Abstract
Ischemia/reperfusion (I/R) injury to the heart is accompanied by the upregulation and posttranslational modification of a number of proteins normally involved in regulating cell cycle progression. Two such proteins, cyclin-dependent kinase-2 (Cdk2) and its downstream target, the retinoblastoma gene product (Rb), also play a critical role in the control of apoptosis. Myocardial ischemia activates Cdk2, resulting in the phosphorylation and inactivation of Rb. Blocking Cdk2 activity reduces apoptosis in cultured cardiac myocytes. Genetic or pharmacological inhibition of Cdk2 activity in vivo during I/R injury led to a 36% reduction in infarct size (IFS), when compared to control mice, associated with a reduction in apoptotic myocytes. To confirm that Rb was the critical target in Cdk2-mediated I/R injury, we determined the consequences of I/R injury in cardiac-specific Rb-deficient mice (CRbL/L). IFS was increased 140% in CRbL/L mice compared to CRb+/+ controls. TUNEL positive nuclei and caspase-3 activity were augmented by 92% and 36%, respectively, following injury in the CRbL/L mice demonstrating that loss of Rb in the heart significantly exacerbates I/R injury. These data suggest that Cdk2 signaling pathways are critical regulators of cardiac I/R injury in vivo and support a cardioprotective role for Rb.
Keywords: Apoptosis, Cyclin-dependent kinase 2, Retinoblastoma gene, Myocardial Ischemia
1. Introduction
A number of cell cycle proteins are reexpressed in dying cardiac myocytes in the adult heart, particularly after ischemic injury. Ischemia in vivo (1) or hypoxia in vitro (2;3) results in a rapid induction of Cdk2 activity but its relationship to infarct size is unknown. Some investigators have interpreted this finding as evidence of post-mitotic cardiac myocytes reentering the cell cycle and undergoing proliferative growth. However, an increasing amount of data indicates that proteins classically thought to be involved in cell cycle regulation also play a critical role in the control of cell death, particularly through apoptotic pathways. While this was originally thought to be an evolutionarily conserved mechanism to link proliferation and cell death, it is now thought that this effect is independent of cell cycle progression (4). Numerous stimuli unrelated to cell cycle progression induce Cdk2 activity and subsequently apoptosis such as irradiation (5), Tumor necrosis factor-α (6), Fas (7), staurosporine (6), hypoxia (2;8),and ischemia (9). Although Cdk2 activity is elevated during apoptosis, activation of the mitotic processes does not occur even in cells capable of proliferating (7). Likewise, interventions that inhibit cell cycle progression, without effecting Cdk2 activity, fail to block apoptosis (10) supporting the notion that Cdk2 activation is not simply indicative of aberrant mitosis but, instead, that Cdk2 plays a direct role in cell death.
Increased Cdk2 activity has also been implicated in regulating apoptotic signaling pathways initiated by hypoxia in cardiac myocytes, at least in vitro (2;8). Increasing Cdk2 activity in cardiac myocytes by itself does not provoke cell cycle reentry (11) but does appear to provoke apoptosis (2;8;12). Alternatively, blocking Cdk2 activity in cultured myocytes inhibited apoptosis in response to hypoxia (2;8). Interestingly, a role for Cdk2 in cardiac preconditioning has also been reported. Pretreating cultured cardiac myocytes with NO donors attenuated hypoxia-induced elevations in Cdk2 activity by repressing Cyclin A (CycA) gene expression and promoting p21 accumulation (3).
The mechanism whereby Cdk2 mediates its proapoptotic effect is unclear but inactivation of Rb is a critical step for the induction of apoptosis in many cell lines, whereas overexpression of Rb attenuates apoptosis induced by a variety of stimuli (13–15) including hypoxia in cardiac myocytes (8;14). Germline disruption of Rb in mice leads to embryonic lethality in midgestation accompanied by apoptosis in a number of tissues including the central nervous system (CNS) (16–20). This apoptosis required an additional cofactor, which in the case of CNS apoptosis, was thought to be hypoxia related to defective erythropoiesis (21). Rb can be inactivated through phosphorylation by kinases such as Cdk2 or cleavage by caspases, releasing free, transcriptionally active E2F (22;23). In vitro studies have suggested that hypoxia-induced Cdk2 activation in cardiac myocytes promotes apoptosis through phosphorylation of Rb and induction of E2F-dependent genes (8). E2F-1 regulates a number of genes involved in the apoptotic cascade including p53 family member, p73 (24), Apaf-1 (25;26), cytochrome c (27) and caspase-3, -7, -8 and -9 (28).
To clarify the role of Cdk2 and Rb in myocardial I/R injury in vivo, we have utilized a combination of genetic mouse mutants and pharmacological inhibitors. Our results demonstrate that Cdk2 activity is upregulated in vivo and attenuation of this activity prevents I/R injury. This effect is likely mediated through an Rb-dependent apoptotic pathway since loss of Rb in the heart significantly exacerbates I/R injury. These data suggest that the cell cycle proteins, Rb and Cdk2, are critical regulators of cardiac I/R injury in vivo and that Rb antagonizes the proapoptotic signals that accompany this stress.
2. Materials and Methods
2.1 Mouse strains and genotyping
Cdk2 deficient (29) and cardiac-restricted Rb null mice (CRbL/L) (30) have been described. RbL/+ were mated to the α-MHC-Cre transgenic line (αMHC-Cre+/+) and backcrossed until homozygous for Cre (CRbL/+). These mice were crossed and Control (CRb+/+) or Rb null mice (CRbL/L) used for the indicated studies. Mice were maintained on a Sv129 (Cdk2) or FVB (CRbL/+) background and littermate controls were used throughout the study. Genotypes of mice were determined by polymerase chain reaction as described (31). All experimental protocols conform to The National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 86-23).
2.2 Myocardial ischemia/reperfusion surgery and infarct size analysis
Male mice were subjected to myocardial ischemia/reperfusion as previously described (32). Full details of the ischemia/reperfusion surgery are provided in the Supplemental Data. Infarct size was measured after 24 hours of coronary artery reperfusion by planimetry with NIH Image and expressed as a percentage of the area at risk (32). For Cdk inhibitor studies, Roscovitine (Sigma) was dissolved in dimethyl sulfoxide (DMSO, Sigma) and mice were injected with 2.8 µg/gm intraperitoneally two hours prior to coronary ligation or 20 minutes into the ischemic period.
2.3 Cell culture
Neonatal rat cardiac myocytes were prepared as previously described (33). Cultured neonatal were serum-starved for 24 h prior to virus infection. The media used to simulate ischemia utilizing 2-deoxyglucose, NaCN and pH 6.6 has been described(34). Recombinant adenoviruses were constructed, propagated and titered as previously described (35).
2.4 Assessment of mitochondrial membrane potential (ΔΨm)
Cardiomyocytes were loaded with 0.5 mM of JC-1 (a lipophilic and cationic dye that exhibits potential-dependent accumulation in negatively charged mitochondria) in DMEM at 37°C for 15 min and washed twice with DMEM. The cells were visualized under a fluorescent microscope equipped with red and green filters. Fluorescent images were taken with a CCD camera (Q Imaging Micropublisher) and analyzed with Image-J software (NIH). At low concentration (low ΔΨm,) JC-1 exists mainly in a monomeric form, which emits green fluorescence. JC-1 at high concentration (high ΔΨm) forms aggregates called “J” complexes, which emit red fluorescence. Thus, a reduction in the ratio of red to green fluorescence indicates a fall in ΔΨm.
2.5 Western blotting and kinase assays
Western assays and Cdk kinase assays were performed as described (30), immunoprecipitating the specific kinase from mouse heart extracts. Western blotting was performed using the identical protein lysates. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz). Protein expression was visualized using horseradish peroxidase–conjugated second antibodies and enhanced chemiluminescence reagents (Amersham Biosciences). Nonsaturated autoradiographs were quantitated with SigmaScan and expression levels normalized to internal control (α-tubulin).
2.6 Histology and caspase assays
Hearts were either freshly frozen or fixed overnight in buffered 4% paraformaldehyde buffered and routinely processed. Evidence of apoptosis was assessed by detection of nuclear DNA fragmentation by terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (Trevigen Inc.). At least 3,500 nuclei were examined per animal. Colorimetric caspase-3 assays were purchased from Promega and used according to the manufacturer’s instructions.
2.7 Statistical analysis
All data are presented as mean ± SEM. Results were compared by unpaired t-tests or analysis of variance with Fisher’s PLSD tests, using significance at a P value <0.05.
3. Results
3.1 Cdk2 activity is increased in ischemia-reperfusion injury and induces cardiac myocyte apoptosis
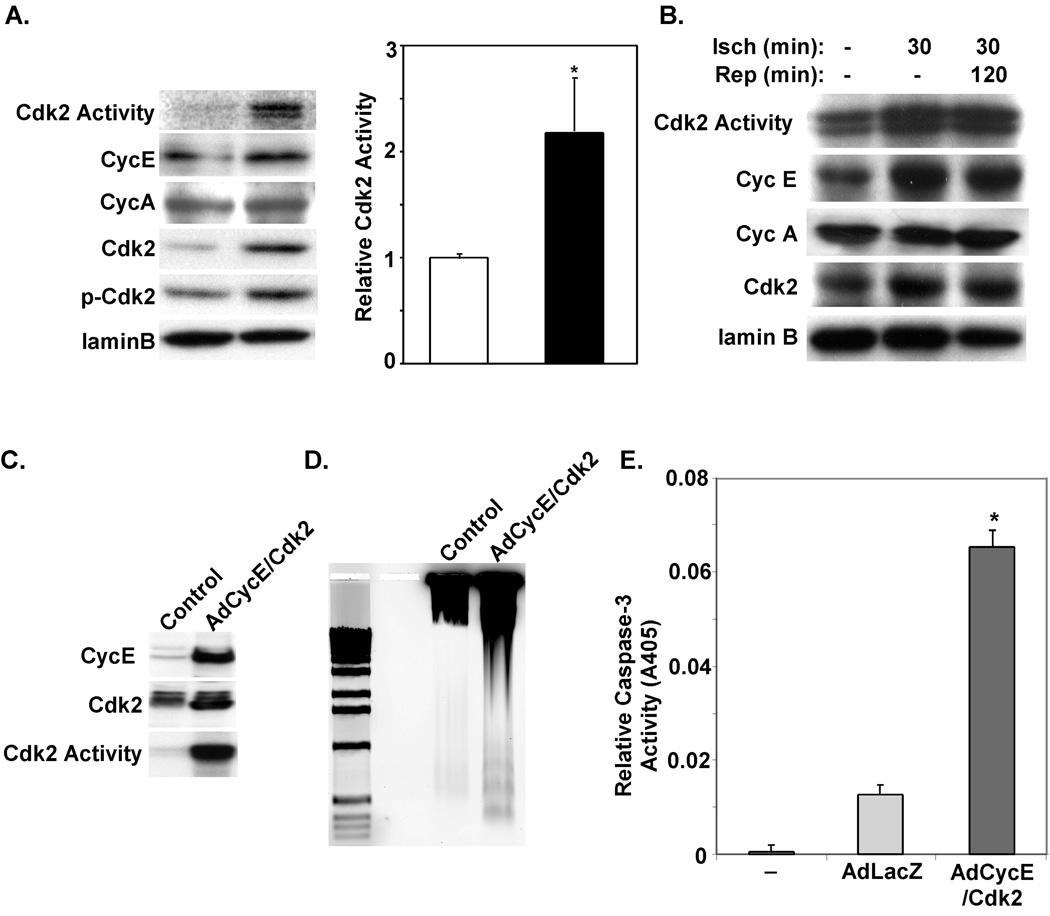
Cdk2 has been reported to be upregulated in response to a number of stress signals including hypoxia in cardiac myocytes in vitro. To confirm that Cdk2 activity is upregulated in response to stress signals we exposed primary cultures of neonatal rat ventricular myocytes (NRVM) to media designed to simulate ischemia (36). Protein levels of CycE and Cdk2 levels were increased resulting in a 2.18-fold increase in Cdk2 activity (Fig. 1A; P<0.05). These changes were also associated with phosphorylation of Cdk2 at threonine 160, an additional marker of its activation (Fig. 1A). To determine if Cdk2 activity was also upregulated in vivo with ischemia and/or reperfusion, we subjected adult mice to 30-min of myocardial ischemia via LAD occlusion with or without reperfusion. As shown in Figure 1B, kinase assays to determine Cdk activity were performed on ventricular lysates. Cdk2 kinase activity was upregulated in ischemic ventricles and remained elevated during reperfusion.
Figure 1. Activation of Cdk2 kinase activity with ischemic injury.
A. Western blots and Cdk2-associated kinase activity from protein lysates prepared from NRVMs exposed to control or ischemia media (IM) for 45 minutes. Cdk2-associated kinase activity was analyzed by immune complex kinase assays from 300µg of total protein lysates from each of the indicated treatment groups. 32P-labeling of histones H1 was measured using a standard Cdk2 kinase assay. Cdk2 activity in ischemia media treated NRVMs was increased in comparison with standard media control (*P<0.05, n=3 reps). B. Cdk2-associated kinase activity and Western blots were performed on protein lysates extracted from ventricular tissue harvested at the indicated time points. C. Neonatal cardiac myocytes were isolated and infected with the indicated virus and cultured in serum free media for 48 hours. Protein lysates were prepared, and Western or Cdk assays performed. D. DNA was isolated from NRVMs infected with the indicated virus and separated on a 1.2% agarose gel. E. Caspase-3 activity was determined using a colorimetric assay on lysates prepared from infected NRVMs. Caspase-3 activity in AdCycE/Cdk2 infected NRVMs was increased when compared to uninfected control or AdLacZ infected NRVMs (*P<0.001, n=3 reps).
To determine whether enhanced Cdk2 activity could induce cardiac myocyte apoptosis, we infected NRVM with adenoviruses expressing either LacZ or CycE and Cdk2. As shown in Figure 1C, overexpression of CycA and Cdk2 results in high-levels of Cdk2 kinase activity. This increased Cdk2 activity induces apoptosis in NRVM as documented by DNA laddering (Fig. 1D) and a 3.5-fold increase caspase-3 activity in AdCycE/Cdk2 versus AdLacZ infected NRVMs (P<0.001, Fig. 1E).
3.2 Cdk2 regulates cardiac myocyte apoptosis in vitro
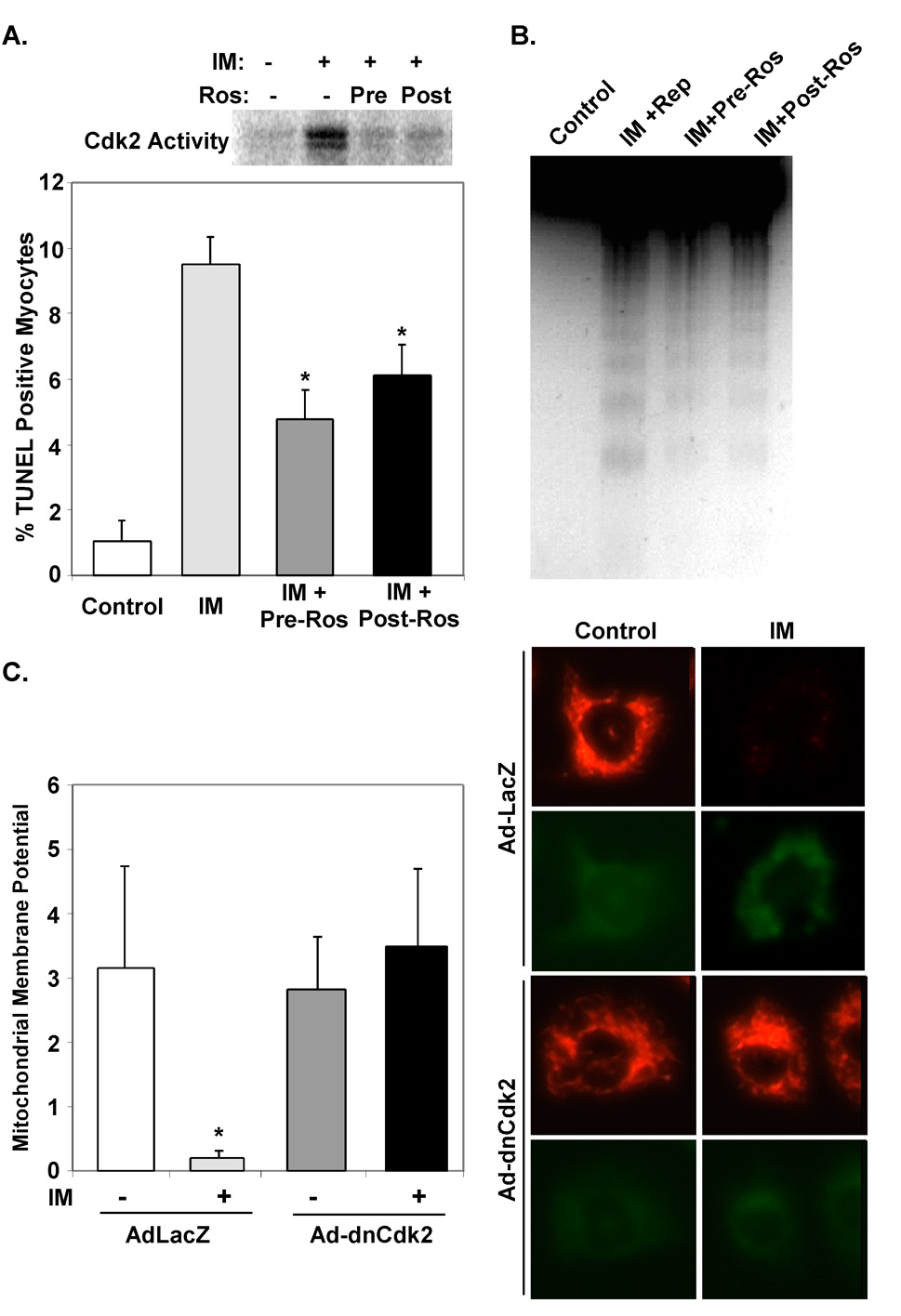
To determine whether pharmacologically inhibiting Cdk2 activity prior to, or even after, an ischemic insult would attenuate cardiac myocyte apoptosis we treated NRVMs with Roscovitine, a well-characterized purine analogue that inhibits Cdk2 activity (37). Roscovitine was a potent inhibitor of Cdk2 activity in ischemic NRVMs (Fig. 2A). Attenuation of Cdk2 activity with Roscovitine prior to exposure to simulated ischemia or immediately afterwards decreased the number of apoptotic NRVMs by 52.7% and 38.3% respectively compared to vehicle-treated cells (4.53±0.58% and 5.9±0.1% versus 9.57±0.66%, P<0.001, Fig. 2A). This correlated with a reduction in DNA laddering in Roscovitine-treated NRVMs (Fig. 2B). Infection of NRVMs with an adenovirus expressing a dominant-negative Cdk2 (dnCdk2) likewise reduced TUNEL staining in response to ischemia media (data not shown). This reduction in apoptosis correlated with the ability of dnCdk2 to prevent the expected mitochondrial membrane depolarization seen in response to ischemia media (Fig 2C).
Figure 2. Inhibition of Cdk2 activity attenuates apoptosis and mitochondrial membrane permeability transition in cardiac myocytes.
A & B. Primary cultures of NRVMs were exposed to ischemia media (IM) for 45 minutes and then returned to standard serum free media for the indicated reperfusion times. Roscovitine (10 µM) was added 15 minutes before (Pre) or immediately after (Post) exposure to ischemia medium. Cells were cultured an additional 2 hrs in serum-free media and then protein lysates were prepared. Results of Cdk2 kinase assays are shown (A). TUNEL positive nuclei (A) and DNA laddering (B) was reduced in NRVMs treated with Roscovitine (*P<0.001, n=3 reps). C. NRVMs were isolated and infected with the indicated virus and cultured in serum free media for 48 hours. NRVMs were labeled with mitochondrial dye, JC-1, and exposed to ischemia media (IM) for 45 minutes. Dncdk2 rescued ΔΨm in myocytes subjected to IM when compared to control virus infected myocytes (*P<0.05, n=3 reps).
3.3 Inhibition of Cdk2 activity attenuates myocyte death and reduces apoptosis
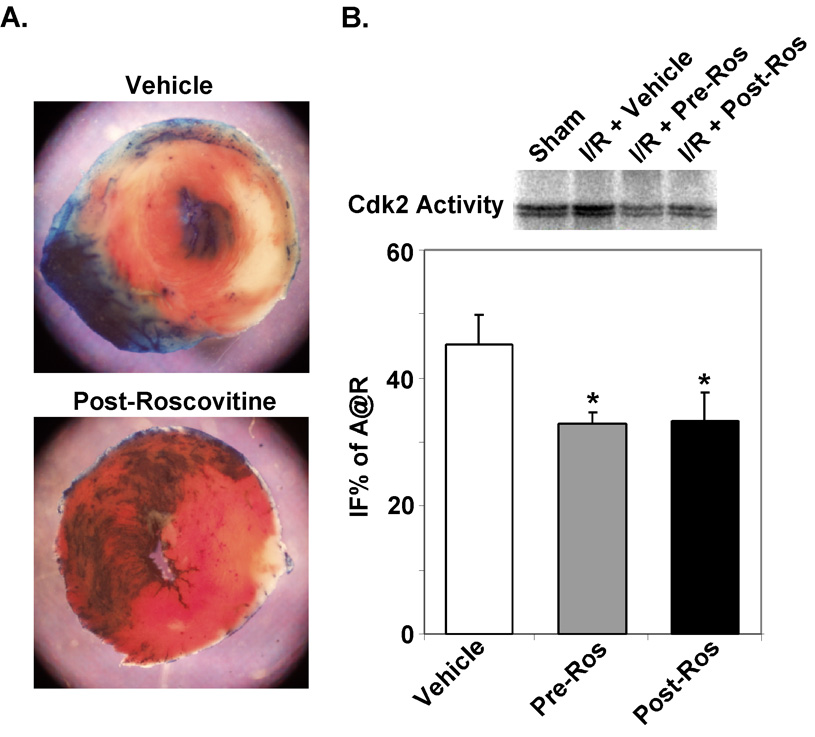
Although blocking Cdk2 activity in vitro in response to hypoxia attenuates cell death (8); whether this also protects cardiac myocytes in vivo is unknown. Thus, to test whether inhibition of Cdk2 activity would attenuate cell death in myocardium during I/R injury, mice were injected intraperitoneally two hours prior to ischemia (Pre) or 20 minutes into the ischemic period prior to reperfusion (Post). The control group received vehicle injections (DMSO). To ensure that Roscovitine was inhibiting the increase in Cdk2 activity we had observed in ischemic myocardium, we measured Cdk2 kinase activity in extracts prepared from ventricular tissue (Fig. 3B). Treatment with Roscovitine inhibited the expected increase in Cdk2 activity. This inhibition of Cdk2 activity led to a 33.5% and 35.4% reduction in infarct size in Pre- or Post-Roscovitine treated mice when compared to vehicle treated mice (34.2±2.6 or 33.2±1.7 versus 51.4±2.2 IFS% of A@R, P<0.05, Fig 3A & B).
Figure 3. Inhibition of Cdk2 activity with Roscovitine attenuates infarct size in vivo.
C57/BL6 mice were subjected to 30-min coronary occlusion followed by 24h of reperfusion. Mice were injected intraperitoneally two hours prior to ischemia (Pre) or 20 minutes after coronary ligation (Post) with 2.8 µg/gm of Roscovitine or DMSO carrier. A. Representative TTC-stained hearts from vehicle or pretreated hearts after I/R injury are shown. B. Cdk2 kinase assays demonstrate reduced 32P-labeling of purified histones consistent with decreased Cdk2 activity in Roscovitine-treated hearts after I/R when compared to vehicle-treated hearts. IFS in Pre- or Post-Roscovitine-treated mice were reduced compared to control vehicle-treated mice (*P<0.001; n=6 per group).
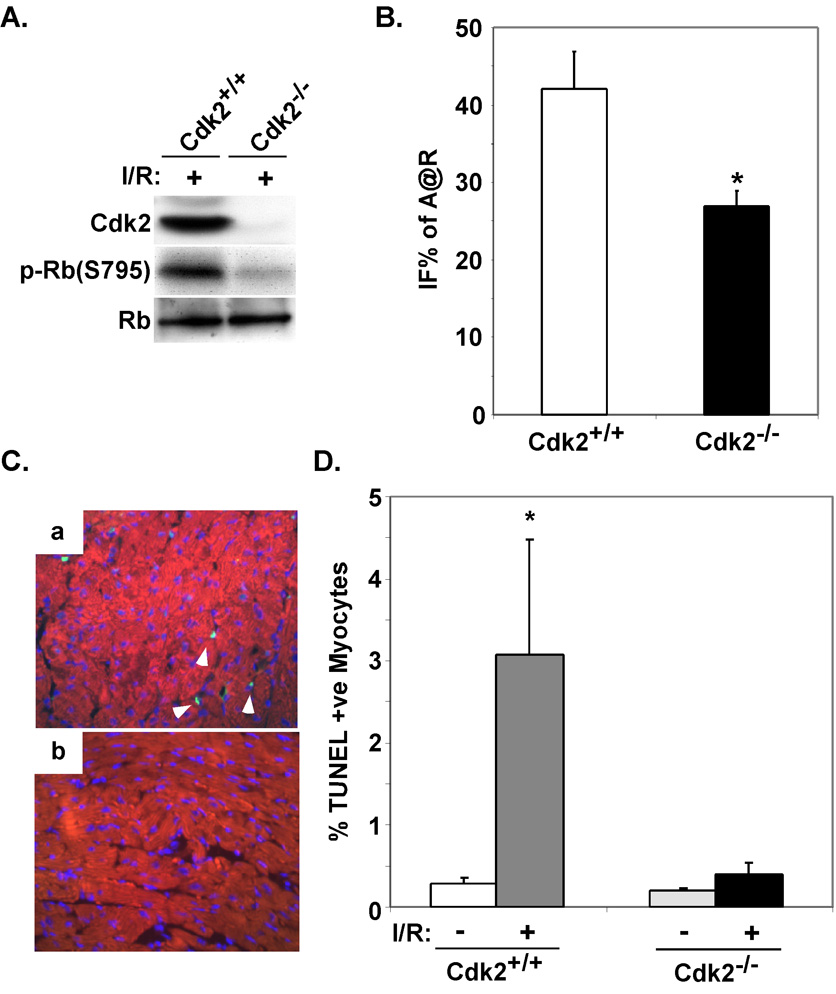
Although these data suggest that Cdk2 is a critical regulator of cardiac myocyte injury in vivo in response to I/R, Roscovitine has been reported to inhibit the activity of other kinases, Cdc2 albeit at significantly lower affinity. Therefore, to confirm that this reduction in infarct size was specifically related to a decrease in Cdk2 kinase activity, we subjected Cdk2-defient mice to I/R injury. As shown, Cdk2-null mice did not demonstrate increased Cdk2 activity nor was the downstream target, Rb, phosphorylated (Fig. 4A). Cdk2-deficient mice had a 36.1% reduction in infarct size when compared to littermate controls (26.9±2.0 versus 42.1±4.8 IFS% of A@R, P<0.05, Fig 4B&C). To determine if inhibition of Cdk2 activity attenuates I/R-induced apoptosis in vivo we determined the number of TUNEL positive nuclei in the infarct border zone Cdk2+/+ of Cdk2−/− mice. The relative number of TUNEL positive nuclei in the border zone was 7.8-fold higher in Cdk2+/+ ventricles when compared to Cdk2−/− hearts after I/R injury (3.08±1.4 versus 0.39±0.14 %TUNEL positive nuclei; P=0.05, Fig 4C & D).
Figure 4. Cdk2-null mice demonstrate reduced infarct size in vivo and apoptosis.
A. Western blots were performed on protein lysates extracted from ventricular tissue from wildtype or Cdk2-null mice subjected to I/R injury. B. Wildtype or Cdk2-null mice were subjected to I/R injury. Mean IFS sizes after 24 hours of reperfusion are shown. (*P<0.05 for Cdk2+/+ versus Cdk2−/− IFS; n=5 per group). C. Immunofluorescent staining for TUNEL (green) and cardiac-specific marker MF20 (red) was performed on myocardial sections from wildtype or Cdk2-null mice subjected to I/R. D. The percentage of TUNEL positive nuclei was quantified on myocardial sections from the indicated genotypes and treatments. The results from examination of at least 3,500 nuclei per animal are shown. (*P<0.05 Cdk2+/+ after I/R versus Cdk2+/+ or Cdk2−/− at baseline and P= 0.05 for Cdk2+/+ versus Cdk2−/− after I/R; n=4 per group).
3.4 Rb is cardioprotective in ischemia-reperfusion injury
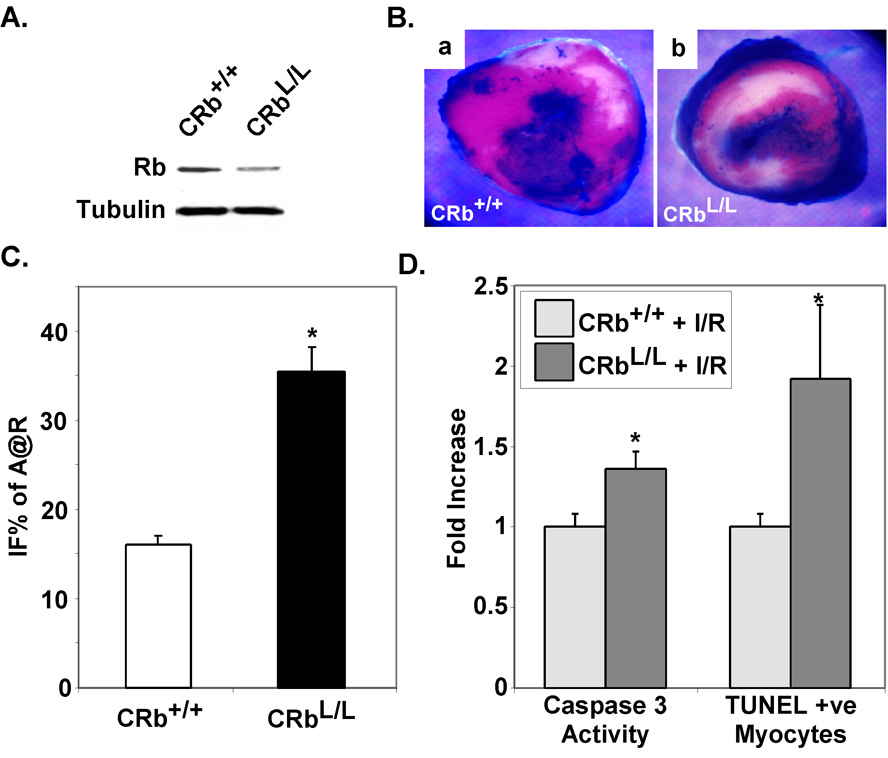
Rb is one of the primary substrates of Cdk2 kinase activity during cell cycle progression, although its role as a Cdk2-target in apoptosis signaling pathways is less clear. Our data demonstrate that Rb is phosphorylated and presumably inactivated in ischemic wildtype myocardium (Fig. 1A) but not in myocardium deficient for Cdk2 (Fig. 4A). To determine if Rb is an important target for Cdk2-dependent cell death in the heart, we subjected cardiac-restricted Rb-deficient mice we had previously created to I/R injury (30). These mice appeared phenotypically and biochemically normal at baseline (Fig. 5 A & B). I/R injury resulted in a 2.4-fold increase in IFS in CRbL/L mice when compared to CRb+/+ controls (37.2+4.9% versus 15.5+1.1%, P<0.001, Fig. 5C & D), demonstrating significant exacerbation of ischemic injury in CRbL/L mice. To explore possible mechanisms underlying the enhanced injury seen in CRbL/L mice after I/R, we determined the number of TUNEL positive nuclei in CRb+/+ versus CRbL/L ventricles after ischemic injury to determine if apoptosis might be altered. At baseline there were no significant differences in TUNEL positive nuclei between genotypes. However, the relative number of TUNEL positive myocyte nuclei in the border zone was increased in CRbL/L ventricles when compared to CRb+/+ hearts after ischemic injury (1.92±0.46 versus 1.0±0.08-fold; P<0.05). Caspase-3 activity was also augmented in ischemic myocardium from CRbL/L compared to that from CRb+/+ mice (1.36±0.11 versus 1.0±0.08-fold; P<0.05). Thus, it is likely that the increased infarct size observed represents, at least in part, an increased susceptibility to apoptosis.
Figure 5. Ischemic injury is enhanced in Rb-null myocardium.
A. Ventricular lysates from control (CRb+/+) or cardiac-restricted Rb-deficient (CRbL/L) mice were probed for the indicated proteins. B. Representative TTC-stained hearts from CRb+/+ or CRbL/L mice after I/R injury are shown. C. CRb+/+ or CRbL/L mice were subjected to ischemia reperfusion injury. Mean IFS sizes after 24 hours of reperfusion are shown. (*P<0.001 for CRbL/L versus CRb+/+ IFS; n=8 per group). D. The frequency of TUNEL positive myocyte nuclei for each animal was determined by examining at least 7,500 myocardial nuclei. Caspase 3 activity was determined on ventricular lysates from ischemic CRb+/+ or CRbL/L mice and the results shown. (*P<0.05 for CRbL/L versus CRb+/+, n=4 per group).
3.5 Bax upregulation is exaggerated in Rb-null myocytes with ischemia
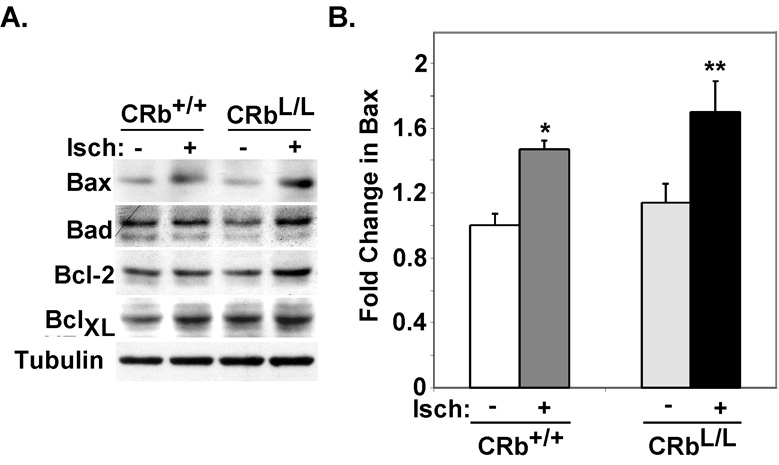
Although dysregulated E2F activity is an obvious downstream target of the Cdk2-Rb pathway, expression of a number of validated E2F-1 target genes involved in the control of apoptosis were unchanged in Cdk2-null mice after exposure to I/R injury compared to wildtype mice (Suppl. Fig. 2). To determine if the susceptibility of Rb-deficient myocytes to apoptosis in response to I/R injury in vivo was related to altered expression of apoptotic regulatory proteins we examined Bcl-2, BclXL, Bax and Bad in total ventricular lysates from CRb+/+ or CRbL/L mice at baseline or after ischemia by quantifying the protein expression levels (Fig. 6A and Suppl. Table 1). There were no statistically significant differences in expression of any apoptotic proteins at baseline in CRb+/+ versus CRbL/L hearts. Expression of Bcl-2 and Bad did not change significantly in CRb+/+ versus CRbL/L hearts after 30 minutes of ischemia. Levels of BCLXL and Bax increased in both CRb+/+ and CRbL/L hearts after thirty minutes. However, there was no difference in the induced levels of BCLXL in ischemic CRb+/+ versus CRbL/L myocardium (1.47+0.05 versus 1.7+0.19; P=NS). In contrast, ischemia-induced levels of Bax were significantly higher in CRbL/L versus CRb+/+ hearts (1.62+0.24 versus 1.15+0.07; P<0.05, Fig. 6B).
Figure 6. Altered expression of apoptotic regulatory proteins in ischemic Rb-deficient hearts.
A. Representative Westerns performed on ventricular lysates from the indicated mice. B. Hearts from each genotype and condition were probed and the results were quantified using enhanced chemiluminescence (*P<0.05 for ischemic CRb+/+ versus nonischemic CRb+/+ ventricles, **P<0.05 for ischemic CRbL/L versus nonischemic CRbL/L or ischemic CRb+/+ ventricles; n=4 per group).
4. Discussion
Growing evidence suggests that cell cycle proteins, and Cdk2 activity in particular, are reexpressed in dying cells and play a central role in the regulation of programmed cell death (37–39). The data we have presented here establishes a similar role for Cdk2 and its downstream targets in the regulation of myocardial I/R injury. It has been appreciated for some time that myocardial ischemia in vivo (1) or hypoxia in vitro (2;3) results in an induction of Cdk2 activity but this has often been interpreted as evidence of cell cycle reentry in cardiac myocytes. Although increasing Cdk2 activity is sufficient to promote proliferation in many cell types, this does not seem to be the case in adult cardiac myocytes, as increasing Cdk2 activity in adult cardiac myocytes was ineffective to provoke S phase entry (12). Although apoptosis was a prominent feature of forced Cdk2 expression in cultured cardiac myocytes (2), apoptosis rates were not reported in Cdk2-overexpressing mice although these mice were more susceptible to left ventricular dysfunction in response to hypertrophic stimuli suggesting there was at least a susceptibility to cardiac myocyte death (12). This is consistent with studies demonstrating that enhanced Cdk2 activity could promote apoptotic cell death even when cell cycle reentry was blocked (10). Thus, given the extensive data implicating the Cdk2-Rb pathway in regulating apoptosis, it is likely that the effects of the interventions we described on infarct size represents, at least in part, a modulation of apoptosis in the heart. Whether there is also increased sensitivity to other forms of cell death is unknown.
Until recently, it was believed that Cdk2 and its activator CycE play essential roles in the progression of the cell cycle. However, studies using knockout mouse models revealed that neither Cdk2 (29;40) nor CycE (41;42) are essential in vivo. Loss of Cdk2 had a relatively minor effect on progression through the mitotic cell cycle, phenotypically manifesting only as delayed entry into S phase. One interesting interpretation of our data is that although Cdk2 appears to be dispensable for normal cell cycle progression (29), it plays a critical role in I/R-dependent cell death signaling pathways. Its importance in mediating apoptosis is likely developmentally and cell-type specific as Cdk2 is not required in vivo to induce apoptosis in lymphocytes (43). This is the first in vivo evidence for a direct role of Cdk2 in cell death pathways in the heart. The mechanisms by which re-activation of Cdk2 in postmitotic cardiac myocytes could propagate an apoptotic signal to the cell death machinery are poorly understood. The primary phosphorylation target of Cdk2 kinase, at least in cell cycle progression, is the retinoblastoma protein (Rb). Our data demonstrating Rb is phosphorylated in ischemic wildtype but not Cdk2-null myocardium suggests that Rb may be one of the main targets of Cdk2 in I/R as well. Nonetheless, given the intense interest in developing pharmacological inhibitors of Cdk2 for cancer treatment (37;44), targeting Cdk2 activity in clinical situations where apoptotic pathways are activated such as myocardial infarction, may prove beneficial.
A role for Rb in the regulation of apoptosis has been well established although this is the first study to demonstrate a susceptibility to myocardial I/R injury in vivo. Widespread programmed cell death was seen with germline deletion of Rb (19), but this was related to its role in extraembryonic lineages since neurogenesis and erythropoiesis was normal and apoptosis restricted with a wildtype placenta (20). CNS-specific excision of Rb in vivo did not provoke significant apoptosis (21;45;46) and apoptosis Rb-null mice required concomitant hypoxia (21). Overexpression of Rb can also attenuate hypoxia-induced apoptosis in cardiac myocytes (8). Therefore, Rb’s normal physiological role in the heart might be to prevent apoptosis in response to environmental stressors. While Rb is postulated to inhibit apoptosis in many cell culture systems, this is the first evidence directly implicating Rb in cell survival in adult post-mitotic myocardium. The present study demonstrates that cardiac-specific Rb-deficient mice are more susceptible to myocardial I/R injury in vivo.
We observed that Rb is phosphorylated on a known Cdk2 site, Ser-795, during myocardial ischemia (Fig. 4A). This site was also phosphorylated during neuronal ischemia; however, the authors concluded that this elevated Rb phosphorylation was dependent upon Cdk4 activity (47). They demonstrated that inhibition of Cdk4, and its activator, cyclin D1, attenuates neuronal cell death in an animal model of brain ischemic (47). Our data does not rule out either an independent or potentially cooperative role for Cdk4 in mediating I/R injury in the heart. In some settings CycD/Cdk4 complexes were critical for inducing Cdk2 activity and proliferation; however, they were dispensable for apoptosis (48;49). We are currently examining CycD/Cdk4 complexes in ischemic myocardium. Our studies exclude the possibility that caspase-dependent inactivation of Rb plays a major role in the setting of I/R injury, since mice with a mutated Rb gene preventing it from being cleaved by caspases showed similar IFS as compared to wildtype mice (Suppl. Fig 1). This contrasts with the increased susceptibility of Rb-MI mice to endotoxin-induced apoptosis (13). Although, even in this model, the protective effects of Rb-MI were tissue specific suggesting that the mechanism of inactivation of Rb with apoptotic inducers may be both signal- and cell type-dependent.
Several reports have suggested loss of Rb leads to apoptosis through E2F-dependent mechanisms (17). E2F-1 deficient mice showed reduced brain injury after ischemic insult (50) suggesting that E2F may also play a pivotal role in inducing cell death by apoptosis even in post-mitotic tissue. E2F activity was also critical for hypoxia-induced apoptosis in cardiac myocytes in vitro and Rb mutants that are unable to bind and inactivate E2F did not demonstrate any protective effect (8). However, when we analyzed the expression of a number of candidate E2F-1 target genes in Cdk2-null mice after exposure to I/R injury only the upregulation of Cyclin E seemed to be significantly affected (Suppl. Fig. 2). Recently, it has been reported that E2F-1’s proapoptotic effect in cardiac myocytes was mediated through the upregulation of Bnip3 (51). However, similar to previous studies in adult myocytes (52), Bnip3 mRNA levels were only induced 2.2-fold in wildtype ventricles after I/R, which was not significantly different from Cdk2-deficient mice (Suppl. Fig. 2). The major difference we observed between ischemic wildtype and Rb-deficient hearts was Bax levels. This finding is consistent with reports on neuronal death in Rb-deficient mice where apoptosis in peripheral nervous system was less dependent upon the E2Fs and also independent of p53 but dependent on Bax (53). Although the mechanisms that link Rb and Bax are unclear, Bax plays a central role in mediating cell death after myocardial I/R injury (54). Our data do not rule out a role for E2F-dependent pathways in I/R injury in vivo but it does suggest that the downstream effectors likely differ from those identified from in vitro models. We are presently exploring the exact role of E2F in myocardial I/R injury in vivo and potential gene targets.
While our data suggest that Rb is a critical protective molecule in the heart and that Cdk2, most likely through phosphorylation of Rb, is a key regulator of myocardial I/R injury several potential limitations exist. Given the complexity of the transgenic lines described in the manuscript it was not practical to backcross the different lines into a single strain. However, although myocardial IFS is well known to be strain-dependent; comparison of groups within a strain is valid (55). Further, although these data support a model where the Cdk2-Rb signaling module plays an important role in regulating myocardial I/R injury through apoptotic pathways we have not shown direct data implicating Rb as the critical and only relevant downstream substrate of Cdk2. It is possible that additional Cdk2-dependent targets exist and we are currently attempting to identify these factors. Nonetheless, these models should prove useful to identify the mechanism(s) underlying Rb’s anti-apoptotic effect and the role of E2F activity in this process. Apoptosis is known to play a pivotal role in a number of cardiovascular diseases (56) and it will be critical in the future to determine the role that cell cycle regulators play in this process.
Supplementary Material
Acknowledgments
We thank J. Chen for technical assistance and Dr. J. Wang for reagents. This work was supported by gifts from the Glazer and Laubisch Funds as well as NIH grants R01 HL70748 to W.R.M, R01 HL65431 to P.P. and P01 HL080111 to W.R.M and P.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reiss K, Cheng W, Giorando A, DeLuca A, Li B, Kajstura J, et al. Myocardial infarction is coupled with activation of cyclin and cyclin-dependent kinases in myocytes. Experimental Cell Res. 1996;225:44–54. [PubMed] [Google Scholar]
- 2.Adachi S, Ito H, Tamamori-Adachi M, Ono Y, Nozato T, Abe S, et al. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res. 2001;88(4):408–414. doi: 10.1161/01.res.88.4.408. [DOI] [PubMed] [Google Scholar]
- 3.Maejima Y, Adachi S, Ito H, Nobori K, Tamamori-Adachi M, Isobe M. Nitric oxide inhibits ischemia/reperfusion-induced myocardial apoptosis by modulating cyclin A-associated kinase activity. Cardiovasc Res. 2003;59(2):308–320. doi: 10.1016/s0008-6363(03)00425-5. [DOI] [PubMed] [Google Scholar]
- 4.Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, et al. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol. 2006;17(9):2434–2442. doi: 10.1681/ASN.2006020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JA, Lewellyn AL, Maller JL. Ionizing radiation induces apoptosis and elevates cyclin A1-Cdk2 activity before but not after the midblastula transition in Xenopus. Mol Biol Cell. 1997;8(7):1195–1206. doi: 10.1091/mbc.8.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey KJ, Lukovic D, Ucker DS. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol. 2000;148(1):59–72. doi: 10.1083/jcb.148.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou BB, Li H, Yuan J, Kirschner MW. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc Natl Acad Sci USA. 1998;95(12):6785–6790. doi: 10.1073/pnas.95.12.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck L, Hansmann G, Dietz R, von Harsdorf R. Inhibition of hypoxia-induced apoptosis by modulation of retinoblastoma protein-dependent signaling in cardiomyocytes. Circ Res. 2002;91(9):782–789. doi: 10.1161/01.res.0000041030.98642.41. [DOI] [PubMed] [Google Scholar]
- 9.Katchanov J, Harms C, Gertz K, Hauck L, Waeber C, Hirt L, et al. Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J Neurosci. 2001;21(14):5045–5053. doi: 10.1523/JNEUROSCI.21-14-05045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakem A, Sasaki T, Kozieradzki I, Penninger JM. The cyclin-dependent kinase Cdk2 regulates thymocyte apoptosis. J Exp Med. 1999;189(6):957–968. doi: 10.1084/jem.189.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akli S, Zhan S, Abdellatif M, Schneider MD. E1A can provoke G1 exit that is refractory to p21 and independent of activating cdk2. Circ Res. 1999;85(4):319–328. doi: 10.1161/01.res.85.4.319. [DOI] [PubMed] [Google Scholar]
- 12.Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, et al. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88(4):443–450. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 13.Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol. 2002;4(10):757–765. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Guo K, Wills KN, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57(3):351–354. [PubMed] [Google Scholar]
- 15.Ip SM, Huang TG, Yeung WS, Ngan HY. pRb-expressing adenovirus Ad5-Rb attenuates the p53-induced apoptosis in cervical cancer cell lines. Eur J Cancer. 2001;37(18):2475–2483. doi: 10.1016/s0959-8049(01)00308-2. [DOI] [PubMed] [Google Scholar]
- 16.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 17.Almasan A, Yin Y, Kelly RE, Lee EYHP, Bradley A, Li W, et al. Deficiency of retinoblastoma protein leads to inappropriate s-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci USA. 1995;92:5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, et al. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 19.Macleod KF, Hu Y, Jacks T. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 1996;15(22):6178–6188. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421(6926):942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- 21.MacPherson D, Sage J, Crowley D, Trumpp A, Bronson RT, Jacks T. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol Cell Biol. 2003;23(3):1044–1053. doi: 10.1128/MCB.23.3.1044-1053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janicke RU, Walker PA, Lin XY, Porter AG. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 1996;15(24):6969–6978. [PMC free article] [PubMed] [Google Scholar]
- 23.Fattman CL, An B, Dou QP. Characterization of interior cleavage of retinoblastoma protein in apoptosis. J Cell Biochem. 1997;67(3):399–408. [PubMed] [Google Scholar]
- 24.Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L, et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol. 2003;5(6):552–558. doi: 10.1038/ncb998. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa Y, Nishimura N, Furukawa Y, Satoh M, Endo H, Iwase S, et al. Apaf-1 is a mediator of E2F-1-induced apoptosis. J Biol Chem. 2002;277(42):39760–39768. doi: 10.1074/jbc.M200805200. [DOI] [PubMed] [Google Scholar]
- 26.Moroni MC, Hickman ES, Denchi EL, Caprara G, Colli E, Cecconi F, et al. Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol. 2001;3(6):552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 27.Luciakova K, Barath P, Li R, Zaid A, Nelson BD. Activity of the human cytochrome c1 promoter is modulated by E2F. Biochem J. 2000;351(Pt 1):251–256. doi: 10.1042/0264-6021:3510251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002;4(11):859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 29.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13(20):1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 30.MacLellan WR, Garcia A, Oh H, Frenkel P, Jordan MC, Roos KP, et al. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol Cell Biol. 2005;25(6):2486–2497. doi: 10.1128/MCB.25.6.2486-2497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100(1):169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, et al. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2005;288(3):H1290–H1295. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- 33.MacLellan WR, Lee T-C, Schwartz RJ, Schneider MD. Transforming growth factor-β response elements of the skeletal α-actin gene. J Biol Chem. 1994;269:16754–16760. [PubMed] [Google Scholar]
- 34.Nijmeijer R, Willemsen M, Meijer CJ, Visser CA, Verheijen RH, Gottlieb RA, et al. Type II secretory phospholipase A2 binds to ischemic flip-flopped cardiomyocytes and subsequently induces cell death. Am J Physiol Heart Circ Physiol. 2003;285(5):H2218–H2224. doi: 10.1152/ajpheart.00887.2002. [DOI] [PubMed] [Google Scholar]
- 35.Graham FL, Prevec L. Methods in Molecular Biology. vol. 7. Clifton, NJ: The Humana Press Inc.; 1991. [Google Scholar]
- 36.Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest. 1999;104(3):239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golsteyn RM. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett. 2005;217(2):129–138. doi: 10.1016/j.canlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Jin YH, Yim H, Park JH, Lee SK. Cdk2 activity is associated with depolarization of mitochondrial membrane potential during apoptosis. Biochem Biophys Res Commun. 2003;305(4):974–980. doi: 10.1016/s0006-291x(03)00870-2. [DOI] [PubMed] [Google Scholar]
- 39.Osuga H, Osuga S, Wang F, Fetni R, Hogan MJ, Slack RS, et al. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A. 2000;97(18):10254–10259. doi: 10.1073/pnas.170144197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35(1):25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 41.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114(4):431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 42.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, et al. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22(18):4794–4803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berthet C, Rodriguez-Galan MC, Hodge DL, Gooya J, Pascal V, Young HA, et al. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol Cell Biol. 2007;27(14):5079–5089. doi: 10.1128/MCB.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirai H, Kawanishi N, Iwasawa Y. Recent advances in the development of selective small molecule inhibitors for cyclin-dependent kinases. Curr Top Med Chem. 2005;5(2):167–179. doi: 10.2174/1568026053507688. [DOI] [PubMed] [Google Scholar]
- 45.Marino S, Vooijs M, van Der GH, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14(8):994–1004. [PMC free article] [PubMed] [Google Scholar]
- 46.de Bruin A, Wu L, Saavedra HI, Wilson P, Yang Y, Rosol TJ, et al. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc Natl Acad Sci USA. 2003;100(11):6546–6551. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, et al. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102(39):14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18(19):5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Roger I, Kim SH, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1) EMBO J. 1999;18(19):5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacManus JP, Koch CJ, Jian M, Walker T, Zurakowski B. Decreased brain infarct following focal ischemia in mice lacking the transcription factor E2F1. Neuroreport. 1999;10(13):2711–2714. doi: 10.1097/00001756-199909090-00004. [DOI] [PubMed] [Google Scholar]
- 51.Yurkova N, Shaw J, Blackie K, Weidman D, Jayas R, Flynn B, et al. The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circ Res. 2008;102(4):472–479. doi: 10.1161/CIRCRESAHA.107.164731. [DOI] [PubMed] [Google Scholar]
- 52.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14(1):146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 53.Keramaris E, Ruzhynsky VA, Callaghan SM, Wong E, Davis RJ, Flavell R, et al. Required roles of Bax and JNKs in central and peripheral nervous system death of retinoblastoma-deficient mice. J Biol Chem. 2008;283(1):405–415. doi: 10.1074/jbc.M701552200. [DOI] [PubMed] [Google Scholar]
- 54.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284(6):H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 55.Miller DL, Van Winkle DM. Ischemic preconditioning limits infarct size following regional ischemia-reperfusion in in situ mouse hearts. Cardiovasc Res. 1999;42(3):680–684. doi: 10.1016/s0008-6363(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 56.MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81(2):137–144. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.