Abstract
Viruses as obligate intracellular parasites use host cell proteins to ensure efficient replication and spread. Cellular proteins are required for several stages of a virus life cycle. Here, we identify BAG3, a co-chaperone, as a regulator of herpes virus immediate early gene expression. We report that a herpes simplex virus lacking the gene encoding a potent transcriptional activator, ICP0, is compromised for replication in cells silenced for BAG3 in a multiplicity of infection-dependent manner. We also show a requirement for BAG3 to augment virus gene expression and demonstrate that the co-chaperone acts independently of promyelocytic leukemia to increase herpes simplex virus replication.
Keywords: Hsc70, HSV, ICP0, VZV
Productive infection by herpes simplex virus (HSV) is temporally regulated in a tightly coordinated cascade. Control of this process involves a complex and largely unknown network of interactions between host- and virus-encoded proteins. HSV genes are classified as immediate early (α), early (β), and late (γ) based on their temporal expression during productive replication. A virion protein (VP16) interacts with the host transcription machinery to initiate expression from α genes. Three of the α proteins (ICP0, ICP4, and ICP27) cooperatively regulate expression of all classes of virus genes (1).
ICP0 is a C3HC4 RING finger containing nuclear phosphoprotein with an apparent molecular mass of 110 kDa (2). Virus mutants lacking the ICP0 gene have an increased particle to pfu ratio, substantially lower yield, and decreased levels of α gene expression. These properties manifest themselves in a multiplicity of infection (moi) and cell type-dependent manner (3, 4). ICP0 behaves as a promiscuous activator of viral and cellular genes (5–7), stimulating expression, at least in part, indirectly by hijacking host activators to use in virus replication and/or by helping the virus bypass innate cellular repression pathways (8, 9).
ICP0 also functions as an E3 ubiquitin ligase to target a growing list of host proteins for proteasomal degradation (10–14). Through this activity, ICP0 promotes degradation of components of nuclear domain 10 (ND10) bodies, including the promyelocytic leukemia (PML) proteins, Sp100 and Daxx. Incoming HSV genomes are decorated with components of ND10s before ICP0-directed dissociation of these bodies and degradation of their constituents occurs (15). Proteins that normally reside within these structures are implicated in silencing of herpesvirus genomes (16–19). Therefore, ICP0-mediated degradation of host proteins may disrupt silencing of HSV genes and enable efficient virus gene expression. The RING finger of ICP0 is required for degradation of ND10 localized proteins and for its transactivation activity (20).
After infection with HSV, virus-induced chaperone-enriched (VICE) nuclear bodies form adjacent to virus replication/transcription foci. The presence of ICP0 facilitates formation of Hsc70 containing domains and results in augmentation of virus growth (21–23).
Unlike HSV, varicella-zoster virus (VZV), a closely related herpesvirus, does not form VICE domains, and the host chaperone machinery localizes within nuclear replication/transcription factories (24). Hsc70 and also BAG3, a host co-chaperone that is involved in regulation of Hsc/Hsp70 activity (25), reside inside replication compartments. Previously, we demonstrated that siRNA-mediated depletion of BAG3 resulted in decreased plaquing efficiency and slow spread of VZV. Interestingly, growth and spread of wild-type HSV were unaffected by silencing of BAG3 (24).
Here, we investigate the role of HSV encoded gene products that confer resistance to BAG3 silencing and study the function of BAG3 in HSV replication. Biochemical analysis of HSV gene expression suggests a requirement for BAG3 in efficient expression and accumulation of α proteins. We show that BAG3 distribution is altered during infection, and that expression of ICP0 is sufficient for redistribution. Moreover, an ICP0 null virus fails to replicate efficiently in cells silenced for BAG3. Mutational analysis shows that ICP0's RING finger is required for replication in the absence of BAG3. Genetic and biochemical evidence reveals that BAG3 acts antagonistic to and independent of PML, a negative regulator of α gene expression. Thus, the co-chaperone BAG3 is a previously undescribed host protein that like ICP0 positively regulates virus α gene expression.
Results
BAG3 Forms Cytoplasmic Perinuclear Bodies During HSV Infection.
HSV infection causes a dramatic redistribution of the host chaperone machinery. Nuclear VICE domains, marked by the presence of Hsc70 (22, 23), decorate the periphery of replication/transcription factories. Here, we investigate the localization of BAG3, a co-chaperone that interacts with Hsc70 and regulates its activity (25), during HSV infection.
Localization of viral and cellular proteins was examined in human melanoma (MeWo) cells infected with HSV. Hsc70-stained VICE domains formed as described (22) (Figs. 1A and S1). Unlike its chaperone partner, the majority of BAG3 accumulated in perinuclear bodies and a small subset of BAG3 localized as discrete nuclear foci within and adjacent to virus replication/transcription compartments (Figs. 1B and S1). This is in contrast to its diffuse cytoplasmic distribution in uninfected cells (Fig. S2A). Partitioning of BAG3 begins between 2 and 4 hours postinfection (hpi), when ICP0 begins to accumulate in the cytoplasm (Fig. S2), suggesting a link between ICP0 and co-chaperone translocation.
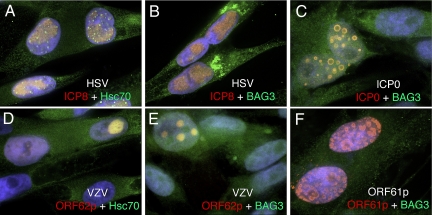
Fig. 1.
Localization of Hsc70 and BAG3 during VZV and HSV infection or ICP0 transformation. MeWo cells were infected with HSV (A and B) or cell-free VZV (D and E) or transformed with a plasmid expressing HSV-ICP0 (C) or VZV-ORF61p (F). Cells were fixed at 6, 24, or 48 hpi, respectively, and the indicated proteins were visualized by indirect immunofluorescence microscopy. Images were captured with a 100× objective and analyzed by volume deconvolution. The individual separated channels are presented as Fig. S1.
Because expression of ICP0 results in aggregation of Hsc70 (22), we asked whether it was also sufficient for redistribution of its co-chaperone BAG3. MeWo cells were transformed with a plasmid expressing ICP0, and the intracellular localization of ICP0 and BAG3 was monitored. Expression of ICP0 caused redistribution of BAG3 as discrete nuclear foci that colocalize with the “ring-like” structures that stained with antibody to ICP0 (Figs. 1C and S1). Therefore, in cells infected with HSV or transformed with DNA encoding ICP0, BAG3 followed the localization of ICP0.
In MeWo cells infected with VZV, VICE domains did not form, and Hsc70 was found inside replication/transcription bodies (Figs. 1D and S1). Moreover, BAG3 did not concentrate in the cytoplasm (Figs. 1E and S1) and ORF61p, the ICP0 ortholog in VZV, failed to mobilize BAG3 or Hsc70 (Figs. 1F and S1 and data not shown). Thus, functions of HSV ICP0 account for differences in localization of chaperone and co-chaperone proteins during infection with these 2 viruses.
ICP0 Expression Is Required for Efficient Growth of HSV in Cells Depleted of BAG3.
Depletion of BAG3 has no effect on the yield of wild-type HSV, whereas VZV growth is restricted (24). Unlike its VZV ortholog, ICP0 is able to redistribute BAG3. Therefore, we hypothesized that it is responsible for allowing HSV to overcome the effects of BAG3 depletion.
To test our hypothesis, wild-type HSV and an ICP0 null mutant were titrated on monolayers of MeWo or siBAG3 cells (24) (a stable cell line depleted of BAG3). The relative plaquing efficiency on these cell lines was expressed as: number of plaques formed in BAG3 cells/number of plaques formed in MeWo cells × 100. Reduction of BAG3 did not alter wild-type HSV plaquing efficiency. In contrast, the ICP0 null virus titer decreased by 1 log when measured on BAG3 depleted cells, mimicking what was seen with VZV (Fig. 2A). Thus, HSV requires ICP0 to replicate to wild-type levels in the absence of BAG3. Interestingly, overexpression of BAG3 in MeWo cells resulted in a 5-fold increase in ICP0 null virus yield (Fig. S4), providing further evidence for its positive role in virus replication.
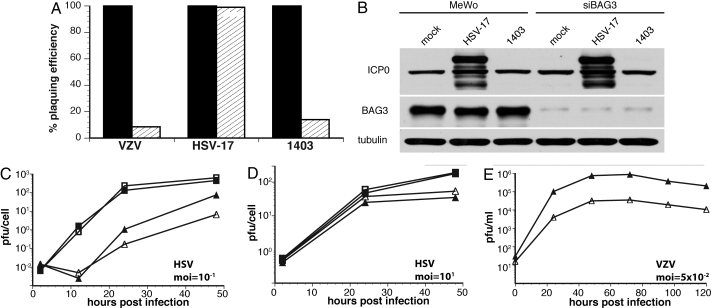
Fig. 2.
Growth of wild-type and ICP0 null HSV in the presence and absence of BAG3. (A) Confluent MeWo (solid bars) or siBAG3 (striped bars) cells were infected with serial dilutions of cell free VZV, HSV-17, or ICP0 null virus. Upon plaque formation, cells were fixed, stained, and plaques were counted. (B) Confluent MeWo or siBAG3 cells were either mock infected or infected with HSV or ICP0 null virus at an moi of 5. At 24 hpi, cells were harvested, and total protein lysates were analyzed by Western blot analysis. (C and D) MeWo (closed shapes) or siBAG3 (open shapes) were infected with HSV (squares) or ICP0 null (triangles) at different mois, as shown. At the indicated times after infection, viruses were harvested and titrated on L7 cells. (E) MeWo (closed triangles) or siBAG3 (open triangles) were infected with cell-free VZV. At the indicated times after infection cells were harvested and titrated on MeWo cells.
ICP0 is a transcriptional activator of viral and cellular genes. In our experiments, we depleted BAG3 using posttranscriptional gene silencing. Therefore, we asked whether ICP0 restored BAG3 levels to enable replication in siBAG3 cells during wild-type virus infection. To address this, MeWo and siBAG3 cells were either mock infected or infected with wild-type HSV or ICP0 null virus, and ICP0 and BAG3 levels were evaluated by Western blot. BAG3 levels were unchanged in cells infected with either virus in both cell lines (Fig. 2B). Thus, ICP0 (or other viral proteins) does not stimulate BAG3 expression, and resistance of wild-type virus to BAG3 depletion cannot be explained by restoration of BAG3 levels in the knockdown cell line.
High MOIs Rescue Growth of ICP0 Null HSV in Cells Depleted of BAG3.
Although ICP0 null viruses have defects in replication, even in wild-type cells, growth is partially restored at high moi (4). Therefore, we asked whether increasing the moi rescued replication of ICP0 null virus in siBAG3 cells. MeWo and siBAG3 cells were infected with HSV or ICP0 null virus at mois of 0.1 or 10, and the kinetics of virus replication were determined. These growth curve analyses demonstrated that the kinetics of wild-type virus replication were identical at high and low mois (Fig. 2 C and D). However, replication of ICP0 null virus was delayed, and yield was decreased after infection at low mois in siBAG3 cells (Fig. 2C). This result mimics replication of wild-type VZV in the same cell lines (Fig. 2E). In contrast, infection with ICP0 null virus at an moi of 10 resulted in restoration of wild-type replication kinetics in siBAG3 cells (Fig. 2D).
To exclude the possibility that faster progression through the lytic cycle by wild-type virus accounts for differences in sensitivity to BAG3, control and BAG3 silenced cells were infected at an moi of 0.001 with wild-type and ICP0 null virus. Growth of wild-type virus was unaffected. Thus, the mutant phenotype does not result from its slower replication kinetics (Fig. S3A).
BAG3 Is Required for Efficient Accumulation of Immediate Early Gene Products.
HSV lytic gene expression is temporally regulated. Expression of α proteins precedes and regulates transcription of β and γ genes (1). Thus, study of the gene expression profile during virus infection can identify when BAG3 is required.
MeWo and siBAG3 cells were mock infected or infected with either HSV or ICP0 null virus at 2 different mois, and α gene protein levels were monitored. In cells infected with the wild-type virus at a low moi, ICP4 and ICP27 were detected at the same time points in control and siBAG3 cells, and accumulation of these proteins in the absence of BAG3 was only slightly reduced (Fig. 3). This reduction in protein abundance had no effect on wild-type virus yields (Fig. 2), suggesting that an excess of regulatory α proteins accumulates in fully permissive cell lines. Unlike ICP4 and ICP27, expression of ICP0 was not affected by depletion of BAG3. However, infection with ICP0 null virus resulted in distinct BAG3-dependent differences in the expression profile of α gene products. In MeWo cells, α proteins were detected at late times after infection, although their abundance was severely decreased when compared with what accumulated in cells infected with wild-type virus. However, in siBAG3 cells, both ICP4 and ICP27, which are required for virus replication, were not detected even at 12 hpi (Fig. 3). Therefore, silencing of BAG3 enhances the ICP0 null phenotype.
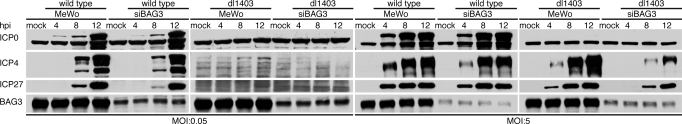
Fig. 3.
Immediate early protein accumulation during wild-type and ICP0 null HSV infection in the presence and absence of BAG3. MeWo or siBAG3 cells were either mock infected or infected with HSV-17 or ICP0 null virus at an moi of 0.05 or 5, as shown. At the indicated times after infection, the cells were lysed, and ICP0, ICP4, ICP27, and BAG3 levels were monitored by Western blot analysis.
At high moi with wild-type virus, expression of all α gene products tested was identical in control and siBAG3 cells. Accumulation of both ICP4 and ICP27 in cells infected with ICP0 null virus was similar to wild-type virus infection at low moi. Although these proteins were expressed with similar kinetics in both cell types, their maximum level of accumulation was decreased (Fig. 3). Therefore, BAG3 is required for temporal regulation and accumulation of α proteins to augment HSV replication.
To investigate whether BAG3 plays a role in transcriptional regulation, we tested the activity of all HSV α gene promoters using transcriptional fusions to firefly luciferase in control and siBAG3 cells. No significant difference in reporter activity was detected between these cell lines (data not shown).
The RING Finger of ICP0 Is Required for Efficient Replication of HSV in siBAG3 Cells.
The RING finger domain of ICP0 is required for its transactivation activity and efficient expression and accumulation of viral proteins, including α gene products (20). Because depletion of BAG3 also resulted in decreased accumulation of α gene proteins, we asked whether the RING finger was essential for ICP0-mediated resistance to silencing.
The efficiency of plaque formation by wild-type HSV, ICP0 null virus, and mutant viruses lacking the RING finger domain (vCM2/7) or with single (vC116A) and double amino acid substitutions (vC116A/C156G) of conserved cysteine residues in the domain in the presence or absence of BAG3 was determined. The RING finger mutant viruses were also compared with their parental virus that expresses ICP0 from a cDNA copy of the gene (vCPc0). Similar to the ICP0 null mutant, all RING finger mutants tested displayed a plaquing defect in siBAG3 cells when compared with vCPc0 (Fig. 4A).
Fig. 4.
Growth of wild-type and HSV ICP0 zinc-finger mutants in the presence and absence of BAG3. (A) Confluent monolayers of MeWo or siBAG3 cells were infected with serial dilutions of HSV-17, ICP0 null virus, vCPc0, vCM2/7, vC116A, or vC116A/C156G. After plaque formation, cells were fixed, stained, and plaques were counted. (B) MeWo (closed shapes) or siBAG3 (open shapes) cells were infected with HSV-17 (squares), vCPc0 (triangles) or vC116A/C156G (circles) at an moi of 0.1. At the indicated times after infection viruses were harvested and titrated on L7 cells.
We next asked whether the plaquing defect also reflected growth impairment of the RING finger mutant in the absence of BAG3. Accordingly, MeWo and siBAG3 cells were infected with wild-type HSV, vCPc0, or vC116A/C156G viruses at an moi of 0.1, and virus replication kinetics were determined. This analysis confirmed that vCPc0 replicates less well than wild-type virus (20). Similar to wild-type virus, its growth was identical in the presence and absence of BAG3, whereas the RING finger mutant replicated with slower kinetics and yielded 10-fold less virus in these cells (Fig. 4B). This result mirrored the phenotype of ICP0 null virus (Fig. 2C). To further demonstrate the specificity for the RING finger, another transactivation-deficient mutant of ICP0 (N/X) (26) was surveyed by using this assay. There was no growth defect in siBAG3 cells infected with N/X (Fig. S3B). We conclude that the RING finger of ICP0 is required to overcome the growth defect in BAG3 depleted cells.
BAG3 and PML Independently Regulate Immediate Early Gene Expression.
Studies by Everett et al. (17) revealed that ICP0 is required to overcome a PML-mediated cellular repression mechanism. It does so by directing degradation of PML, resulting in more efficient expression of α genes (17). Our results demonstrated that BAG3 expression is also required to boost α protein accumulation. We next asked how these 2 host proteins regulate expression of α genes, and whether they functioned independently of each other.
A pseudotyped retrovirus expressing a functional siRNA sequence targeting PML mRNA (19) was used to produce stable MeWo and siBAG3 cell lines depleted of PML and/or BAG3. The intracellular levels and localization of BAG3 and PML were evaluated by indirect immunofluorescence microscopy and Western blot analysis after selection and expansion. Depletion of each protein had no effect on the expression profile or intracellular distribution of the other (Figs. 5 A and B and S5). Thus, there is no direct correlation between the levels or localization of the 2 proteins.
Fig. 5.
Growth of wild-type and ICP0 null HSV in the presence and absence of BAG3 and/or PML. (A) MeWo, siPML, siBAG3, and siPML/siBAG3 cells were fixed and BAG (red) and PML (green) were visualized by indirect immunofluorescence microscopy. Images were captured with a 100× objective and analyzed by volume deconvolution. The individual separated channels are presented as Fig. S5. (B) MeWo, siPML, siBAG3, and siPML/siBAG3 cells were lysed and total cell lysates were analyzed by SDS/PAGE. The levels of PML, BAG3, and GAPDH were monitored by Western blot analysis. (C) MeWo (solid blue), siPML (solid red), siBAG3 (dashed blue), and siPML/siBAG3 (dashed red) with HSV (squares) or ICP0 null virus (triangles) at an moi of 0.1. At the indicated times after infection viruses were harvested and titrated on L7 cells.
We next examined HSV growth without one or both of these proteins. Confluent monolayers of MeWo, siBAG3, siPML, and siBAG3/siPML cells were infected with either wild-type HSV or ICP0 null virus. At the indicated times postinfection, virus yields were determined (Fig. 5C). The growth kinetics of wild-type virus was unaffected by the presence or absence of either protein. However, infection with ICP0 null virus revealed a role for PML and BAG3. As described, depletion of PML resulted in an increased yield of the mutant virus (17). In contrast, in the absence of BAG3, yields of ICP0 null virus were decreased. Thus, these proteins have antagonistic effects on HSV replication. PML acts to limit virus growth, whereas BAG3 functions as an activator. Silencing of PML in a BAG3-depleted background resulted in partial restoration of ICP0 null virus growth. Virus replication is affected by silencing either PML or BAG3 whether or not the other protein is present. These data suggest that PML and BAG3 function independently. This is further supported by the fact that degradation of PML and disruption of ND10 bodies during infection is independent of BAG3 (Fig. S6).
Discussion
Viruses have evolved to use many host proteins to ensure efficient replication and spread. During infection, synthesis of potent viral activators can mask a requirement for cellular proteins and hide the function of host components that modulate virus replication. However, mutations that inactivate these virus proteins often give rise to novel phenotypes that unveil steps in virus replication where the host assists or intervenes. Here, we use a HSV lacking the multifunctional activator ICP0 to identify a role for BAG3, a co-chaperone, in efficient replication and regulation of viral α gene expression.
Human α herpesviruses remodel the nuclear microenvironment during replication. In HSV-infected cells, Hsc70 concentrates predominantly within VICE domains that form at the periphery of replication sites (Fig. 1A) (22). In contrast, during infection with the closely related virus VZV, Hsc70 localizes within nuclear replication/transcription factories (Fig. 1D). BAG3, an Hsc70 interaction partner, does not localize within VICE domains but concentrates predominantly outside the nuclear membrane in cells infected with HSV (Fig. 1B). This localization occurs in cells infected with wild-type virus at 4 hpi, when a portion of ICP0 translocates from the nucleus into the cytoplasm (Fig. S2) (27), which suggests a link between ICP0 and redistribution of BAG3. Although expression of ICP0 is sufficient for reorganization of BAG3 (Fig. 1C), infection at high enough mois with an ICP0 null virus or a virus whose ICP0 fails to accumulate in the cytoplasm (28), also result in relocalization. Thus, in the context of a lytic infection, ICP0 is only required for efficient redistribution at low moi (data not shown).
Although BAG3 is required for efficient replication of VZV, it does not affect plaquing efficiency or spread of wild-type HSV (24). We therefore asked whether ICP0, which independently can redistribute BAG3 (Fig. 1C), is needed for HSV growth in BAG3 silenced cells. We showed that a mutant virus lacking ICP0 replicates less efficiently in cells depleted of BAG3.
Although VZV encodes an ortholog of ICP0 (ORF61p) (29), it cannot efficiently replicate in the absence of BAG3 and thus does not provide ICP0's function in this setting. Similar to ICP0, ORF61p transactivates viral promoters (29). However, amino acid alignment of these proteins reveals that, although the RING finger domains are conserved, ORF61p lacks the N-terminal acidic domain of ICP0 and their C termini are widely divergent. Furthermore, unlike ICP0, expression of ORF61p does not result in formation of Hsc70 or BAG3 inclusions (Fig. 1F and data not shown). Thus, ICP0 and ORF61p possess distinct functions.
Western blot analysis of HSV α gene expression in MeWo and siBAG3 cells revealed a requirement for BAG3 for accumulation of α proteins (Fig. 3). Infection of siBAG3 cells with wild-type virus at a low moi, or with ICP0 null virus at all mois tested, demonstrated that temporal control of viral gene expression was altered, and the abundance of α proteins was significantly decreased compared with the control. However, transcriptional fusions of α gene promoters to luciferase did not reveal a requirement for BAG3 for increased reporter activity. Thus, we posit that BAG3 enhances accumulation of α gene products via a posttranscriptional mechanism.
Expression and accumulation of ICP0 differ from other α proteins, and ICP0 is unaffected by depletion of BAG3. Consistent with this observation, ICP0 is one of the first virus proteins expressed and acts upstream of all other α proteins to ensure their efficient expression (20, 30). It is thus advantageous for HSV to first express ICP0, thus evading the host silencing machinery, and then use this protein to facilitate robust α gene expression.
The replication defect of a mutant virus lacking ICP0 in the absence of BAG3 was verified both for growth and efficiency of plaquing. We also showed that growth impairment in siBAG3 cells was overcome by infection at high mois. We envision 2 hypotheses to explain this observation. Possibly, VP16, a virus tegument protein that is transported with viral DNA to the nucleus to activate all α gene promoters (31, 32), might substitute for loss of BAG3. Both ICP0 and VP16 are required to efficiently overcome an intracellular barrier to viral gene expression (33). Because infection at high mois results in increased levels of VP16, we posit that this bypasses the BAG3 requirement for efficient activation of α gene promoters and initiation of virus replication. Alternatively, increased numbers of incoming genomes may improve the likelihood of expression of all viral gene classes as described (9, 34). Consistent with this hypothesis, at high mois, the host contribution to viral gene expression and protein accumulation is minimal, thus greatly limiting the requirement for high intracellular BAG3 levels.
The precise molecular details of BAG3's contribution to accumulation of α proteins remain unknown. Our results demonstrate that BAG3 is a positive regulator of these processes. We envision 2 different scenarios that are consistent with our observations. Perhaps, similar to other host proteins such as HCF and Oct1 or virus proteins such as VP16 and ICP0, BAG3 can activate gene expression. Alternatively, BAG3 might be required for release of a block that leads to gene silencing. The latter hypothesis suggests that BAG3 is an antirepressor and thus an upstream positive regulator of virus replication. In the presence of ICP0, the requirements for host proteins are different (Figs. 2 and 3), and BAG3 levels do not affect virus yield, although they alter the abundance of viral α proteins. Because ICP0 and BAG3 accumulate in the cytoplasm at the same time after infection (Fig. S2), and BAG3 is not involved in virus transcription, we suggest a link between the co-chaperone and the proposed cytoplasmic functions of ICP0 that interfere with host posttranscriptional regulation to increase virus protein accumulation (27, 28, 35).
Herpesvirus genomes are silenced upon entry, and virus-encoded activities are required for release of host repression (9, 17, 19, 34). Studies by Everett et al. (17) and others showed that proteins that normally reside within ND10s are components of a host innate antiviral defense mechanism. For HSV, these proteins block expression of α genes (17), the same step that requires BAG3 activity. The major component and docking site of all these proteins is PML. Here, we demonstrate that BAG3 and PML have antagonistic effects on virus growth. Unlike PML, the major component of ND10 bodies, BAG3 functions as a positive regulator of α gene expression and does not normally localize within nuclear bodies, and its levels and localization are unaffected by PML. Thus, BAG3 is a previously undescribed regulator of HSV gene expression.
We demonstrate that a predominantly cytoplasmic co-chaperone, BAG3, plays an important role in events that occur immediately after infection with HSV. BAG3, similar to other BAG family members alters the activity of the Hsp/Hsc70 chaperone complex by changing its ability to use ATP (36). Furthermore, it is implicated in regulation of proteasomal degradation orchestrated by Hsp90 (37). There is increasing evidence that chaperone activity is required for efficient growth of viruses (23, 24, 38–43). Our results emphasize the importance of BAG3, a regulator of chaperone activity, in the life cycle of herpesviruses, raising the interesting possibility that co-chaperones might function to control replication of other animal viruses.
Materials and Methods
Mammalian Cells.
MeWo, siBAG3 (24), L7 (44), and 293T cells were maintained as described (24).
To generate stable cell lines expressing siRNAs targeting PML mRNA, cells were transduced with retroviruses and selected with hygromycin.
Viruses.
HSV.
Strains used were wild-type HSV-1 (Glasgow strain 17), vCPc0 (45), ICP0 null virus (dl1403) (4), vCM2/7 (46), vC116G, and vC116G/C156A (20).
VZV.
Jones, a wild-type clinical isolate, was propagated and titrated as described (47). Cell-free virus was prepared as described (48, 49).
Retroviruses.
Retroviruses were constructed as described in ref. 24.
Virus Growth Assays: Plaque Assays.
MeWo or siBAG3 cells were infected with 10-fold serial dilutions of virus stocks and infected cells were fixed, stained and plaques counted.
Growth Curves.
HSV.
The titers of all HSV stocks were determined before analysis by titration on the ICP0-complementing cell line L7. Virus yield in MeWo or siBAG3 cells was calculated from at least 2 independent experiments, and titrations were done in duplicate. (b) The titer of cell associated VZV after infection of MeWo or siBAG3 cells was determined by mixing infected cells with uninfected MeWo cells and counting the resulting plaques.
Transformations.
Transformations were performed by using Fugene HD (Roche).
DNA Plasmids.
pCK-Super.retro.hygro was created by inserting a XhoI (filled)/ClaI (filled) fragment of pI-tk-hygro (50) into a StuI site of pSuper.retro.puro (51). To generate pCK-siPML, annealed oligos for siPML2 (19) were ligated into BglII/HindIII digested pCK-Super.retro.hygro.
Plasmid pSS-7 expressing ICP0 and pCK-flag-ORF61 expressing ORF61p were described (52, 53).
Antibodies.
Polyclonal antibodies to BAG3, ICP0 and ICP27 were described (24, 45, 54). Monoclonal ICP0 antibody was purchased from the Rumbaugh–Goodwin Institute. Monoclonal antibodies to PML, GAPDH and tubulin, were from Santa Cruz Biotechnology. Polyclonal antibody against PML was purchased from Chemicon. Alexa Fluor 488-conjugated anti-mouse and Alexa Fluor 546-conjugated anti-rabbit antibodies were from Molecular Probes. Anti-rabbit and -mouse conjugated to horseradish peroxidase for immunoblotting were from KPL.
Indirect Immunofluorescence Microscopy.
Cells on glass coverslips were treated as described (24).
SDS/PAGE and Western Blot Analysis.
Cells were washed twice with cold PBS, lysed in 1.5 × SDS sample buffer (75 mM Tris·HCl, pH 6.8, 150 mM DTT, 3% SDS, 0.15% bromophenol blue, 15% glycerol), boiled, and proteins analyzed as described (24).
Supplementary Material
Acknowledgments.
We thank Drs. Matthew Walters and Daniel Wolf for helpful discussions and Drs. Stephen Goff and Vincent Racaniello for critical comments on the manuscript. This work was supported by the Public Health Service (Grant AI-024021, to S.J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810656105/DCSupplemental.
References
- 1.Weir JP. Regulation of herpes simplex virus gene expression. Gene. 2001;271:117–130. doi: 10.1016/s0378-1119(01)00512-1. [DOI] [PubMed] [Google Scholar]
- 2.Pereira L, Wolff MH, Fenwick M, Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977;77:733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- 3.Everett RD. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J Gen Virol. 1989;70:1185–1202. doi: 10.1099/0022-1317-70-5-1185. [DOI] [PubMed] [Google Scholar]
- 4.Stow ND, Stow EC. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 5.Everett RD. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 1985;4:1973–1980. doi: 10.1002/j.1460-2075.1985.tb03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelman IH, Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci USA. 1985;82:5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinlan MP, Knipe DM. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985;5:957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc Natl Acad Sci USA. 2007;104:17134–17139. doi: 10.1073/pnas.0707266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chelbi-Alix MK, de The H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18:935–941. doi: 10.1038/sj.onc.1202366. [DOI] [PubMed] [Google Scholar]
- 11.Everett RD, Earnshaw WC, Findlay J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett RD, Maul GG. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomonte P, Sullivan KF, Everett RD. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J Biol Chem. 2001;276:5829–5835. doi: 10.1074/jbc.M008547200. [DOI] [PubMed] [Google Scholar]
- 14.Gu H, Roizman B. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc Natl Acad Sci USA. 2003;100:8963–8968. doi: 10.1073/pnas.1533420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett RD, Sourvinos G, Leiper C, Clements JB, Orr A. Formation of nuclear foci of the herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: Localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes. J Virol. 2004;78:1903–1917. doi: 10.1128/JVI.78.4.1903-1917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett RD, Parada C, Gripon P, Sirma H, Orr A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J Virol. 2008;82:2661–2672. doi: 10.1128/JVI.02308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett RD, et al. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J Virol. 2006;80:7995–8005. doi: 10.1128/JVI.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maul GG. Nuclear domain 10, the site of DNA virus transcription and replication. BioEssays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Tavalai N, Papior P, Rechter S, Leis M, Stamminger T. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J Virol. 2006;80:8006–8018. doi: 10.1128/JVI.00743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lium EK, Silverstein S. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J Virol. 1997;71:8602–8614. doi: 10.1128/jvi.71.11.8602-8614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston CM, DeLuca NA, Wilkinson DE, Weller SK. Oligomerization of ICP4 and rearrangement of heat shock proteins may be important for herpes simplex virus type 1 prereplicative site formation. J Virol. 2008;82:6324–6336. doi: 10.1128/JVI.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burch AD, Weller SK. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J Virol. 2004;78:7175–7185. doi: 10.1128/JVI.78.13.7175-7185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Johnson LA, Dai-Ju JQ, Sandri-Goldin RM. Hsc70 Focus Formation at the Periphery of HSV-1 Transcription Sites Requires ICP27. PLoS ONE. 2008;3:e1491. doi: 10.1371/journal.pone.0001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyratsous CA, Silverstein SJ. BAG3, a host cochaperone, facilitates varicella-zoster virus replication. J Virol. 2007;81:7491–7503. doi: 10.1128/JVI.00442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 26.Lium EK, Panagiotidis CA, Wen X, Silverstein SJ. The NH2 terminus of the herpes simplex virus type 1 regulatory protein ICP0 contains a promoter-specific transcription activation domain. J Virol. 1998;72:7785–7795. doi: 10.1128/jvi.72.10.7785-7795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez P, Van Sant C, Roizman B. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J Virol. 2001;75:3832–3840. doi: 10.1128/JVI.75.8.3832-3840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriuchi H, Moriuchi M, Smith HA, Straus SE, Cohen JI. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992;66:7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon AP, Silverstein SJ, Roizman B. An early regulatory function required in a cell type-dependent manner is expressed by the genomic but not the cDNA copy of the herpes simplex virus 1 gene encoding infected cell protein 0. J Virol. 2002;76:9744–9755. doi: 10.1128/JVI.76.19.9744-9755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston CM, Frame MC, Campbell ME. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 32.Kristie TM, LeBowitz JH, Sharp PA. The octamer-binding proteins form multi-protein–DNA complexes with the HSV alpha TIF regulatory protein. EMBO J. 1989;8:4229–4238. doi: 10.1002/j.1460-2075.1989.tb08608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock MH, Corcoran JA, Smiley JR. Herpes simplex virus regulatory proteins VP16 and ICP0 counteract an innate intranuclear barrier to viral gene expression. Virology. 2006;352:237–252. doi: 10.1016/j.virol.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc Natl Acad Sci USA. 2005;102:7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawaguchi Y, Matsumura T, Roizman B, Hirai K. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J Virol. 1999;73:4456–4460. doi: 10.1128/jvi.73.5.4456-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doong H, Vrailas A, Kohn EC. What's in the ‘BAG’?–A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32. doi: 10.1016/s0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 37.Doong H, et al. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: Accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003;278:28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- 38.Liu JS, et al. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J Biol Chem. 1998;273:30704–30712. doi: 10.1074/jbc.273.46.30704. [DOI] [PubMed] [Google Scholar]
- 39.Jeon YK, et al. The heat-shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein–Barr virus-positive NK/T cell lymphoma by Akt down-regulation. J Pathol. 2007;213:170–179. doi: 10.1002/path.2219. [DOI] [PubMed] [Google Scholar]
- 40.Stahl M, Retzlaff M, Nassal M, Beck J. Chaperone activation of the hepadnaviral reverse transcriptase for template RNA binding is established by the Hsp70 and stimulated by the Hsp90 system. Nucleic Acids Res. 2007;35:6124–6136. doi: 10.1093/nar/gkm628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basha W, et al. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibits gene expression and replication of human cytomegalovirus. Antivir Chem Chemother. 2005;16:135–146. doi: 10.1177/095632020501600206. [DOI] [PubMed] [Google Scholar]
- 42.Connor JH, McKenzie MO, Parks GD, Lyles DS. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362:109–119. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto T, et al. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006;25:5015–5025. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panagiotidis CA, Lium EK, Silverstein SJ. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J Virol. 1997;71:1547–1557. doi: 10.1128/jvi.71.2.1547-1557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J Virol. 1992;66:2916–2927. doi: 10.1128/jvi.66.5.2916-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grose C, Brunel PA. Varicella-zoster virus: Isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect Immun. 1978;19:199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koyama K, Osame J. 2000. USPTO 6039958.
- 49.Provost PJ, Krah DL, Friedman PA. 1997. USPTO 5607852.
- 50.Stolarov J, et al. Design of a retroviral-mediated ecdysone-inducible system and its application to the expression profiling of the PTEN tumor suppressor. Proc Natl Acad Sci USA. 2001;98:13043–13048. doi: 10.1073/pnas.221450598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 52.Gelman IH, Silverstein S. Herpes simplex virus immediate-early promoters are responsive to virus and cell trans-acting factors. J Virol. 1987;61:2286–2296. doi: 10.1128/jvi.61.7.2286-2296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walters MS, Kyratsous CA, Wan S, Silverstein S. Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J Virol. 2008;82:8673–8686. doi: 10.1128/JVI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lium EK, Panagiotidis CA, Wen X, Silverstein S. Repression of the alpha0 gene by ICP4 during a productive herpes simplex virus infection. J Virol. 1996;70:3488–3496. doi: 10.1128/jvi.70.6.3488-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.