Abstract
Hereditary cases of breast and ovarian cancer are often attributed to germ-line mutations of the BRCA1 tumor suppressor gene. Although BRCA1 is involved in diverse cellular processes, its role in the maintenance of genomic integrity may be a key component of its tumor suppression activity. The protein encoded by BRCA1 interacts in vivo with the related BARD1 protein to form a heterodimeric complex that acts as a ubiquitin E3 ligase. Because the enzymatic activity of the BRCA1/BARD1 heterodimer is conserved over a broad phylogenetic range, it is thought to be critical for the central functions of BRCA1. To test this hypothesis, we have generated isogenic clones of embryonic stem cells that do or do not express an enzymatically proficient Brca1 polypeptide. Surprisingly, cells lacking the ubiquitin ligase activity of BRCA1 are viable and do not accumulate spontaneous cytogenetic rearrangements. Gene targeting efficiencies are modestly reduced in these cells, and chromosomal rearrangements arise at elevated rates in response to genotoxic stress. Nonetheless, cells lacking Brca1 enzymatic activity are not hypersensitive to the DNA cross-linking agent mitomycin C. They also form Rad51 focus in response to ionizing radiation and repair chromosome breaks by homologous recombination at wild-type levels. These results indicate that key aspects of BRCA1 function in genome maintenance, including its role in homology-directed repair of double-strand DNA breaks, do not depend on the E3 ligase activity of BRCA1.
Keywords: breast cancer, BRCA1/BARD1 heterodimer, E3 ubiquitin ligase, homologous recombination, DNA double-strand break repair
Familial susceptibility to breast and ovarian cancer is often mediated by germ-line mutations of the BRCA1 tumor suppressor gene (1). Because BRCA1 has been implicated in a broad range of cellular processes, it has an especially important role in the maintenance of genome integrity (2, 3), and studies of genetically engineered mice support a direct role for Brca1 in preserving chromosome stability (4). Indeed, murine ES cells and mouse embryonic fibroblasts (MEFs) with homozygous Brca1 mutations readily accumulate extensive cytogenetic lesions (5, 6). Also, BRCA1 is required for homology-directed repair (HDR) (5, 7, 8), a process that allows for error-free repair of double-stranded DNA breaks (DSBs). In accord with these findings, BRCA1 associates with proteins that mediate DSB repair, such as Rad51, CtIP, and the RAD50/MRE11/NBS1 complex, and is phosphorylated by protein kinases that regulate the cellular response to genotoxic stress, including ATM, ATR, and Chk2/Cds1 (2, 3). As such, a deficiency in DSB repair may contribute to the chromosomal instability characteristic of BRCA1 mutant cells.
In vivo, most BRCA1 polypeptides exist as a heterodimeric complex with the related BARD1 protein (9, 10). Both proteins harbor an N-terminal RING domain, an motif required for the enzymatic function of many E3 ubiquitin ligases (11). Although the individual BRCA1 and BARD1 polypeptides display modest E3 ligase activity in vitro (12, 13), this activity is dramatically enhanced on heterodimerization (14). BARD1 has also been implicated in a number of BRCA1 functions, including its ability to promote HDR of DSBs (15, 16). Also, Brca1-null, Bard1-null, and double Brca1/Bard1-null mice display embryonic lethality phenotypes that are essentially indistinguishable, suggesting that the early developmental functions of both proteins are mediated by the Brca1/Bard1 heterodimer (17). The tumor suppression activity of BRCA1 also appears to be mediated by the BRCA1/BARD1 heterodimer, because mammary-specific inactivation of either Brca1 or Bard1 elicits breast tumors in mice that arise with identical kinetics and a common histopathology (18).
Although the existing data support an important role for the BRCA1/BARD1 heterodimer in the repair (15, 16), developmental (17), and tumor suppression (18) functions of BRCA1, the extent to which these processes depend on the E3 ubiquitin ligase activity of the heterodimer is not known. Thus, we have generated isogenic ES cells that express either wild-type Brca1 (Brca1FH-WT) or an enzymatically inactive form (Brca1FH-I26A) that retains the ability to heterodimerize with Bard1. Although Brca1-null ES cells are not viable, ES cells expressing enzymatically inert Brca1 can be readily cultured in vitro. The levels of damage-induced, but not spontaneous, chromosomal abnormalities are elevated in Brca1FH-I26A/− cells relative to control (Brca1FH-WT/−) and wild-type (Brca1+/+) ES cells. However, these cells resist genotoxic stress, undergo damage-induced Brca1 hyperphosphorylation, accumulate Rad51 at damage-induced nuclear foci, and mediate HDR of chromosomal DNA breaks at levels comparable with those of wild-type ES cells. Thus, the E3 ligase activity of Brca1 is dispensable for critical aspects of its function in the maintenance of genome stability.
Results
ES Cells Expressing Enzymatically Inactive Brca1 Are Viable.
The E3 ligase activity may be required for some, all, or none of the functions of the BRCA1/BARD1 heterodimer. To distinguish between these possibilities, we sought to generate cells that only express a catalytically deficient form of Brca1. The BRCA1/BARD1 heterodimer mediates ubiquitin conjugation through its association with a subset of E2 enzymes that bind with similar affinities to a common surface cleft on the RING domain of BRCA1 (19, 20). Mutations of this surface cleft ablate the ability of BRCA1 to interact with its cognate E2 proteins and consequently impair the enzymatic activity of the BRCA1/BARD1 heterodimer. Among these, the I26A mutation is of particular interest, because it can abolish the E3 activity of BRCA1 without disturbing the overall structure of its RING domain or formation of the BRCA1/BARD1 heterodimer (19, 20). Therefore, to generate cells that express enzymatically-inactive heterodimers, knock-in targeting constructs containing mouse Brca1 genomic DNA were prepared. As illustrated in supporting information (SI) Fig. S1, a promoterless hygromycin-resistance gene cassette preceded by a slice acceptor signal and flanked by FRT (FLP recombinase target) sites was inserted into the intron upstream of exon 2. Also, a short sequence (“FH”) encoding an artificial initiator methionine and the FLAG and HA protein epitopes was inserted immediately upstream of the natural translation initiator codon in exon 2. Two forms of this construct were prepared: a wild-type version (pBrca1FH-WT-hyg) and a mutant version (pBrca1FH-I26A-hyg), in which the I26A mutation was introduced into exon 2. After electroporation of these constructs into 129Sv ES cells, several independent hygromycin-resistant ES clones harboring the Brca1FH-I26A-hyg and Brca1FH-WT-hyg knock-in alleles were identified (Fig. S2). To excise the hygromycin gene cassette from the knock-in alleles, the targeted ES cells were transiently transfected with a plasmid encoding the FLPe recombinase, and properly recombined ES clones bearing the desired genotypes (Brca1FH-I26A/+ and Brca1FH-WT/+) were identified by Southern blot analysis and confirmed by sequence analysis (data not shown).
To assess whether the E3 ligase activity of the BRCA1/BARD1 is required at the cellular level, we sought to inactivate the remaining wild-type allele of the Brca1FH-I26A/+ and Brca1FH-WT/+ ES cells. Thus, a null targeting construct (pBrca1ex2-hyg) was prepared by replacing exon 2 (which includes coding sequences for the initiator methionine and part of the RING domain) with a hygromycin gene cassette (Fig. S3). In principle, this construct can generate a null allele (Brca1−) by recombining with either the endogenous (Brca1+) or knock-in (Brca1FH-I26A or Brca1FH-WT) allele of the heterozygous ES cells. Indeed, Southern blot analysis of the hygromycin-resistant colonies obtained by electroporation of the null construct into Brca1FH-WT/+ cells identified 38 independent subclones that had undergone homologous recombination at the Brca1 locus; of these, 27 resulted from targeting the Brca1+ allele (to yield Brca1FH-WT/− subclones), and 11 from targeting the Brca1FH-WT allele (to yield Brca1−/+ subclones) (Fig. 1 and Table S1). Likewise, both alleles were also targeted on electroporation of Brca1FH-I26A/+ cells with the Brca1-null construct; of the 29 homologous recombinants recovered, 22 arose by targeting the Brca1+ allele (to yield Brca1FH-I26A/− subclones), and 7 by targeting the Brca1FH-I26A allele (to yield Brca1−/+ subclones). The viability and undifferentiated status of individual subclones was confirmed by examination of cell morphology and alkaline phosphatase staining, as well as colony formation assays, as illustrated in supporting information Fig. S4 and Fig. S5.
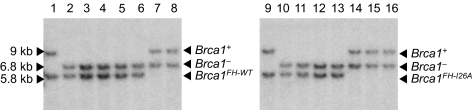
Fig. 1.
Isogenic ES cells that express either a wild-type (Brca1FH-WT/−) or enzymatically-deficient (Brca1FH-I26A/−) Brca1 protein. Heterozygous Brca1FH-I26A/+ and Brca1FH-WT/+ ES cells were electroporated with the null targeting construct pBrca1ex2-hyg, and hygromycin-resistant subclones were screened by Southern blot analysis of NcoI-digested genomic DNA with probe B. The introduction of additional NcoI sites in the targeting vectors reduces the 9 kb NcoI germ-line fragment to a 5.8-kb fragment in the Brca1FH-I26A and Brca1FH-WT knock-in alleles and to a 6.8 kb in the null Brca1− allele. Electroporation of Brca1FH-WT/+ cells (lane 1) with pBrca1ex2-hyg generated hygromycin-resistant subclones with either the Brca1FH-WT/+ (lanes 7 and 8) or Brca1FH-WT/− (lanes 2–6) genotypes, whereas electroporation of Brca1FH-I26A/+ cells (lane 9) yielded subclones of the Brca1FH-I26A/+ (lanes 14–16) or Brca1FH-I26A/− (lanes 10–13) genotypes.
The fact that Brca1FH-I26A/− subclones, which can only express the catalytically-deficient FH-tagged Brca1-I26A polypeptide, were readily generated indicates that the enzymatic activity of Brca1 is not required for the viability of ES cells. For comparison, we also attempted to produce ES cells homozygous for either a null or hypomorphic Brca1 allele. To obtain Brca1-null cells, a heterozygous Brca1+/− ES clone (21) was electroporated with a hygromycin-based Brca1-null construct (pBrca1ex2-hyg); of the 11 homologous recombinants obtained, all involved targeting of the nonfunctional Brca1− allele (Table S1), suggesting that Brca1−/− ES cells are not viable. We also examined ES cells harboring a truncating mutation (Brca1tr) that eliminates the carboxy-terminal half (amino acids 901-1812) of Brca1 (22). When heterozygous Brca1+/tr ES cells were reelectroporated with the pBrca1tr-neo targeting construct (22), each of the 31 independent homologous recombinants analyzed involved targeting of the mutant Brca1tr allele (Table S1), indicating that Brca1tr/tr ES cells are also not viable. Likewise, by using a similar approach, we were not able to obtain ES subclones bearing homozygous null mutations of the Bard1 gene (17). Together, these data indicate that ES cell viability depends on the Brca1/Bard1 heterodimer, but not on the E3 ligase activity of the heterodimer.
The E3 Ligase Activity of FH-Brca1 Polypeptides from Brca1FH-I26A/− Cells Is Defective.
To observe expression of the knock-in FH-Brca1 polypeptides, lysates were prepared from several independent subclones of Brca1FH-I26A/− and Brca1FH-WT/− ES cells. Immunoblotting with HA-specific antibodies revealed that the steady-state levels of the mutant FH-Brca1-I26A polypeptide (Fig. 2A, lanes 9–14) are reproducibly lower than those of the wild-type polypeptide (lanes 3–8). Consistent with the notion that the in vivo stabilities of BRCA1 and BARD1 are interdependent (14, 17), a corresponding reduction in the levels of endogenous Bard1 protein was also observed in Brca1FH-I26A/− cells, whereas the levels of another Brca1-associated protein (Ctip) were not affected. Of note, when cells were exposed to ionizing radiation (IR), both the FH-Brca1-WT and FH-Brca1-I26A polypeptides displayed electrophoretic mobility shifts indicative of hyperphosphorylation, suggesting that damage-induced phosphorylation of Brca1 is largely independent of its E3 ligase activity.
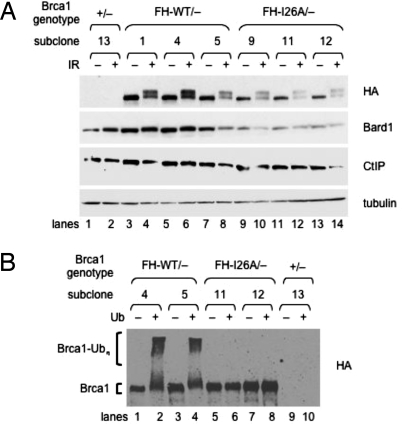
Fig. 2.
Expression and enzymatic activity of the knock-in FH-Brca1 polypeptides. (A) ES cells were subjected to 10 Gy of IR or left untreated; 2 h later, lysates were prepared from Brca1+/− cells (lanes 1 and 2) and from several independent subclones of Brca1FH-WT/− (lanes 3–8) and Brca1FH-I26A/− (lanes 9–14) cells. The ES cell lysates were then fractionated by PAGE and immunoblotted with antibodies specific for the HA epitope (to detect FH-Brca1 polypeptides), mouse Bard1, mouse Ctip, or α-tubulin. (B) I26A-mutant FH-Brca1 polypeptides from Brca1FH-I26A/− cells lack autoubiquitination activity. Ubiquitination reactions were conducted, in the presence (even lanes) or absence (odd lanes) of ubiquitin, by using FH-Brca1 complexes immunoprecipitated on M2 beads from independent subclones of either Brca1FH-WT/− (lanes 1–4) or Brca1FH-I26A/− (lanes 5–8) knock-in ES cells or from control Brca1+/− ES cells (lanes 9 and 10). The PAGE-fractionated reaction products were then immunoblotted with HA-specific antibodies to reveal autoubiquitinated conjugates of the wild-type (lanes 2 and 4), but not the I26A-mutant (lanes 6 and 8), FH-Brca1 polypeptides.
To confirm that the FH-Brca1 polypeptides of Brca1FH-I26A/− cells are enzymatically inert, we conducted in vitro ubiquitination reactions by using FH-Brca1 complexes from knock-in ES cells as an E3 ligase. These complexes were purified from ES cell lysates by immunoprecipitation on M2 beads, an agarose resin conjugated with Flag-specific monoclonal antibodies. To assess enzymatic function, the purified resin-bound FH-Brca1 complexes were incubated with ATP, ubiquitin monomer, E1 enzyme, and E2 enzyme UbcH5c, and the reaction products fractionated by PAGE. As shown in Fig. 2B, autoubiquitinated forms of the wild-type FH-Brca1 polypeptide from Brca1FH-WT/− cells were readily observed by immunoblotting with HA-specific antibodies (lanes 2 and 4). In contrast, autoubiquitinated I26A-mutant polypeptides could not be detected in Brca1FH-I26A/− cells (lanes 6 and 8).
Cells Lacking Brca1 Enzymatic Activity Are Resistant to Mitomycin C (MMC).
To ascertain whether the enzymatic activity of BRCA1 is required for cellular resistance to genotoxic stress, a clonogenicity assay was used to evaluate the response of isogenic ES subclones to MMC, a DNA cross-linking agent. As shown in Fig. 3, the MMC survival curve of Brca1FH-I26A/− cells is completely overlapping with those of the Brca1FH-WT/− and Brca1+/− control cells. In parallel, we also analyzed ES cells homozygous for a hypomorphic allele that encodes a Brca1 polypeptide that retains the RING and BRCT domains but lacks the large central segment specified by exon 11. Although designated Brca1Δ223–763 (23), this allele has also been referred to as Brca1− in some studies (5, 7, 8, 24). In any case, homozygous Brca1Δ223–763/Δ223–763 ES cells are hypersensitive to MMC and deficient for HDR of DSBs (5, 7, 8). In accord with the published data (5), we found that these cells, unlike Brca1FH-I26A/− cells expressing enzymatically inactive Brca1, displayed a pronounced hypersensitivity to MMC (Fig. 3). Thus, ES cell resistance to MMC depends on some aspect of Brca1 function, but not its ubiquitin ligase activity.
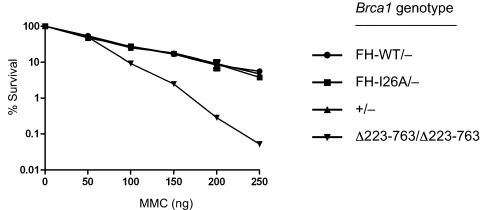
Fig. 3.
Cells lacking Brca1 enzymatic activity are resistant to MMC-induced genotoxic stress. Isogenic ES cells that are proficient (Brca1FH-WT/−) or deficient (Brca1FH-I26A/−) for Brca1 E3 ligase activity were examined for MMC sensitivity in clonogenic survival assays, together with ES cells homozygous for a hypomorphic Brca1 mutation (Brca1Δ223–763/Δ223–763) and control ES cells (Brca1+/−). Cells were plated and treated with various concentration of MMC for 4 h. After 8–10 days, the surviving colonies were stained with Giemsa and counted. Survival is calculated as a percentage of colonies in the mock-treated plates. Each subclone was tested in triplicate, and the error bars represent SE of the mean of survival for each subclone. Similar results were also observed in separate experiments by using additional independently derived Brca1FH-WT/− and Brca1FH-I26A/− ES subclones.
Brca1FH-I26A/− ES Cells Do Not Accumulate Spontaneous Chromosome Abnormalities.
Cytogenetic analyses have shown that chromosomal rearrangements spontaneously arise at high rates in Brca1Δ223–763/Δ223–763 ES cells (5). To ascertain whether the enzymatic activity of Brca1 is required for chromosomal stability, we examined the karyotypes of several different ES subclones. As expected, Brca1Δ223–763/Δ223–763 cells harbored high levels of spontaneous defects, predominantly chromosome exchanges, relative to the parental Brca1+/+ cells (Table S2 and Fig. S6). Significantly, Brca1FH-I26A/− cells displayed low levels of chromosomal rearrangement, comparable with those of the control (Brca1FH-WT/−) and wild-type (Brca1+/+) ES cells. Thus, although Brca1 suppresses spontaneous chromosomal instability, its ubiquitin ligase activity is not required for this function.
DNA Damage Elicits High Levels of Chromosomal Rearrangements in Brca1FH-I26A/− Cells.
Despite their resistance to spontaneous chromosomal rearrangements, Brca1FH-I26A/− cells accumulated cytogenetic defects at high rates when subjected to DNA damage by MMC exposure (Table S2). Indeed, these rates are greatly elevated relative to those of control (Brca1FH-WT/− and Brca1+/+) ES cells, although they do not approach the higher levels observed in MMC-treated Brca1Δ223–763/Δ223–763 cells. Because the steady-state expression of mutant FH-Brca1-I26A is lower than that of the wild-type FH-Brca1-WT polypeptide (Fig. 2), we do not know whether the heightened chromosomal instability of MMC-treated Brca1FH-I26A/− cells is due to the enzymatic deficiency or the reduced expression levels of FH-Brca1-I26A.
Although Gene Targeting Efficiencies Are Modestly Reduced in Brca1FH-I26A/− ES Cells, These Cells Support Homology-Directed Repair of Chromosomal Breaks at Wild-Type Levels.
In light of the known requirement for BRCA1 in homology-directed repair, we used 2 distinct assays to compare homologous recombination in Brca1FH-I26A/− and Brca1FH-WT/− ES cells. Gene targeting measures the ability of cells to support homologous integration of exogenous DNA. Although the relationship between homologous integration and HDR is not fully understood, cells bearing mutations in certain DSB repair genes, including Brca1 and Brca2, often display deficiencies in both processes (5, 7, 8, 25). To measure homologous integration at the Pim1 locus, Brca1FH-I26A/− and Brca1FH-WT/− cells were electroporated with p31kDR-GFP, a DNA construct that contains a DR-GFP recombination substrate (described below) and a promoterless neomycin-resistance marker flanked by targeting arms comprised of Pim1 genomic DNA. After neomycin selection, the surviving subclones were examined for homologous integration at the Pim1 locus by Southern blot analysis (5). As illustrated in Table S3, Pim1 targeting was observed in 86% of the neomycin-resistant Brca1FH-WT/− clones and 40–48% of the drug-resistant Brca1FH-I26A/− clones. Similar modest reductions (2–4 fold) of the gene-targeting efficiency were also observed in Brca1FH-I26A/− cells with constructs designed to integrate homologously at 2 distinct chromosomal loci, Rb and Rosa26 (Table S3). Thus, cells lacking Brca1 enzymatic function display a reproducible defect in their capacity to support homologous integration. Again, we cannot distinguish whether this deficiency is due to the enzymatic inactivity or the reduced levels of FH-Brca1-I26A in Brca1FH-I26A/− cells. However, this defect is modest relative to the striking effects seen in Brca1Δ223–763/Δ223–763 ES cells, where targeting efficiencies were reduced 23-fold at the Pim1 locus and 13-fold at the Rb locus (7).
We next examined HDR of DSBs within a defined chromosomal locus by using an integrated DR-GFP recombination substrate (26). The DR-GFP substrate has 2 nonfunctional copies of the GFP gene: SceGFP, which is disrupted by an 18-bp insertion recognized by the I-SceI endonuclease; and iGFP, which lacks the N- and C-terminal coding sequences of GFP. When a DSB triggered by I-SceI cleavage of SceGFP undergoes HDR using iGFP as the template, a functional GFP gene is generated, and such events can be quantitated by flow cytometry (26). Because the p31kDR-GFP targeting construct contains the DR-GFP substrate, the G418-resistant Brca1FH-I26A/− and Brca1FH-WT/− subclones that arose by homologous integration of this construct (Table S3) possess a DR-GFP substrate at identical positions of the Pim1 locus. To measure HDR of an induced chromosomal DSB, Brca1FH-I26A/− and Brca1FH-WT/− subclones were evaluated for the appearance of GFP-positive cells after transient transfection with an expression vector encoding I-SceI. In the absence of I-SceI expression, very few GFP-positive cells were generated in either the Brca1FH-I26A/− and Brca1FH-WT/− subclones (0 to 0.01%; data not shown). As expected, I-SceI expression increased the number of Brca1FH-WT/− GFP-positive cells (2.9%), consistent with its ability to induce HDR by producing chromosomal DSBs within the integrated DR-GFP substrate (Fig. 4A). Surprisingly, I-SceI expression induced a similar proportion of Brca1FH-I26A/− GFP cells (2.5%), suggesting that the enzymatic activity of Brca1 is not required for HDR. In contrast, HDR efficiency is significantly reduced in Brca1Δ223–763/Δ223–763 ES cells (0.6% GFP cells) relative to isogenic Brca1+/+ cells (2.3% GFP cells) harboring a DR-GFP substrate integrated at the same position of the Pim1 locus (Fig. 4A) (5). Thus, although Brca1 and Bard1 are both required for homology-directed repair of chromosomal DNA breaks (5, 7, 15, 16), the ubiquitin ligase activity of the Brca1/Bard1 heterodimer appears to be dispensable.
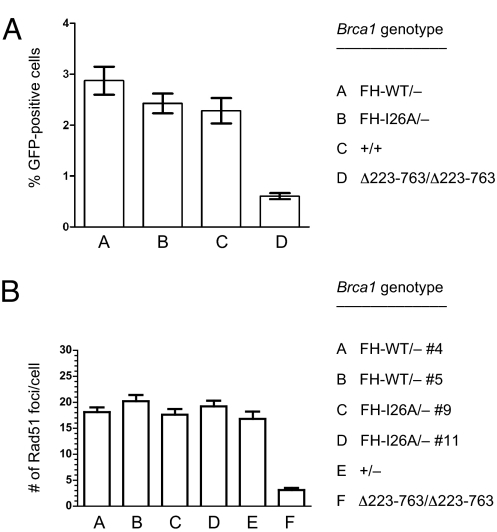
Fig. 4.
Cells lacking Brca1 enzymatic activity are proficient for HDR of DSBs and assembly of Rad51 nuclear foci in response to DNA damage. (A) Brca1+/+, Brca1FH-WT/−, Brca1FH-I26A/−, and Brca1Δ223–763/Δ223–763 ES subclones containing the DR-GFP substrate integrated into the Pim1 locus were transiently transfected with either the I-SceI expression vector or the empty vector. GFP-positive cells were rarely generated (<0.01%; data not shown) on transfection with the empty vector. I-SceI expression strongly induced the number of GFP-positive cells in the Brca1+/+, Brca1FH-WT/−, and Brca1FH-I26A/− ES subclones, indicating efficient DSB repair by gene conversion, but not in the Brca1Δ223–763/Δ223–763 ES cells, which are known to be deficient in HDR of DSBs (5). Each ES subclone was assayed in triplicate, and the error bars represent SE of the mean. Similar results were also observed in separate experiments by using additional independently derived Brca1FH-WT/− and Brca1FH-I26A/− ES subclones. (B) Brca1FH-WT/−, Brca1FH-I26A/−, Brca1+/−, and Brca1Δ223–763/Δ223–763 ES cells were exposed to IR (10 Gy) and IRIF formation was assessed 3 h later by immunostaining with Rad51-specific antibodies. Rad51-containing IRIFs were counted in 50 nuclei of each genotype, and the error bars represent SE of the mean. IR treatment strongly induced the number of IRIFs in the Brca1FH-WT/−, Brca1FH-I26A/−, and Brca1+/− ES subclones, but not in Brca1Δ223–763/Δ223–763 ES cells, which are known to be deficient for IRIF assembly of Rad51 (24).
The Ubiquitin Ligase Activity of Brca1 Is Not Required for Assembly of Rad51 Nuclear Foci in Response to DNA Damage.
In cells subjected to IR, Rad51 polypeptides accumulate at sites of DNA damage to form IR-induced foci (IRIFs). These nuclear structures, which can be visualized by immunofluorescent microscopy, likely reflect assembly of the Rad51 nucleofilaments necessary for homology-dependent DSB repair. A requirement for Brca1 in this process was established by the fact that Rad51 accumulation into IRIFs is dramatically reduced in Brca1Δ223–763/Δ223–763 ES cells relative to isogenic Brca1+/− cells (24). In accord with this study, we also observe deficient formation of Rad51-staining IRIFs in Brca1Δ223–763/Δ223–763 cells (Fig. 4B). However, IRIFs occurred at normal levels in ES cells expressing either the wild-type or enzymatically-deficient FH-Brca1 polypeptide (Fig. 4B). These data indicate that Rad51 recruitment to sites of DNA damage, a key step in DSB repair by homologous recombination, depends on Brca1, but not its ubiquitin ligase activity.
Discussion
In living cells, most BRCA1 polypeptides exist in association with the related BARD1 protein (9, 10), and the resulting BRCA1/BARD1 heterodimer displays an ubiquitin E3 ligase activity that is dramatically (>100-fold) higher than that of either BRCA1 or BARD1 alone (14). Indeed, as the only known enzymatic activity intrinsic to BRCA1, it was reasonable to propose that the E3 ligase activity is critical to BRCA1 function. To test this hypothesis, we have generated isogenic ES cells that express either a wild type (Brca1FH-WT) or enzymatically-deficient (Brca1FH-I26A) form of Brca1. Surprisingly, we found that the E3 ligase activity of BRCA1 is dispensable for mammalian cell viability, as well as for critical aspects of BRCA1 function in genome stability, including cellular resistance to genotoxic stress, suppression of spontaneous chromosomal rearrangements, damage-induced BRCA1 hyperphosphorylation, Rad51 recruitment to damaged DNA, and HDR of DSBs.
BRCA1 is thought to be essential for the viability of mammalian cells (27). This is consistent with the early embryonic lethality of mice bearing homozygous null Brca1 mutations (21, 28), the failure to culture MEFs from Brca1-null mice (21, 28), and the inability to generate viable ES cells by targeted inactivation of both alleles of Bard1 (17). BRCA1-null cells do exist in, and have been cultured from, human and mouse tumors; however, these malignant cells harbor additional genetic lesions that presumably allow for viability in the absence of BRCA1 (27). Nevertheless, sustained growth of normal diploid mammalian cells that lack BRCA1 has not been demonstrated. Thus, the relative ease with which we were able to generate ES cells lacking the E3 ligase activity of Brca1 immediately suggested that this activity is not required for all cellular Brca1 functions.
Brca1-deficient ES cells generated by gene targeting typically display a karyotypic instability manifested by aneuploidy and/or gross chromosomal rearrangements (5, 6, 29). In contrast, we found that ES cells expressing enzymatically-inactive Brca1 (Brca1FH-I26A/−) develop chromosome defects at low rates that are indistinguishable from those of isogenic cells with wild-type Brca1 (Brca1FH-WT/−). These results suggest that Brca1 acts to suppress spontaneous chromosomal rearrangements, but that its E3 ligase activity is not essential for this function. Nevertheless, gross chromosome aberrations are induced on exposure of Brca1FH-I26A/− cells, but not Brca1FH-WT/− cells, to the DNA-cross-linking agent, MMC. At least 2 possible explanations can be invoked to account for the increased chromosomal instability of MMC-treated Brca1FH-I26A/− cells. As discussed below, the enzymatic activity of Brca1 may be required for some, but not all, Brca1 functions in the DNA damage response. Alternatively, the reduced levels of FH-Brca1-I26A polypeptides in Brca1FH-I26A/− cells (Fig. 2A) may be insufficient to maintain chromosomal stability in face of the excess DNA damage associated with acute MMC exposure.
There is substantial evidence that BRCA1 is required for HDR of double-strand DNA breaks (5, 7, 8). Because HDR defects can lead to chromosomal rearrangements and aneuploidy, loss of HDR function may be a key source of genomic instability in BRCA1-deficient cells. Interestingly, the BRCA2 tumor suppressor, which is also implicated in the hereditary breast and ovarian cancer syndrome, is required for HDR (25), and has been ascribed a specific biochemical role in this process during assembly of the Rad51 nucleoprotein filament (30). Thus, it is conceivable that HDR deficiency is a common determinant of breast cancer susceptibility in both BRCA1 and BRCA2 mutation carriers. Unlike BRCA2, precise molecular functions for BRCA1 in HDR have not yet been determined. To ascertain whether the E3 ligase activity of BRCA1 is required for HDR, we used 2 distinct assays to measure this process in the isogenic ES cells. First, the ability of these cells to homologously integrate transfected DNA fragments was evaluated in gene-targeting assays. In this manner, we observed a 2–3-fold reduction in the gene-targeting efficiency of Brca1FH-I26A/− cells relative to the control Brca1FH-WT/− cells. Although this effect was consistent when analyzed at 3 distinct chromosomal loci, it is considerably smaller than the 10–25-fold reductions observed in Brca1Δ223–763/Δ223–763 ES cells. Again, we cannot distinguish whether the modest gene-targeting deficiency of Brca1FH-I26A/− cells is due to the enzymatic inactivity or the reduced expression levels of FH-Brca1-I26A.
Gene targeting occurs through an “omega” intermediate in which the 2 targeting arms of the linearized construct align with homologous sequences in the chromosomal locus. As such, homologous integration of the targeting construct entails 2 distal recombination reactions, each involving chromosomal invasion by a 1-ended DSB (26). However, HDR of a genomic DNA break is mediated primarily by recombination with an intact sister chromatid through a single recombination reaction that involves 2 proximal DSB ends (26). When these events were measured by using an integrated DR-GFP recombination substrate, we observed equivalent levels of HDR in ES cells that do or do not express enzymatically proficient Brca1. As the Brca1 and Bard1 polypeptides have both been implicated in HDR (5, 7, 15, 16), these results indicate that the Brca1/Bard1 heterodimer, but not its ubiquitin ligase activity, is required for HDR of chromosomal DNA breaks.
Our results show that the enzymatic activity of BRCA1 is dispensable for ES cell viability, cellular resistance to MMC, damage-induced Brca1 hyperphosphorylation, suppression of spontaneous chromosomal rearrangements, and HDR of chromosomal breaks. What, then, are the functions of its E3 ligase activity? Because enzymatically-deficient FH-Brca1 polypeptides are expressed at lower levels than wild-type FH-Brca1 (Fig. 2), one possibility is that autoubiquitination serves to increase the stability of BRCA1. This notion would be consistent with the ability of BRCA1 and BARD1 polypeptides to stabilize one another in a reciprocal fashion (14, 17, 31), and the fact that autoubiquitinated BRCA1 is conjugated to K6-linked chains that do not target proteins for proteasomal degradation (32–34). Accordingly, the reduced levels of enzymatically-deficient FH-Brca1 in Brca1FH-I26A/− cells may allow for suppression of chromosomal rearrangements in the absence, but not the presence, of overt genotoxic stress (Table S2). Likewise, these reduced levels may also be sufficient for HDR when measured by using DR-GFP assays, in which each transfected cell experiences a single chromosomal break (Fig. 4), but not gene-targeting assays, in which each transfected cell is exposed to multiple double-strand DNA ends (Table S3). Nonetheless, we cannot preclude the possibility that BRCA1 promotes some aspects of the DNA damage response, such as as suppression of MMC-induced chromosome lesions, through enzymatic activity, and others, including homology-directed repair of DSBs, through nonenzymatic means. It will be especially important to determine whether the E3 ligase activity is required for the critical role of BRCA1 in suppressing tumor formation in mammary and ovarian epithelial cells. Meanwhile, the current results indicate that significant aspects of BRCA1 function in genome maintenance are independent of its E3 ligase activity.
Materials and Methods
Detailed methods for ES cell culture, targeted mutagenesis experiments, MMC survival assays, HDR assays, and karyotype analysis are provided as SI Materials and Methods.
Expression of FH-Brca1 Polypeptides.
ES cells were subjected to 10 Gy of IR or left untreated, incubated at 37 °C for 2 h, and harvested in PBS supplemented with 2 mM PMSF and 1× phosphatase inhibitors (20 mM NaF/1 mM sodium pyrophosphate/1 mM sodium orthovanadate). The cells were then lysed in low salt Nonidet P-40 buffer (10 mM Hepes, pH 7.6/250 mM NaCl/0.1% Nonidet P-40/5 mM EDTA/10% glycerol), supplemented with 1 mM DTT, 25 mM NaF, and complete protease inhibitor mixture tablet (Roche); and the lysates were clarified by high-speed centrifugation. For direct Western blot analysis, 50 μg of whole cell lysate were fractionated by SDS/PAGE on 4–20% Tris·HCl Criterion precast gels (BioRad). Immunoblotting was performed with monoclonal antibodies specific for the HA epitope (rat clone 3F10, Roche) and α-tubulin (mouse clone DM1A, Calbiochem), and rabbit polyclonal antisera raised against mouse Bard1 and mouse Ctip (17).
Brca1 Autoubiquitination Reactions.
ES cells were suspended in lysis buffer (20 mM Tris·HCl, pH 8.0/150 mM NaCl/0.5% Nonidet P-40/1 mM EDTA/10% glycerol/1 mM DTT/5 mM NaF/Roche Complete Mini EDTA-free protease inhibitor) on ice for 20 min. The lysates were then centrifuged at 13,000 rpm for 10 min at 4 °C, and the supernatants incubated with Flag M2 resin (Sigma) for 3 h at 4 °C. Each resin was washed 3 times with lysis buffer, once with Tris buffer (25 mM Tris·HCl, pH 7.9/50 mM NaCl/10% glycerol/1 mM DTT), and once with ubiquitination buffer (50 mM Tris·HCl, pH 7.4/5 mM MgCl2/2 mM NaF/10 nM okadaic acid/2 mM ATP/6 mM DTT). The resin-immobilized protein was then incubated for 45 min at 37 °C in ubiquitination buffer with 40 ng of His-6-tagged E1 ubiquitin activating enzyme (Biomol) and 200 ng of 6H-UbcH5c, in the presence or absence of 1 μg of ubiquitin (32). After adding protein sample dye, each resin was boiled for 5 min, centrifuged, and the supernatant was fractionated on a 4–20% gradient electrophoresis gel. The proteins were transferred to a nitrocellulose membrane and the FH-Brca1 polypeptides visualized by immunoblotting with high-affinity anti-HA antibody (clone 3F10, Roche).
Rad51 Immunostaining.
Three hours after IR treatment (10 Gy), ES cells were fixed and permeabilized as described (24). IRIFs were stained by using rabbit anti-Rad51 antibody (Oncogene-EMD) at a 1:500 dilution and the secondary fluorophore-conjugated antibody (goat anti-rabbit; Southern Biotech) at a 1:200 dilution. The nuclei were then counterstained with Toto-3 (Invitrogen), and the mounted cells were inspected by confocal laser microscopy. IRIFs were counted in 50 nuclei of each genotype.
Supplementary Material
Acknowledgments.
This work was supported by the Henrietta Milstein Foundation and National Cancer Institute Grant 1P01-CA97403 Projects 4 (to R.B.) and 5 (to T.L.). L.J.R. was supported by a Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship 1F31-CA132626; R.S. by the New York State Breast Cancer Research Fund Fellowship C020909 and a Susan G. Komen Breast Cancer Foundation fellowship; A.P.M. by National Cancer Institute Fellowship T32-CA09503 and Department of Defense Breast Cancer Research Program Fellowship BC050560; M.L. by National Cancer Institute Fellowship T32-CA09503; and J.-T.C. by Republic of China National Science Council Grant NSC 96-2918-I-110-001.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811203106/DCSupplemental.
References
- 1.Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 2.Deng CX. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaraju G, Scully R. Minding the gap: The underground functions of BRCA1 and BRCA2 at stalled replication forks. DNA Repair (Amsterdam) 2007;6:1018–1031. doi: 10.1016/j.dnarep.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: Past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 5.Moynahan ME, Cui TY, Jasin M. Homology-directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 2001;61:4842–4850. [PubMed] [Google Scholar]
- 6.Xu X, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 7.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 8.Snouwaert JN, et al. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a brca1 transgene. Oncogene. 1999;18:7900–7907. doi: 10.1038/sj.onc.1203334. [DOI] [PubMed] [Google Scholar]
- 9.Wu LC, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Baer R. Nuclear localization and cell cycle-specific expression of CtIP, a protein that associates with the BRCA1 tumor suppressor. J Biol Chem. 2000;275:18541–18549. doi: 10.1074/jbc.M909494199. [DOI] [PubMed] [Google Scholar]
- 11.Baer R, Ludwig T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr Opin Genet Dev. 2002;12:86–91. doi: 10.1016/s0959-437x(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 12.Lorick KL, et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. Cancer-predisposing mutations within the RING domain of BRCA1: Loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci USA. 2001;98:5134–5139. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume R, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 15.Westermark UK, et al. BARD1 participates with BRCA1 in homology-directed repair of chromosome breaks. Mol Cell Biol. 2003;23:7926–7936. doi: 10.1128/MCB.23.21.7926-7936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laufer M, et al. Structural requirements for the BARD1 tumor suppressor in chromosomal stability and homology-directed DNA repair. J Biol Chem. 2007;282:34325–34333. doi: 10.1074/jbc.M705198200. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy EE, Celebi JT, Baer R, Ludwig T. Loss of Bard1, the heterodimeric partner of the Brca1 tumor suppressor, results in early embryonic lethality and chromosomal instability. Mol Cell Biol. 2003;23:5056–5063. doi: 10.1128/MCB.23.14.5056-5063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakya R, et al. The basal-like mammary carcinomas induced by Brca1 or Bard1 inactivation implicate the BRCA1/BARD1 heterodimer in tumor suppression. Proc Natl Acad Sci USA. 2008;105:7040–7045. doi: 10.1073/pnas.0711032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzovic PS, et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc Natl Acad Sci USA. 2003;100:5646–5651. doi: 10.1073/pnas.0836054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen DE, Brzovic PS, Klevit RE. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14:941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: Lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig T, Fisher P, Ganesan S, Efstratiadis A. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 2001;15:1188–1193. doi: 10.1101/gad.879201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snouwaert JN, Gowen LC, Lee V, Koller BH. Characterization of Brca1 deficient mice. Breast Disease. 1998;10:33–44. doi: 10.3233/bd-1998-101-206. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross- linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 25.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 26.Pierce AJ, Hu P, Han M, Ellis N, Jasin M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 2001;15:3237–3242. doi: 10.1101/gad.946401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elledge SJ, Amon A. The BRCA1 suppressor hypothesis: An explanation for the tissue-specific tumor development in BRCA1 patients. Cancer Cell. 2002;1:129–132. doi: 10.1016/s1535-6108(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 28.Hakem R, et al. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 29.Shen SX, et al. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Li Q, Fan J, Holloman WK, Pavletich NP. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction. Nature. 2005;433:653–657. doi: 10.1038/nature03234. [DOI] [PubMed] [Google Scholar]
- 31.Joukov V, Chen J, Fox EA, Green JB, Livingston DM. Functional communication between endogenous BRCA1 and its partner, BARD1, during Xenopus laevis development. Proc Natl Acad Sci USA. 2001;98:12078–12083. doi: 10.1073/pnas.211427098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem. 2003;278:34743–34746. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa H, et al. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:3916–3924. doi: 10.1074/jbc.M308540200. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Koike A, Takeshita T, Ohta T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div. 2008;3:1–10. doi: 10.1186/1747-1028-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.