]. Toll signaling in the immune system leads to the the translocation of DL and Dif to the nucleus and the consequent activation of a subset of anti-microbial defense genes, including drosomycin (drs) and Immune induced molecule 1[1, 12–14]. DL and Dif are believed to have redundant roles in this process and thus either one alone is sufficient for the induction of drs [15, 16]. Activation of the Imd signal transduction pathway, leads to proteolytic cleavage of Rel [17–19]. The N-terminal region of Rel, which contains the RHD, then translocates into the nucleus where it activates expression of anti-bacterial genes, such as diptericin (dipt), cecropin-A1 (cec-A), and attacin-A. DL, Dif, and Rel homo- and heterodimerize to activate different subsets of the anti-microbial defense genes in response to signals from the Toll and Imd pathways [4, 20–23].
Very little is known about the identity of factors that assist the RHD proteins in the activation of the anti-microbial defense genes. Proteins that modulate expression of these genes include transcription factors such as the GATA factor Serpent (Srp,[24–26]), Hox factors [27], Helicase89B [28], and an unknown protein that binds region 1 (R1,[29]), a regulatory module in cec-A and other anti-microbial defense genes. In addition, a recent screen identified several POU domain proteins as potential regulators of anti-microbial defense genes [30].
To date, about a dozen proteins that interact directly with DL and modulate its regulatory functions have been identified by genetic and biochemical means. For example, an interaction between DL and Twist (Twi) enhances the activation of DL target genes, while an interaction between DL and Groucho (Gro) is essential for DL-mediated repression [31, 32]. A yeast two-hybrid screen to identify DL interacting proteins yielded, in addition to the well characterized DL-interactors Twi and Cactus [3], four novel DL-interactors (Dip1, Dip2, Dip3, and Dip4/Ubc9) [33]. Conjugation of SUMO to DL by Ubc9 was subsequently shown to result in more potent activation by DL [33, 34].
Dip3 belongs to a family of proteins that contain both MADF (for Myb/SANT-like in ADF) and BESS (for BEAF, Stonewall, SuVar(3)7-like) domains. While MADF-BESS domain proteins are found in both insects and vertebrates, only a few [35] have been characterized and their functions are largely unknown. The Drosophila genome encodes 14 MADF-BESS domain factors. In addition to Dip3, these include Adf-1, which was initially found as an activator of Alcohol dehydrogenase, and Stonewall, which is required for oogenesis [36, 37]. The Dip3 MADF domain mediates sequence specific binding to DNA, while the Dip3 BESS domain mediates binding to a subset of TATA binding protein associated factors as well as to the DL RHD and to Twi [38]. In addition to functioning as an activator, Dip3 can function as a coactivator to stimulate synergistic activation by DL and Twi in S2 cells.
In this study we show that Dip3 assists RHD proteins during both embryonic development and the innate immune response. By stimulating the expression of antimicrobial defense genes, Dip3 improves survival of both larvae and adults following septic injury. The presence of Dip3 near the promoters of antimicrobial defense genes depends upon Rel family proteins suggesting that Dip3 functions as a coactivator at these promoters.
Materials & Methods
Molecular cloning
S2 cell vectors encoding FLAG-tagged Dip3, GFP-tagged Dip3, FLAG-tagged DL, DL, and Twi have been described previously [31, 33, 38, 39]. The vectors encoding Rel (pSH-FLAG-Rel) and Dif (pSH-His-Dif) were provided by T. Ip [20]. Plasmids bearing the sna and rho promoters were obtained from T. Ip and subcloned (Bhaskar & Courey, Unpublished) into -37tkLuc, which encodes firefly luciferase under the control of the HSVtk core promoter [32, 40]. The -37tk Renilla Luc reporter [33] served as an internal control for the luciferase assays. The Dip3 coding region was subcloned into the UASp vector [41] for maternal and zygotic expression in flies via the Gal4/UAS system.
Cell culture, co-immunoprecipitation, luciferase assays and dsRNA interference
Drosophila S2 cells were maintained in 6 well plates at 25°C in Schneider's Drosophila medium (Sigma) supplemented with heat-inactivated 10% fetal bovine serum (HyClone). Cells were transfected with either calcium phosphate or the Effectene reagent (Qiagen).
Co-immunoprecipitation experiments were carried out in interaction buffer (25 mM HEPES pH=7.9, 1.5 mM MgCl2 100 mM NaCl, 10% Glycerol, 0.5% NP40, 0.5 mg BSA/ml) or in 1X PBS (pH=7.5) as described previously [32, 34]. Approximately 25 µl of FLAG beads (Sigma Chemical Co.) bound to 500 ng of FLAG-Rel or FLAG-Dorsal were incubated with 35S-labeled Dip3. The beads were extensively washed with interaction buffer and bound proteins were eluted by boiling in SDS-PAGE sample buffer and resolved by SDS-PAGE. The 35S labeled proteins were visualized with a phosphorimager.
RNA interference (RNAi) was carried out as described previously [34, 42]. 1–10 ug of purified dsRNA was added during transfection. 10 ug of Dip3 dsRNA and 1 ug each of DL, Dif, and Rel dsRNA were required for effective knockdown of expression in five days.
Luciferase activity was determined with the Dual Luciferase Reporter Assay system (Promega) as described previously [34]. Transiently transfected and stable lines expressing combinations of FLAG-Dip3, Dip3GFP, DL, FLAG-DL, FLAG-Rel, Rel, or 6XHis tagged DL-like-immunity factor (His-Dif) were generated for immunoprecipitation and chromatin immunoprecipitation (ChIP) experiments. Lipopolysaccharide (LPS) induction was carried out by adding 10 ug of LPS (Sigma) per ml of S2 medium for a period of 4 hours.
Fly strains and procedures
Oregon R, Canton S, and w1118 flies were used as wild-type controls. Mutants used in this study are: dorsal1(dl1); dorsal-interacting-protein 31(Dip31); spaetzlerm7 (spzrm7); RelishE20 (RelE20), all of which are strong hypomorphic or null alleles that have been previously described [34, 42] (Duong et. al., 2008, manuscript under submission). The Dip31 allele was created by imprecise excision of a P-element inserted immediately upstream of the Dip3 transcriptional start site. The resulting deletion of genomic DNA removes the Dip3 transcriptional and translational start site and 930 bp of the Dip3 coding region (Duong et. al., manuscript submitted). The mutation was extensively backcrossed to wild-type flies to remove background mutations. The UASpDip3 lines were generated by P-element transformation using standard methods. Maternal Gal4 expression, cuticle preparations and in situ hybridization were performed as described [31]. The maternal Gal4 driver (obtained from Daniel St. Johnston, University of Cambridge, UK) encodes Gal4-VP16 under the control of the maternally active α-4-Tubulin promoter. Fat body expression was achieved with by either the Fat body-Gal4 (Lsp2; Bloomington #6357) driver or the Collagen-Gal4 driver (Cg-Gal4)[43].
Antibody staining and microscopy
S2 cells, Drosophila embryos and larval tissues were fixed in 4% Methanol free formaldehyde for 15 –30 minutes and washed in 1X PBS with 0.1% Tween. When required, Taxol was added at a final concentration of 10 µM during fixation to arrest cells at metaphase. Tissues were blocked with 10% BCS (Novacell) for 1 hour and primary and secondary antibodies were incubated with the tissue in 10% BCS for 1 hour at room temperature or overnight at 4° C. Primary antibodies utilized in this study include: rabbit anti-DL (Ratnaparkhi et. al., 2006), mourse anti-DL (DSHB, produced by Ruth Steward), rabbit anti-Dip3 (Duong et. al., under submission), mouse anti-tubulin (Sigma), mouse anti-FLAG (Sigma), and rabbit anti-GFP (Invitrogen). The Dip3 antibodies were preadsorbed to Dip31 embryos before use. Alexa Secondary antibodies used in this study were obtained from Invitrogen and were also preadsorbed to Oregon R embryos before use.
Infection and survival experiments
For survival experiments after septic injury we used Drosophila adults, aged 1–3 days. Septic injury was performed by pricking the thorax of the flies with a thinly pulled capillary previously dipped into a ten-times concentrated overnight culture of Escherichia coli and/or Micrococcus luteus [13, 44]. The entomopathogenic fungus Beauveria bassiana (ATCC #9453) was used for fungal infection. Fungal spores were injected into the fly by pricking with a needle. Post-injury, flies were incubated at 25 °C and monitored and also put in new vials every 2 days for a period of one month. 50 flies were kept per vial and four independent vials were maintained to generate statistical data. Septic injury experiments were also performed on climbing third instar larvae in a manner similar to that described for adult flies.
Antimicrobial gene expression
The levels of the expression of the antimicrobial defense genes were quantified by measuring mRNA levels in the larvae by quantitative real time PCR (QRT-PCR). Infected larvae were maintained on wet filter papers placed on apple juice plates. The larvae were allowed to recover on these plates for 0–4 hours. RNA was extracted from 25 larvae (for each experimental point) using 1 ml of TRIZOL (Invitrogen) according to the manufacturers protocol. The extracted RNA was resuspended in RNase free water, heated at 80 °C for 10 minutes in order to dissolve the RNA pellet, cooled and then treated with DNase I for 30 minutes. Next, the RNA was re-purified on an RNeasy column (Qiagen). The RNA quality was judged by loading on an agarose gel and quantified using a spectrophotometer. 1 ug of this purified RNA was used in a Reverse transcriptase reaction using the M-MLV enzyme (Invitrogen) as per the supplied protocol. 500 ng of cDNA produced from the above reaction was diluted 1:5 in water and used to quantify expression of drs, dipt, cec-A & rp49 by Quantitative Real Time PCR. The amplification reaction was carried out with reagents from the iQ SYBR green Supermix qPCR kit (Bio-Rad Laboratories). Amplification was carried for 40 cycles (95°C for 15 min, followed by cycles of 95°C for 10 s, 65°C for 30 s, and 72°C for 30 s) in an Opticon 2 (MJ Research), followed by a T-melt to confirm homogeneity of the amplified product. All samples were analyzed in duplicate and the amount of mRNA was normalized using a dilution series of rp49. We used the normalized data to quantify the relative levels of antimicrobial defense gene mRNA according to cycling threshold analysis (ΔCt). Fold changes were calculated by using the equation fold change = 2−ΔCT, where CT is threshold cycle and ΔCT = [CT (gene of interest) − CT (rp49)] [45]. CT values for multiple experiments were averaged. The following gene-specific primers pairs [46, 47] were used: drs, 5'-CGTGAGAACCTTTTCCAATATGATG -3' and 5'-TCCCAGGACCACCAGCAT -3'; dipt, 5'- GCTGCGCAATCGCTTCTACT -3' and 5'-TGGTGGAGTGGGCTTCATG -3'; cec-A 5'-TTTCGTCGCTCTCATTCTGG-3' and 5'-GACAATCCCACCCAGCTTCCCGATTG-3'; and ribosomal protein 49(rp49), 5'-GACGCTTCAAGGGACAGTATCTG -3' and 5'- AAACGCGGTTCTGCATGAG -3'.
Chromatin immunoprecipitation (ChIP)
ChIP was carried out using the mouse anti-FLAG (Sigma) and rabbit anti-GFP (Invitrogen) antibodies. The ChIP protocol used was modified from the protocol provided by Upstate Biotechnology (Millipore Life Sciences). In short, 1% Formaldehyde was added to 4 × 106 S2 cells at 24 °C for 1 hour. The formaldehyde was washed away with multiple washes in ice cold PBS and glycine. The cells were resuspended in 300 ul of ice cold lysis buffer (with protease inhibitors) and the genomic DNA was sheared at 4 °C to an average length of 500 bp in a Diagenode Bioruptor (20 minutes total; 30 sec On, 1 minute Off). The shearing was confirmed by loading a small aliquot of the sheared sample on an agarose gel. The extract was diluted 10 fold in dilution buffer and 10% of the total (“the input”) was set aside at −80 °C. The rest of the extract was then incubated with 6 ug of primary antibody overnight at 4–8 °C on a nutator. The next day, 25 ul of Protein A beads (Amersham) was added per extract for 2 hours at 4 °C and washed with increasingly stringent buffers. The chromatin immunoprecipitated by the beads was extracted at 65 °C in elution buffer. The crosslinking was reversed by incubating the eluted chromatin at 65 °C overnight. The eluted DNA was purified using an Invitrogen PCR purification column. Real time-PCR was used to quantitate the amount of DNA immunoprecipitated in each sample. The following gene specific primers pairs were used: drs, 5'-CCTGGGGTTTTTACAATCCAT-3' and 5'-GACGCGAGTTTGGATAGGTC-3'; dipt, 5'-AAGAAAGATCCCCTGGTGGT-3' and 5'-CTTTTATAGGCCGCTTTCCA-3'; cecA 5'-CATTGAAATCCCCGATTGTT-3' and 5'-TGAGGTCTGAGCGACTGATG-3'.
Results
Dip3 in D/V pattern formation
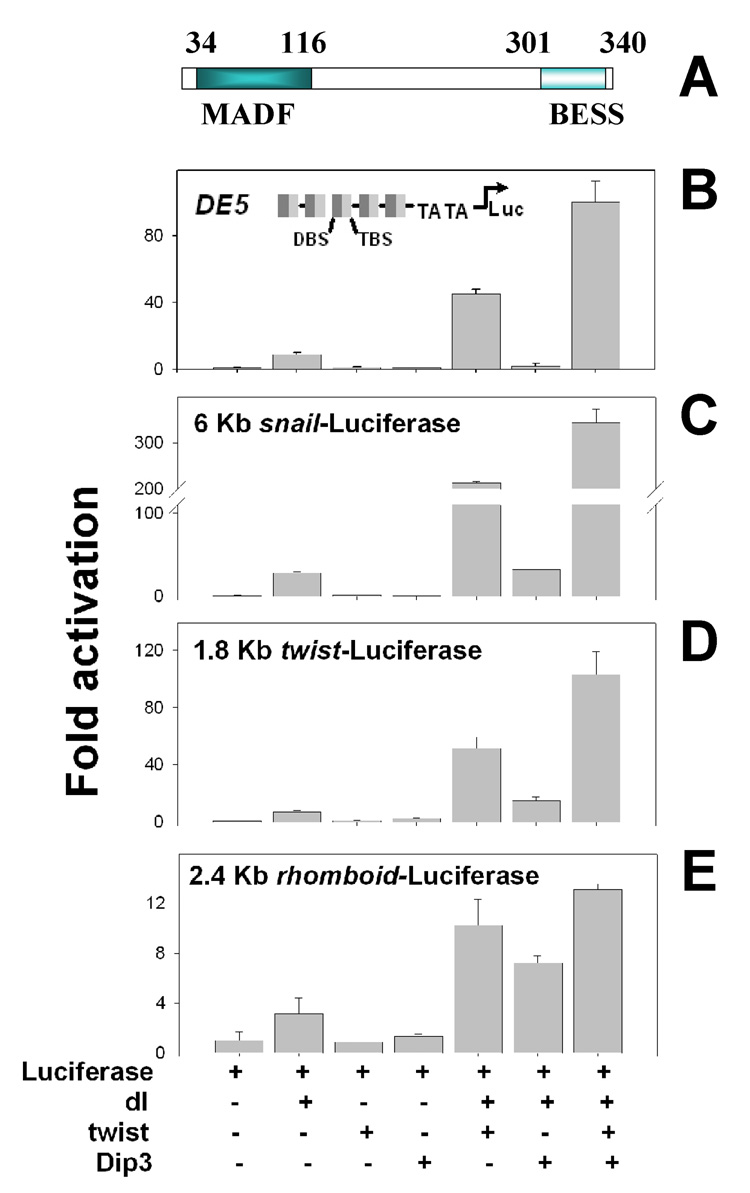
We previously showed that Dip3 (Figure 1A), which binds both DL and Twi via its BESS domain, synergistically enhances the activation of a luciferase reporter with multiple DL and Twi binding sites upstream of the promoter (Figure 1B,[38]). In addition, Dip3 has been implicated as the “mystery protein” which binds to sites adjacent to DL and Twi binding sites in a subset of DL target genes [48]. We therefore examined the ability of Dip3 to enhance the expression of the DL target promoters twi, sna, and rho in S2 cell transient transfection assays. All three promoters require both DL and Twi for full activity. Dip3 was found to synergize with DL and Twi in the activation of the sna (Figure 1C) and twi (Figure 1D) promoters, but not in the activation of the rho promoter (Figure 1E).
Figure 1. Dip3 enhances synergistic activation of DL/Twi target genes.
(A) Dip3 contains an N-terminal MADF domain, which binds a DNA sequence motif with a consensus CCNNNCCNNNCC, and a C-terminal BESS, which mediates interactions with other proteins[38]. (B) Activation of the DE5 reporter, which contains alternating DL binding sites (DBS) and Twi binding sites (TBS) upstream of a minimal TATA-containing promoter by the indicated combinations of vectors encoding DL, Twi, and Dip3. (C–E) Activation of reporters containing the sna core promoter and 6 kb of 5’ flanking sequence (C), the twi core promoter and 1.8 kb of 5’ flanking sequence (D), or the rho core promoter and 2.4 kb of 5’ flanking sequence (E) by the indicated combinations of vectors encoding DL, Twi, and Dip3.
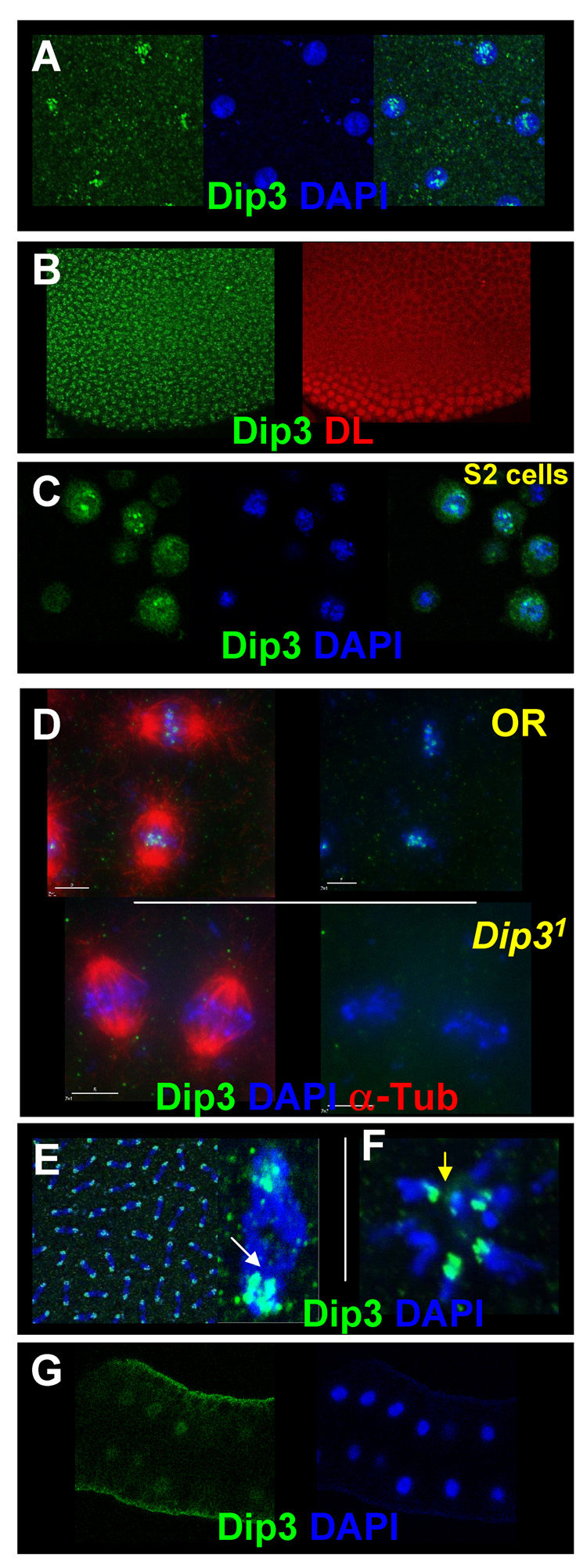
We generated a polyclonal antibody against recombinant Dip3, and used it to determine where and when Dip3 is present in the embryo. Maternally expressed Dip3 is observed in all nuclei as early as nuclear cycle 7 (Figure 2A). We continue to detect it in subsequent nuclear cycles during formation of the DL nuclear concentration gradient (Figure 2B). In interphase embryonic as well as S2 cell nuclei, Dip3 localizes to nuclear speckles of unknown identity (Figure 2A, C). During mitosis Dip3 is enriched on chromosomes (Figures 2D, top panel and 2E). It associates with the centrosome proximal portion of the anaphase chromatids (Figure 2E, arrow) and the inside ring of the polar body rosette (Figure 2F, arrow) suggesting a predominant pericentromeric location at this stage of the cell cycle and hinting at a possible role of Dip3 in centromeric function. Confirming the specificity of the antibodies, the immunoreactivity is absent from Dip31 embryos in which the Dip3 transcriptional and translational start sites as well as a large segment of the Dip3 coding region have been deleted (see Materials and Methods) (Figure 2D, bottom panel). Weak Dip3 expression is also detected in the larval fat body (Figure 2G).
Figure 2. Immunolocalization of Dip3.
A, B Nuclear cleavage stage interphase nuclei showing Dip3 nuclear speckling as revealed by Dip3 antibodies. Nuclear Dip3 is observed during the early nuclear cleavages (A) and in the syncytial blastoderm embryo (B). (B) also shows the same embryo stained with anti-DL antibodies to reveal the DL nuclear concentration gradient. (C) Staining of S2 cells with the Dip3 antibody reveals localization of Dip3 to nuclear speckles in interphase nuclei. (D) Dip3 localizes to chromosomes in taxol-stabilized metaphase embryos (top). The staining is absent in Dip31 flies (bottom). (E) Dip3 predominantly localizes to the pericentromeric region during anaphase (inset shows a single highly magnified anaphase figure). (F) Peri-centromeric localization (arrow) is also observed the polar body rosette. (G) Dip3 is also found in the larval fat body nuclei.
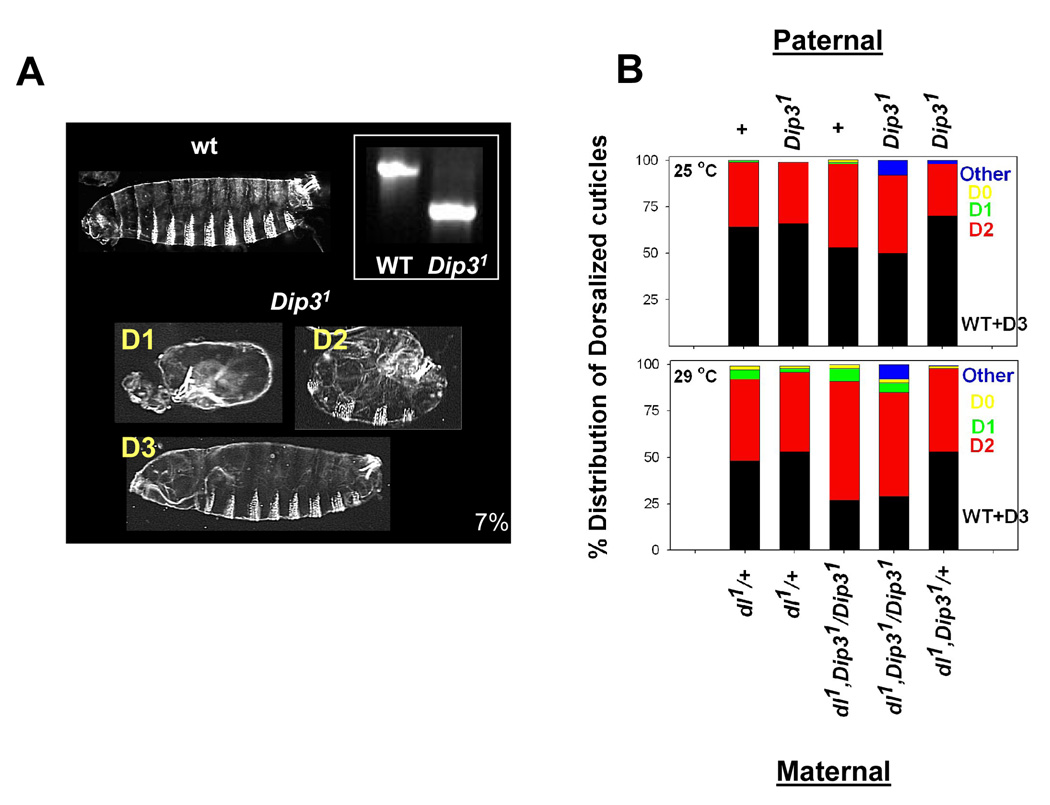
Homozygous Dip31 flies are viable and fertile, indicating that Dip3 cannot have an essential role in embryonic D/V pattern formation. However, a small proportion (7±4%) of the embryos fail to hatch and exhibit D/V patterning defects (Figure 3A). Embryos produced by females transheterozygous for Dip31 and a deficiency that removes a portion of the second chromosome containing the Dip3 gene (Df(PC4) exhibit similar embryonic lethality (10%) and D/V patterning defects (data not shown). Also, maternal overexpression of Dip3 using the Gal4-UAS system leads to 54±9 % embryonic lethality with cuticles of the dead embryos showing both anteroposterior and D/V patterning defects, indicating that Dip3 may have a role in embryonic pattern formation.
Figure 3. The Dip31 mutation enhances the dl haploinsufficiency phenotype.
(A) Dip31 null embryos exhibit a low level of embryonic lethality (~7%). Cuticle preparations show dorsalization of ~5% of the Dip31 embryos. Dorsalized embryos are categorized as D3, D2, D1, or D0 in order of increasing dorsalization (see text for details). Inset: Genomic PCR of wild-type (Oregon-R) and Dip31 flies showing the 930 bp deletion that removes a large portion of the Dip3 coding region.
(B) The Dip31 mutation enhances the maternal dl haploinsufficiency phenotype. Cuticles of embryos produced by dl1/+ females at 25 °C and 29 °C were categorized according to phenotype. The graph displays each phenotypic category (WT, D3, D2, D1, D0, Other) as a percentage of the total. 400 embryos were counted for each genotype. The maternal genotypes are given at the bottom while the paternal genotypes are given at the top. For flies with the maternal genotype dl1,Dip31/Dip31 the increase in the D2 subset was significant (t-test, P<0.005) at 29° C, but not at 25° C (P>0.1).
Consistent with a non-essential role for Dip3 in D/V patterning, a Dip3 mutation enhances the temperature sensitive dl haploinsufficieny phenotype (Figure 3B). The degree of dorsalization is often quantified by categorizing embryos on a scale from D0 (completely dorsalized, lacking all dorsoventral pattern elements other than dorsal epidermis) to D3 (inviable, but with little or no apparent defect in the cuticular pattern). At 29°, about half the dead embryos produced by dl1/+ females exhibit detectable D/V patterning defects and the majority of these fall into the D2 category (moderately dorsalized, exhibiting mildly expanded ventral denticle belts and a twisted germ band) (Figure 3A,B). Removal of maternal Dip3 increases the proportion of dorsalized embryos to about 75% with most of the increase being due to an increase in the number of D2 embryos. The effect seems to be strictly maternal as the paternal genotype does not modulate the dl haploinsufficiency phenotype.
Dip3 activates RHD target genes in the immune system
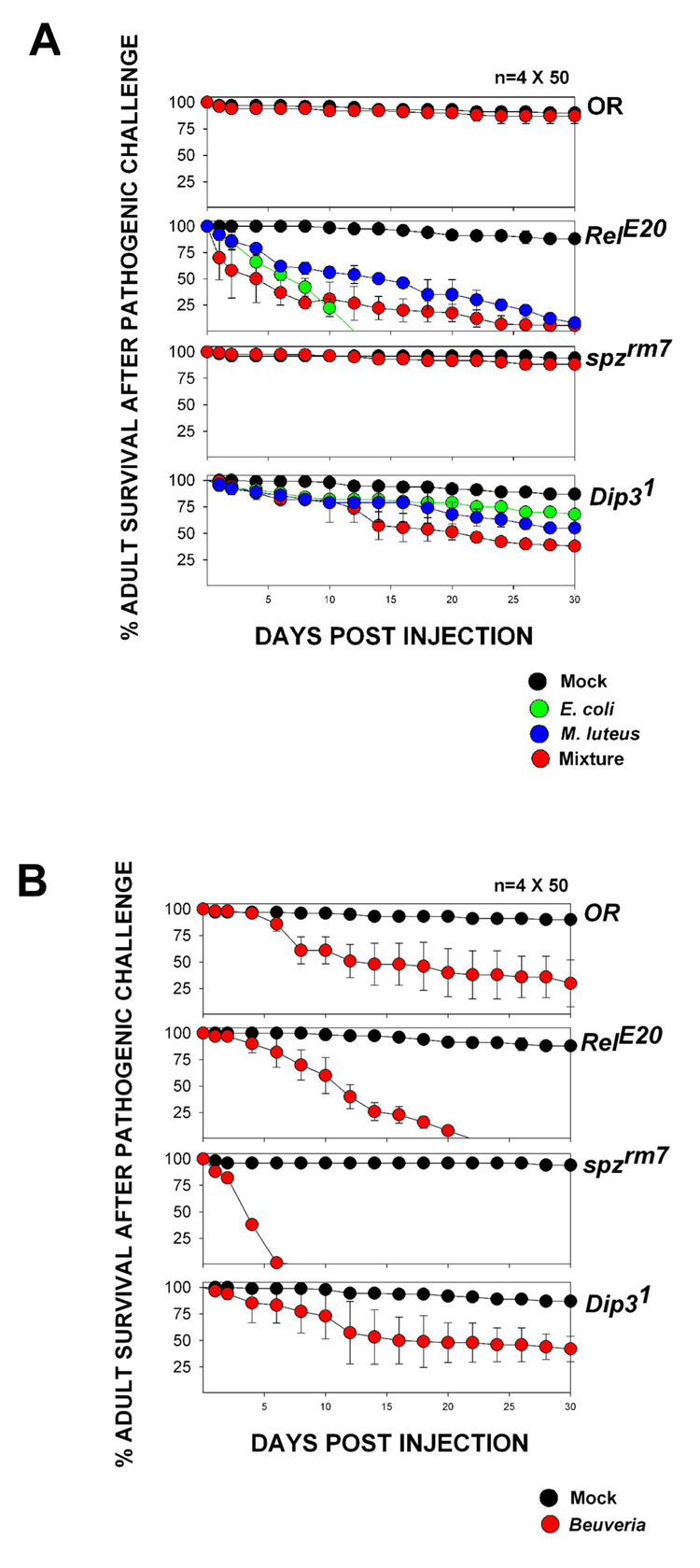
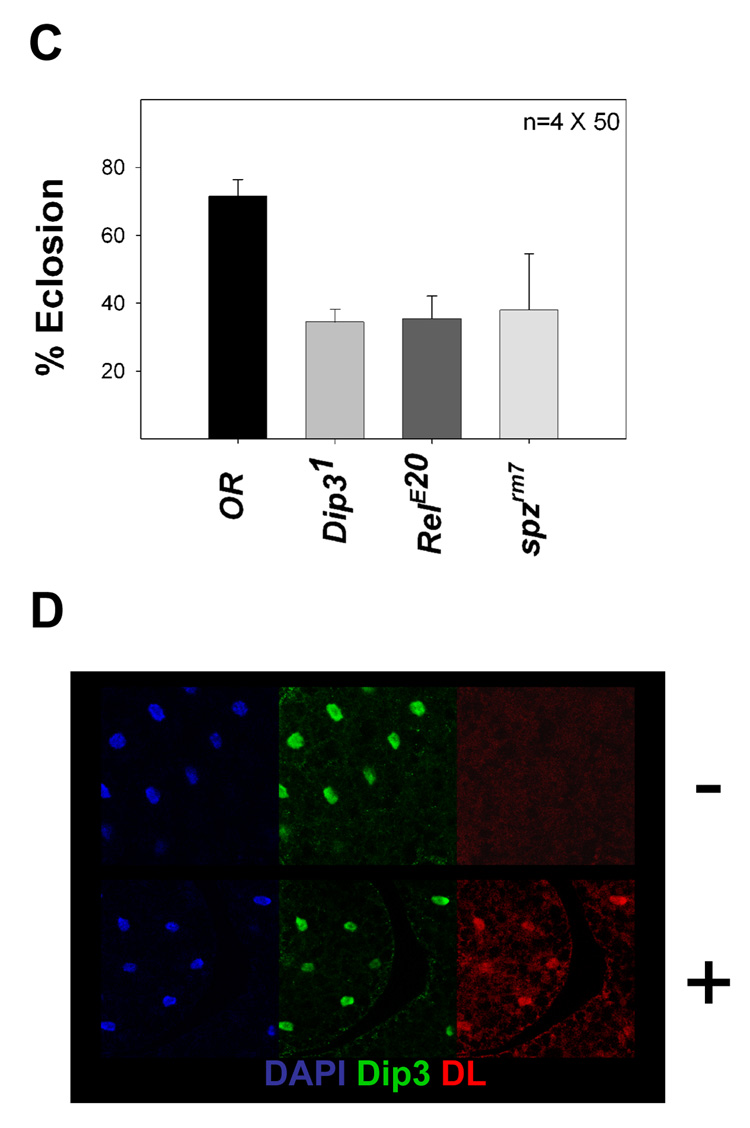
Dip3 is present in the fat body (Figure 2G), the organ in which RHD factors activate antimicrobial defense genes in response to infection. Since Dip3 binds the DL RHD, we examined the role of Dip3 in the innate immune response by assessing the sensitivity of Dip31 flies to bacterial and fungal infection. Wild-type and Dip31 adults and larvae were injected with gram positive bacteria (M. luteus), gram negative bacteria (E. coli), and fungi (B. brassiana). For comparison, we also infected flies containing mutations in known components of the Toll (spzrm7) and Imd (RelE20) pathways. Wild-type, RelE20, spzrm7, and Dip31 adults showed little lethality (<15%) 30 days after mock infection (injection with a sterile needle). However, the Dip31 adult flies exhibited 55% lethality one month after injection with a 1:1 mixture of M. luteus and E. coli, compared to 10% lethality after 30 days for wild-type flies and 98% after 30 days for RelE20 flies (Figure 4A). In contrast, wild-type and Dip31 adults were equally sensitive to fungal infection, both showing 55–70% lethality after 30 days compared to 100% lethality after 22 days for RelE20 adults and 100% lethality after 7 days for spzrm7 adults (Figure 4B). Similar results were seen in larvae (Figure 4C) in which Dip31, RelE20 and spzrm7 mutations resulted in reduced rates of eclosion following septic injury compared to wild-type. The effectiveness of the immune challenge was further verified by an experiment showing that septic injury leads to translocation of DL into the nucleus (Figure 4D).
Figure 4. Dip31 flies are sensitive to bacterial infection.
(A) Adult survival as a function of time post injection for Oregon R (wild-type), Dip31, RelE20, and spzrm7 flies injected with M. luteus, E coli, or a mixture of M. luteus and E. coli. “Mock injected” flies were pricked with a sterile needle.
(B) Adult survival as a function of time post injection for Oregon R (wild-type), Dip31, RelE20, and spzrm7 flies injected with the fungus Beuveria bassiana. “Mock injected” flies were pricked with a sterile needle.
(C) Eclosion rates of Oregon R (wild-type), Dip31, RelE20, and spzrm7 larvae injected with a mixture of M. luteus and E. coli. “Mock injected” larvae were pricked with a sterile needle. The wild-type injected larvae show significant lethality because of trauma to the larvae caused by injection. The decrease in eclosion relative to wild-type for Dip31, RelE20 and spzrm7 larvae was statistically significant (t-test; P<0.001, P<0.001 and P=0.008 respectively).
(D) DL is not detected in the fat body nuclei of an uninfected larva (−), but is accumulated in the fat body nuclei after injection (+). Dip3 nuclear localization is not affected by septic injury.
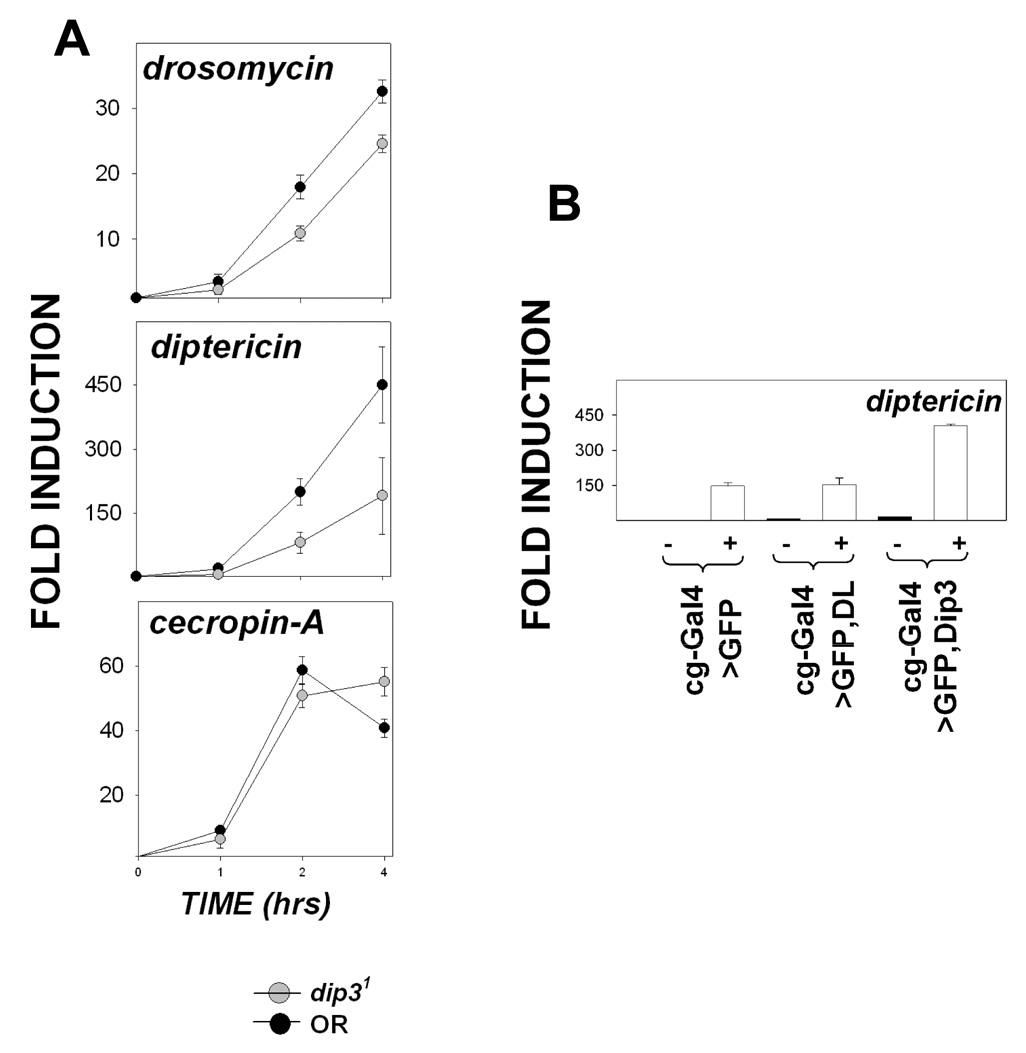
To determine if the sensitivity of Dip31 flies to infection results from reduced induction of antimicrobial peptides, we monitored the expression of dipt, drs and cec-A as a function of time following septic injury (Figure 5A). Relative to uninfected flies, the levels of expression of drs and dipt were reduced by the Dip31 mutation, especially at the 2 and 4 hr time points, while the levels of cec-A expression were not significantly altered. Thus, some, but not all, antimicrobial defense genes that are regulated by RHD family proteins exhibit dependence on Dip3. At the 4 hr time point, relative to infected, wild type flies, the spzrm7 mutation reduced drs expression to basal levels while the RelE20 mutation reduced dipt expression ten fold (data not shown).
Figure 5. Anti-microbial defense gene expression is reduced in Dip31 larvae.
(A) Expression of drs, dipt, and cecA in Dip31 and OR larvae, as assayed by quantitative real time PCR, 0, 1, 2, and 4 hrs after infection with a mixture of gram positive and gram negative bacteria. The fold activation is calculated relative to the 0 hr time point. The data shown is from two independent experiments, each done in duplicate. The expression levels of drs and dipt at the 2 and 4 hour time points were significantly lower in Dip31 larvae than in OR larvae (P ≤ 0.05).
(B) Expression of dipt in Cg-Gal4; UAS-GFP (control), Cg-Gal4; UASp-DL, and Cg-Gal4; UASp-Dip3 larvae as determined by quantitative RT-PCR in uninfected animals (−) and in animals 4 hr after infection (+) with a mixture of gram positive and gram negative bacteria. The data shown is the average of four independent experiments. Fold induction is calculated relative to Cg-Gal4; UAS-GFP uninfected larvae. The expression level for dipt in infected Cg-Gal4; UASp-Dip3 larvae is significantly higher (P <0.001) than in the controls not overexpressing Dip3.
Dip3 was over expressed in the larvae using the Cg-Gal4 driver to examine the effect of increasing levels of Dip3 on the expression of antimicrobial defense genes in the fat body. Cec-A and drs levels were unaffected (data not shown), while dipt levels increased two-fold in infected flies (Figure 5B). Thus, both loss-of-function and over expression data are consistent with the conclusion that Dip3 makes the immune response more robust by elevating the expression of a subset of antimicrobial defense genes.
Dip3 is present at antimicrobial defense genes
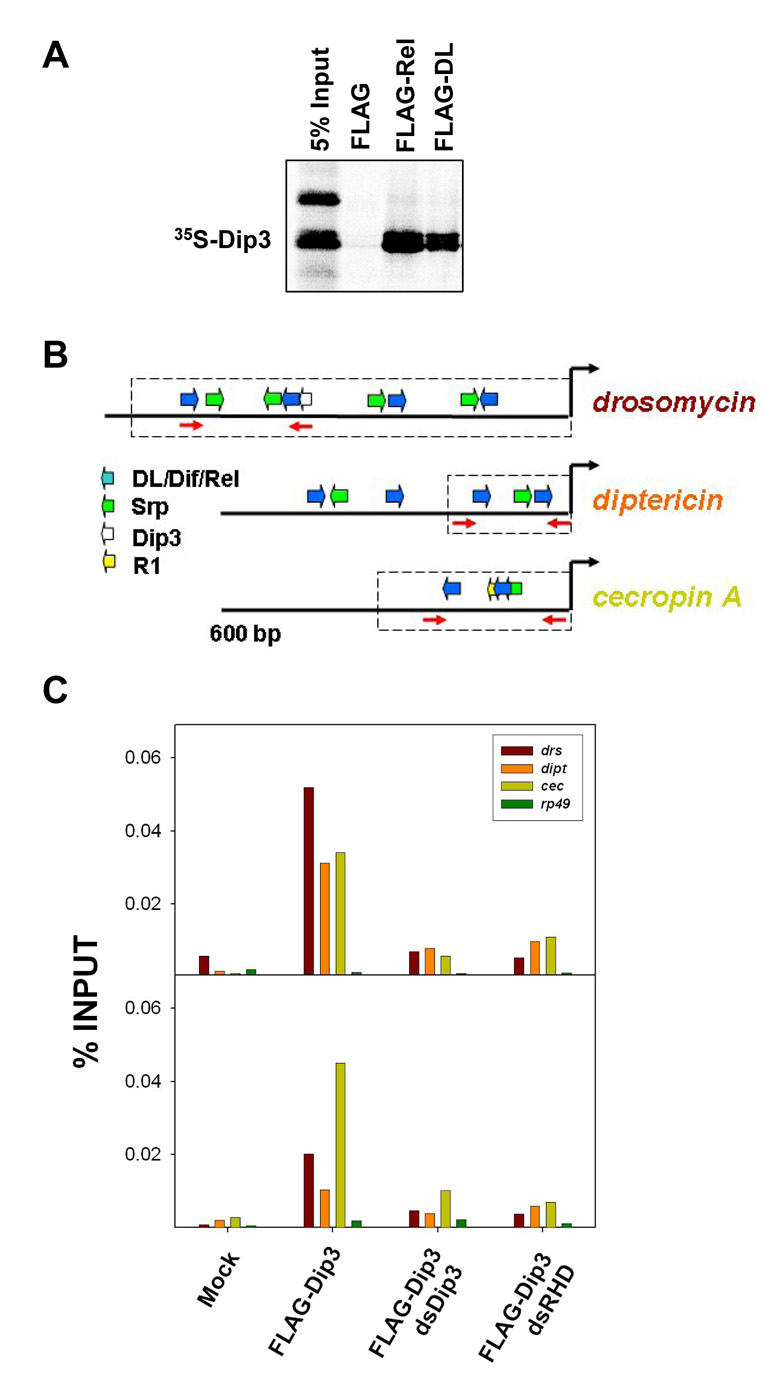
Radiolabeled Dip3 interacts with FLAG-tagged DL and Rel (Figure 6A) immobilized on anti-FLAG beads. Similarly, immobilized FLAG-Dip3 binds DL [38] and Rel (Residues 1–600; data not shown). Dip3 binds to DNA via its MADF domain and to the RHD via its BESS domain, and can thus function either as an activator or as a coactivator [38]. To determine if Dip3 is present at the promoters of antimicrobial defense genes, we carried out ChIP assays in S2 cells transfected with FLAG-Dip3 (Figure 6C - the two graphs show duplicate runs of the same experiment). FLAG antibody was used to immunoprecipitate Dip3 crosslinked to chromatin. Compared both to mock-transfected cells and to the transcribed region of a ribosomal protein-encoding gene (rp49), Dip3 was highly enriched at the drs, dipt and cecA promoters. As expected, dsRNA directed against Dip3 eliminated the ChIP signal verifying antibody specificity. The association of Dip3 with the promoters of the anti-microbial defense genes depended on Rel family proteins as knockdown of these proteins by dsRNAi significantly reduced association of Dip3 with the promoters. Similar results were observed with an anti-GFP antibody and cells expressing a Dip3-GFP fusion protein (data not shown).
Figure 6. Dip3 is present at the enhancers of the antimicrobial defense genes.
(A) Approximately 500 ng of FLAG-DL and FLAG-Rel bound to FLAG-Beads were incubated with equivalent amounts of in vitro translated 35S-Dip3. Dip3 bound to DL and Rel but not to beads alone.
(B) The 5’ flanking regions of three antimicrobial defense genes [26], indicating binding sites for the RHD proteins DL, Dif, and Rel, and binding sites for the GATA factor Serpent (Srp). Also indicated is a potential binding site for Dip3 in drosomyocin and the Region 1 (R1) motif in cecropin A [29]. The dashed boxes indicate regions of the gene that have been experimentally confirmed [26] to direct expression patterns that mimic those of the intact genes. The primer pairs used for amplification in the ChIP assays (Figure 6B) are shown as red arrows.
(C) Chromatin immunoprecipitation assays carried out on cells expressing FLA-Gtagged Dip3, DL, His-Dif, Rel and the indicated double stranded RNAs. Quantitative real time PCR was used to quantify levels of drs, dipt, and, cecA 5’ flanking DNA in the immunoprecipitate (PCR primers are shown in A). The rp49 gene was used as a negative control. The two panels are independent repetitions of the ChIP experiment.
Discussion
The regulation of eukaryotic genes frequently depends on synergistic interactions between transcription factors. This is essential for the rich array of transcriptional responses required for both development and adaptation to the environment. For example, synergy between rel homology domains and other proteins is essential for robust target gene activation in both development [49, 50] and immunity[26]. These interactions include DL/Twi synergy, which mediates spatially and temporally-regulated target gene expression in the early embryo, as well as synergy of multiple Rel family proteins with Srp to enhance activation of antimicrobial defense genes in larvae and adults.
The results presented here suggest that Dip3 may synergize with RHD proteins in multiple developmental contexts possibly through contact with the DL rel homology domain [38]. Dip3 is expressed maternally and present in cleavage stage nuclei at the time that DL is functioning to pattern the D/V axis. Furthermore, Dip3 can potentiate DL-mediated activation of the twist and snail promoters in S2 cells. These observations suggest that Dip3 might have a role in D/V patterning. Consistent with this possibility, we find that removal of maternal Dip3 results in occasional D/V patterning defects and significantly enhances the dl haploinsufficiency phenotype suggesting the Dip3 renders D/V patterning more robust perhaps by assisting in DL-mediated activation.
An important aspect of the immune response is activation in the fat body of genes encoding antimicrobial peptides by the Rel family transcription factors DL, Dif, and Rel. Since Dip3 is expressed in the fat body, we looked at its role in the immune response. We discovered that synergistic killing of flies by a mixture of E.coli and M. luteus is enhanced in Dip31 flies. This suggests roles for Dip3 in the Imd and/or Toll pathways, which mediate the response to microbial infection. In accord with this idea, we find that activation of the Imd pathway target dipt and the Toll pathway target drs are compromised in Dip3 mutant larvae.
To determine if the role of Dip3 at antimicrobial defense gene promoters is direct, we carried out ChIP assays demonstrating that this factor associates directly with the drs, dipt, and cec-A promoters in S2 cells. Since Dip3 contains a DNA binding domain, it is possible that it binds to these promoters through a direct interaction with DNA. However, with one exception in the drs promoter, these promoters lack matches for the consensus Dip3 binding sites. Thus, Dip3 may be acting as a coactivator at these promoters consistent with its ability to bind the rel homology domain. In support of this idea, we found that simultaneous knockdown of all three rel family proteins significantly reduced recruitment of Dip3 to the promoters.
The mechanism of Dip3 co-activation remains unclear. The finding that the Dip3 BESS domain binds TAFs [38] suggests a role for Dip3 in the recruitment of the basal machinery. In addition, the MADF domain is closely related to the SANT domain, which binds histone tails and may have a role in interpreting the histone code[38]. While our analysis of RHD targets suggests roles for Dip3 in activation, Dip3 also associates with pericentromeric heterochromatin during mitosis, consistent with a possible role in silencing. Other heterochromatic proteins including a suppressor of position effect variegation (Su(Var)3–7) also contain BESS domains. However, the loss of Dip3 does not appear to modify position effect variegation (data not shown).
In flies, additional roles for RHD-mediated activation have been demonstrated in haematopoesis [51–53], neural fate specification [54], and glutamate receptor expression [55]. Antimicrobial defense genes are also expressed constitutively in barrier epithelia and in the male and female reproductive tracts ([22] and references therein). It will be interesting to determine if Dip3 is involved in rel protein-dependent and independent gene activation in some or all of these tissues. One tissue in which Dip3 appears to have clear rel-independent functions is in the developing compound eye, where Dip3 overexpression results in conversion of eye to antenna [56], while Dip3 loss-of-function leads to mispatterning of the retina (Duong et. al., 2008, Manuscript submitted).
Acknowledgements
We thank Matthew Smith, Clint Winkler and Minghua Nie for valuable discussion and Vinay Bhaskar, Tony Ip, Mike Levine, Jean-Luc Imler, Pernille Rorth, Shubha Govind, Preeta Guptan, Amy Henkenius, Cory Evans, Sudip Mandal, Lolitika Mandal and the Developmental Studies Hybridoma Bank for reagents. Confocal images were obtained in the UCLA CNSI Advanced Light Microscopy/Spectroscopy Shared Facility. This work was supported by an NIH grant (GM44522) to A.J.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 2.Govind S, Nehm RH. Innate immunity in fruit flies: a textbook example of genomic recycling. PLoS Biol. 2004;2:E276. doi: 10.1371/journal.pbio.0020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 4.Govind S. Control of development and immunity by rel transcription factors in Drosophila. Oncogene. 1999;18:6875–6887. doi: 10.1038/sj.onc.1203223. [DOI] [PubMed] [Google Scholar]
- 5.Tanji T, Ip YT. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 2005;26:193–198. doi: 10.1016/j.it.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Minakhina S, Steward R. Nuclear factor-kappa B pathways in Drosophila. Oncogene. 2006;25:6749–6757. doi: 10.1038/sj.onc.1209940. [DOI] [PubMed] [Google Scholar]
- 7.Stathopoulos A, Van Drenth M, Erives A, Markstein M, Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell. 2002;111:687–701. doi: 10.1016/s0092-8674(02)01087-5. [DOI] [PubMed] [Google Scholar]
- 8.Stathopoulos A, Levine M. Dorsal gradient networks in the Drosophila embryo. Dev Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. [DOI] [PubMed] [Google Scholar]
- 9.Zeitlinger J, Zinzen RP, Stark A, Kellis M, Zhang H, Young RA, et al. Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 2007;21:385–390. doi: 10.1101/gad.1509607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 11.Wu LP, Anderson KV. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature. 1998;392:93–97. doi: 10.1038/32195. [DOI] [PubMed] [Google Scholar]
- 12.Pal S, Wu J, Wu LP. Microarray analyses reveal distinct roles for Rel proteins in the Drosophila immune response. Dev Comp Immunol. 2007 doi: 10.1016/j.dci.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, et al. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- 14.Tauszig S, Jouanguy E, Hoffmann JA, Imler JL. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc Natl Acad Sci U S A. 2000;97:10520–10525. doi: 10.1073/pnas.180130797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. Embo J. 1999;18:3380–3391. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 1999;13:792–797. doi: 10.1101/gad.13.7.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, et al. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoven S, Ando I, Kadalayil L, Engstrom Y, Hultmark D. Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000;1:347–352. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, Maniatis T. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han ZS, Ip YT. Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. J Biol Chem. 1999;274:21355–21361. doi: 10.1074/jbc.274.30.21355. [DOI] [PubMed] [Google Scholar]
- 21.Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity. 2000;12:569–580. doi: 10.1016/s1074-7613(00)80208-3. [DOI] [PubMed] [Google Scholar]
- 22.Uvell H, Engstrom Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 2007;23:342–349. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hedengren-Olcott M, Olcott MC, Mooney DT, Ekengren S, Geller BL, Taylor BJ. Differential activation of the NF-kappaB-like factors Relish and Dif in Drosophila melanogaster by fungi and Gram-positive bacteria. J Biol Chem. 2004;279:21121–21127. doi: 10.1074/jbc.M313856200. [DOI] [PubMed] [Google Scholar]
- 24.Petersen UM, Kadalayil L, Rehorn KP, Hoshizaki DK, Reuter R, Engstrom Y. Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. Embo J. 1999;18:4013–4022. doi: 10.1093/emboj/18.14.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tingvall TO, Roos E, Engstrom Y. The GATA factor Serpent is required for the onset of the humoral immune response in Drosophila embryos. Proc Natl Acad Sci U S A. 2001;98:3884–3888. doi: 10.1073/pnas.061230198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senger K, Armstrong GW, Rowell WJ, Kwan JM, Markstein M, Levine M. Immunity regulatory DNAs share common organizational features in Drosophila. Mol Cell. 2004;13:19–32. doi: 10.1016/s1097-2765(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, et al. Innate Immune Homeostasis by the Homeobox Gene Caudal and Commensal-Gut Mutualism in Drosophila. Science. 2008 doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 28.Yagi Y, Ip YT. Helicase89B is a Mot1p/BTAF1 homologue that mediates an antimicrobial response in Drosophila. EMBO Rep. 2005;6:1088–1094. doi: 10.1038/sj.embor.7400542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uvell H, Engstrom Y. Functional characterization of a novel promoter element required for an innate immune response in Drosophila. Mol Cell Biol. 2003;23:8272–8281. doi: 10.1128/MCB.23.22.8272-8281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junell A, Uvell H, Pick L, Engstrom Y. Isolation of regulators of Drosophila immune defense genes by a double interaction screen in yeast. Insect Biochem Mol Biol. 2007;37:202–212. doi: 10.1016/j.ibmb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Ratnaparkhi GS, Jia S, Courey AJ. Uncoupling dorsal-mediated activation from dorsal-mediated repression in the Drosophila embryo. Development. 2006;133:4409–4414. doi: 10.1242/dev.02643. [DOI] [PubMed] [Google Scholar]
- 32.Dubnicoff T, Valentine SA, Chen G, Shi T, Lengyelz JA, Paroush Z, et al. Conversion of dorsal from an activator to a repressor by the global corepressor Groucho. Genes Dev. 1997;11:2952–2957. doi: 10.1101/gad.11.22.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhaskar V, Valentine SA, Courey AJ. A functional interaction between dorsal and components of the Smt3 conjugation machinery. J Biol Chem. 2000;275:4033–4040. doi: 10.1074/jbc.275.6.4033. [DOI] [PubMed] [Google Scholar]
- 34.Bhaskar V, Smith M, Courey AJ. Conjugation of Smt3 to dorsal may potentiate the Drosophila immune response. Mol Cell Biol. 2002;22:492–504. doi: 10.1128/MCB.22.2.492-504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brideau NJ, Flores HA, Wang J, Maheshwari S, Wang X, Barbash DA. Two Dobzhansky-Muller genes interact to cause hybrid lethality in Drosophila. Science. 2006;314:1292–1295. doi: 10.1126/science.1133953. [DOI] [PubMed] [Google Scholar]
- 36.Clark KA, McKearin DM. The Drosophila stonewall gene encodes a putative transcription factor essential for germ cell development. Development. 1996;122:937–950. doi: 10.1242/dev.122.3.937. [DOI] [PubMed] [Google Scholar]
- 37.England BP, Heberlein U, Tjian R. Purified Drosophila transcription factor, Adh distal factor-1 (Adf-1), binds to sites in several Drosophila promoters and activates transcription. J Biol Chem. 1990;265:5086–5094. [PubMed] [Google Scholar]
- 38.Bhaskar V, Courey AJ. The MADF-BESS domain factor Dip3 potentiates synergistic activation by Dorsal and Twist. Gene. 2002;299:173–184. doi: 10.1016/s0378-1119(02)01058-2. [DOI] [PubMed] [Google Scholar]
- 39.Jia S, Flores-Saaib RD, Courey AJ. The Dorsal Rel homology domain plays an active role in transcriptional regulation. Mol Cell Biol. 2002;22:5089–5099. doi: 10.1128/MCB.22.14.5089-5099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Nguyen PH, Courey AJ. A role for Groucho tetramerization in transcriptional repression. Mol Cell Biol. 1998;18:7259–7268. doi: 10.1128/mcb.18.12.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 42.Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci U S A. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–215. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 45.Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 46.Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, et al. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- 47.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 48.Erives A, Levine M. Coordinate enhancers share common organizational features in the Drosophila genome. Proc Natl Acad Sci U S A. 2004;101:3851–3856. doi: 10.1073/pnas.0400611101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courey AJ. Cooperativity in transcriptional control. Curr Biol. 2001;11:R250–R252. doi: 10.1016/s0960-9822(01)00130-0. [DOI] [PubMed] [Google Scholar]
- 50.Zinzen RP, Senger K, Levine M, Papatsenko D. Computational models for neurogenic gene expression in the Drosophila embryo. Curr Biol. 2006;16:1358–1365. doi: 10.1016/j.cub.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 51.Huang L, Ohsako S, Tanda S. The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev Biol. 2005;280:407–420. doi: 10.1016/j.ydbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Sorrentino RP, Melk JP, Govind S. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics. 2004;166:1343–1356. doi: 10.1534/genetics.166.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matova N, Anderson KV. Rel/NF-kappaB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc Natl Acad Sci U S A. 2006;103:16424–16429. doi: 10.1073/pnas.0605721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayyar S, Pistillo D, Calleja M, Brookfield A, Gittins K, Goldstone C, et al. NF-kappaB/Rel-Mediated Regulation of the Neural Fate in Drosophila. PLoS ONE. 2007;2:e1178. doi: 10.1371/journal.pone.0001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heckscher ES, Fetter RD, Marek KW, Albin SD, Davis GW. NF-kappaB, IkappaB, and IRAK control glutamate receptor density at the Drosophila NMJ. Neuron. 2007;55:859–873. doi: 10.1016/j.neuron.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duong HA, Wang CW, Sun YH, Courey AJ. Transformation of eye to antenna by misexpression of a single gene. Mech Dev. 2008;125:130–141. doi: 10.1016/j.mod.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]