Abstract
The p53 cofactor Strap (stress responsive activator of p300) is directly targeted by the DNA damage signalling pathway where phosphorylation by ATM (ataxia telangiectasia mutated) kinase facilitates nuclear accumulation. Here, we show that Strap regulation reflects the coordinated interplay between different DNA damage-activated protein kinases, ATM and Chk2 (Checkpoint kinase 2), where phosphorylation by each kinase provides a distinct functional consequence on the activity of Strap. ATM phosphorylation prompts nuclear accumulation, which we show occurs by impeding nuclear export, whereas Chk2 phosphorylation augments protein stability once Strap has attained a nuclear location. These results highlight the various functional roles undertaken by the DNA damage signalling kinases in Strap control and, more generally, shed light on the pathways that contribute to the regulation of the p53 response.
Keywords: p53, cofactor, Strap, Chk2
Introduction
Strap (stress responsive activator of p300) is a p300-interacting protein that is required for an effective p53 response (Demonacos et al, 2001). Strap has an unusual structure consisting of a tandem array of tetratricopeptide repeat motifs (Demonacos et al, 2001) that function in the assembly of multiprotein complexes (D'Andrea & Regan, 2003). Strap interacts with various components of the p53 co-activator complex, including JMY (junction mediating and regulatory) and p300, which facilitate p53 activity. This occurs in part because Strap increases the half-life of p53 by preventing the downregulation of p53 by Mdm2 (murine double minute 2). These findings, together with the fact that Strap is DNA damage responsive (Demonacos et al, 2001, 2004), suggest that Strap, as a component of the p53 co-activator complex, has an important function in regulating the cellular response to DNA damage.
Strap is a direct target for the DNA damage signalling pathway; ATM (ataxia telangiectasia mutated) kinase phosphorylates Strap in response to DNA damage, and phosphorylation is required for protein stabilization (Demonacos et al, 2004). It is consistent with the function of ATM kinase in the control of Strap stability that a Strap mutant devoid of the S203 phosphorylation site and endogenous Strap in ataxia telangiectasia cells fail to undergo nuclear accumulation and stabilization in response to DNA damage (Demonacos et al, 2004). However, in the context of the DNA damage signalling cascade, it is widely accepted that Chk2 (Checkpoint kinase 2) lies downstream from ATM, where it becomes activated, in part, through phosphorylation by ATM (Bartek et al, 2001). In turn, Chk2 phosphorylates several effector targets, including p53 and E2F-1, and has a crucial function in mediating the checkpoint response (Hirao et al, 2000).
Here, we report that the response of Strap to DNA damage reflects the coordinated interplay between ATM and Chk2 kinase, each of which phosphorylates Strap on different residues and has distinct functional consequences on Strap activity. Although phosphorylation by ATM kinase causes nuclear accumulation, most probably by impeding nuclear export, Chk2 augments protein stability once Strap has attained a nuclear location. These results highlight the interplay between the main DNA damage signalling kinases in Strap control and, more generally, the intricate pathways involved in cofactor control during the p53 response.
Results
Strap undergoes regulated nuclear export
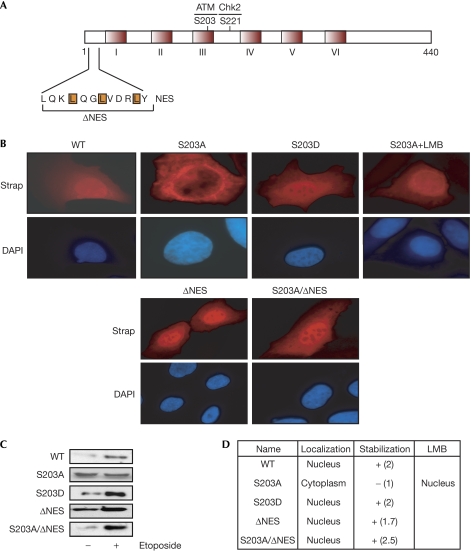
The nuclear accumulation of Strap is influenced by the phosphorylation of residue S203 by ATM kinase (Fig 1A; Demonacos et al, 2004). To explore the mechanisms involved in the control of nuclear accumulation, we studied the properties of a panel of Strap mutant derivatives with the objective of defining motifs and signals that have an impact on the localization of Strap. We confirmed the distribution of the Strap mutant S203A, which, in contrast to wild type, was located mainly in the cytoplasm (Fig 1B; Demonacos et al, 2004). Consistent with the function of S203 phosphorylation in controlling nuclear location, the phospho-mimic S203D mutant was nuclear (Fig 1B). The possibility that Strap contains a nuclear export signal (NES) was investigated by studying the effect of the nuclear export inhibitor, leptomycin B (Ossareh-Nazari et al, 1997), on S203A. In the presence of leptomycin B, S203A underwent significant nuclear accumulation (Fig 1B), suggesting that the activity of NES resides in Strap.
Figure 1.
Strap regulation through nuclear accumulation. (A) Strap contains six TPR motifs (I–VI), with the ATM S203 phosphorylation site located in the third TPR motif and the Chk2 S221 (this study) phosphorylation site indicated. The leucine-rich NES is located in the amino-terminal region (residues 10–21). (B) U2OS cells were transfected with expression vectors (200 ng) encoding Strap or the indicated mutant derivative and, where denoted, treated with leptomycin B (LMB; 10 nM, 4 h). Immunostaining was performed with the anti-HA HA11 as described and DAPI was used to visualize nuclei. (C) Strap or the indicated mutant derivative was transfected into U2OS cells (500 ng) and thereafter left either untreated or treated with etoposide (10 μM). Extracts were prepared after 16 h and immunoblotting was performed using anti-HA antibody to detect ectopic Strap. Co-transfected β-galactosidase expression vector (200 ng) acted as a control for transfection efficiency. (D) Summary of properties of Strap mutant derivatives. The fold increase of Strap and its derivatives under DNA-damaging conditions (derived from C) is indicated in parentheses. ATM, ataxia telangiectasia mutated; Chk2, Checkpoint kinase 2; DAPI, 4,6-diamidino-2-phenylindole; HA, haemagglutinin; NES, nuclear export signal; Strap, stress responsive activator of p300; TPR, tetratricopeptide repeat; WT, wild type.
A candidate NES, characterized by a high leucine content (Stommel et al, 1999), is present in the amino-terminal region of Strap (Fig 1A). To test whether the motif provides NES activity, we made a mutant derivative in which the candidate NES was deleted. In a side-by-side comparison with wild-type Strap, which was predominantly nuclear with some cytoplasmic staining, ΔNES showed increased nuclear localization (Fig 1B), suggesting that the sequence deleted in ΔNES provides functional NES activity. As S203A localizes to the cytoplasm, but undergoes nuclear accumulation on leptomycin B treatment (Fig 1B), we reasoned that the activity of NES might be influenced by S203 phosphorylation. Therefore, we combined both mutations in S203A/ΔNES and found that the combination mutant underwent more marked nuclear accumulation than S203A (Fig 1B). These results suggest that the control of Strap nuclear accumulation occurs indirectly through a process that affects nuclear export.
The nuclear accumulation of Strap is necessary for DNA damage-dependent stabilization of protein (Demonacos et al, 2004). For each Strap mutant derivative, we correlated nuclear location with protein stabilization. As expected, S203A underwent limited DNA damage-dependent stabilization when compared with wild-type Strap (Fig 1C,D; Demonacos et al, 2004). By contrast, both S203D and ΔNES showed DNA damage stabilization of a similar magnitude to wild-type Strap (Fig 1C,D). In a similar manner, S203A/ΔNES also underwent DNA damage-responsive stabilization (Fig 1C,D). Taken together, these results show that the nuclear location of Strap coincides with DNA damage-induced stabilization, and argue that nuclear accumulation occurs through a mechanism dependent on phosphorylation at S203.
Chk2 kinase phosphorylates Strap
The properties of the Strap mutants raised the possibility that other signalling events are also involved in Strap control. For example, S203A/ΔNES undergoes protein stabilization in response to DNA damage, even though it lacks the S203 phosphorylation site (Fig 1C,D). Similarly, NLS-S203A lacks the same phosphorylation site but attains a nuclear location owing to the artificial NLS and undergoes DNA damage-responsive stabilization (Demonacos et al, 2004). As Chk2 is activated by DNA damage and lies downstream from ATM (Bartek et al, 2001), we reasoned that Chk2 might be a candidate kinase.
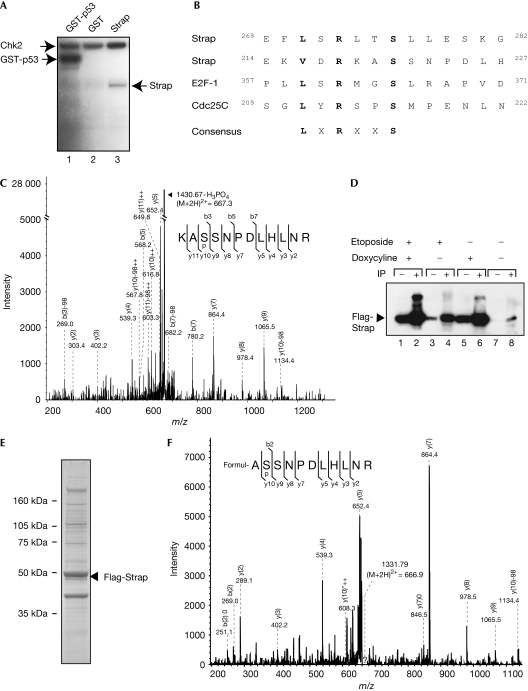
In vitro recombinant His-Strap was phosphorylated by Chk2 kinase (Fig 2A). An established substrate for Chk2, p53 (Hirao et al, 2000), acted as a positive control and glutathione S-transferase protein as a negative control (Fig 2A). A comparison of the Strap sequence with the Chk2 consensus (LXRXXS; Ahn et al, 2004) identified two possible phosphorylation sites: one located at S221 and the other centred around S276; both sites show similarity with physiological Chk2 substrates (Fig 2B). To ascertain whether these residues could be phosphorylated directly by Chk2, we analysed in vitro phosphorylated Strap by reverse-phase liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC-MS/MS), and detected significant phosphorylation at S221 (Fig 2C).
Figure 2.
Strap is phosphorylated by Chk2. (A) Recombinant Chk2 protein (200 ng) was incubated with His-tagged Strap (100 ng; lane 3) and phosphorylation (32P) assessed by using SDS–PAGE as described. Recombinant GST-p53 (lane 1) was used as a positive control and GST (200 ng; lane 2) as a negative control. (B) Comparison of putative Chk2 kinase sites in Strap when compared with known phosphorylation sites of Chk2 in E2F-1 and Cdc25C. (C) His-tagged Strap was incubated with recombinant Chk2 protein and analysed by LC-MS/MS using the Mascot Software package (Matrixscience, London, UK). Both the peptides (M+2H)2+ 716.5 and (M+3H)3+ 477.7 (data not shown) were identified as KASSNPDLHLNR (MW 1430.7) containing a phosphoserine at position 3 (S221). The unmodified peptide KASSNPDLHLNR (M+2H)2+ 676.5 (MW 1351.1) was also detected. (D) Cell lysates from the Strap cell line treated with etoposide (+; 20 μM for 12 h; lanes 1–4) and doxycycline (+; 1 μg/ml for 36 h; lanes 1, 2, 5 and 6) or untreated (–; lanes 7 and 8) were immunoprecipitated (IP) with anti-Flag (lanes 2, 4, 6 and 8) and then blotted with anti-Strap peptide 510. The level of input protein is shown (lanes 1, 3, 5 and 7) by immunoblotting with anti-Strap peptide 510 where equal amounts of protein (50 μg) were loaded. (E) An extract prepared from the Flag-Strap stable cell line treated with etoposide (20 μM for 12 h) and doxycycline (1 μg/ml for 36 h) was immunoprecipitated with anti-Flag, and the immunoprecipitate was separated by using SDS–PAGE and silver stained as described. Flag-Strap is indicated. (F) Flag-tagged Strap (E) purified from the Flag-Strap stable cell line was subjected to in-gel trypsin digestion. Analysis by LC-MS/MS showed not only the unmodified ASSNPDLHLNR peptide (H+H)2+ 612.5 (MW 1222.9) but also the same species with phosphoserine (S221) represented by (M+H)2+ 666.9 (MW 1331.8). Cdc25C, cell division cycle 25C; Chk2, Checkpoint kinase 2; E2F-1, E2F transcription factor 1; GST, glutathione S-transferase; LC-MS/MS, liquid chromatography coupled with electrospray ionization tandem mass spectrometry; MW, molecular weight; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; Strap, stress responsive activator of p300.
To determine whether the same residue was phosphorylated in cells, we analysed ectopic Strap protein that had been affinity purified from a conditionally inducible TET-ON stable cell line treated with etoposide (Fig 2D). Strap accounted for the main polypeptide purified from doxycycline-induced cells (Fig 2E). Purified Strap was subjected to LC-MS/MS, whereupon S221 was also seen to be the site of phosphorylation (Fig 2F). Thus, Strap is phosphorylated at S221 in cells. Although these results do not exclude the possibility that S276 is phosphorylated, it is most likely to occur at reduced efficiency or under different cellular conditions.
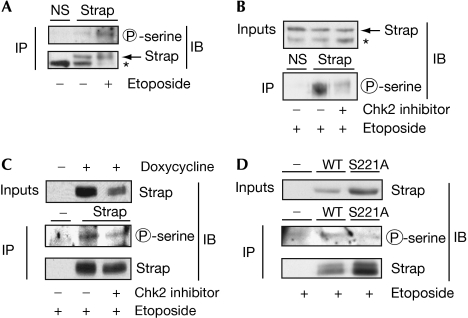
To clarify the relevance of Chk2 to physiological control, we assessed the level of phosphorylation on both endogenous and ectopic Strap. The phosphoserine detected on endogenous Strap increased under DNA damage response conditions (Fig 3A; about sixfold when normalized with the level of Strap), indicating that, as anticipated from previous results (Demonacos et al, 2004), Strap phosphorylation is under DNA damage response control. Furthermore, the phosphorylation of endogenous Strap was dependent on the activity of Chk2 because treating cells with a small molecule Chk2 inhibitor (Sharma & Tepe, 2004) reduced phosphorylation (Fig 3B). Furthermore, the increased level of phosphorylation that occurred on Strap in the inducible cell line (Fig 2E) was dependent on Chk2 activity because, in the presence of the Chk2 inhibitor, phosphorylation also occurred at a reduced level (Fig 3C). It is consistent with these results that, when compared with wild-type Strap, a mutant derivative that lacked the phosphorylation site of Chk2, S221A, showed low levels of phosphorylation (Fig 3D; about fivefold lower than wild-type Strap). Thus, Chk2 kinase phosphorylates Strap in cells.
Figure 3.
Strap phosphorylation in cells. (A) Strap was immunoprecipitated (IP) from U2OS cells using anti-Strap or the nonspecific (NS) control. Strap immunoprecipitates were immunoblotted (IB) using anti-phosphoserine and then reprobed for Strap using the anti-Strap; the asterisk indicates a nonspecific band; n=2. (B) U2OS cells were treated with etoposide (10 μM) and Chk2 inhibitor (15 μM) as indicated. Strap was immunoprecipitated as described above, and immunoblotted with anti-phosphoserine. Input Strap levels are shown; the asterisk indicates a nonspecific band; n=2. (C) Flag-Strap was induced in cells using doxycyline. Cells were treated with etoposide (10 μM) and Chk2 inhibitor (15 μM) as indicated. Strap was immunoprecipitated using Flag-tagged beads and immunoblotted with the anti-phosphoserine. The blot was then reprobed with Flag antibody for Strap levels. Input levels represent 5% of the total used in immunoprecipitation; n=2. (D) U2OS cells were transfected with expression vectors (1 μg) encoding wild-type Strap, S221A or empty vector and treated with etoposide (10 μM for 16 h) before collection. Cell extracts were immunoprecipitated as described above with anti-HA and immunoblotted using anti-phosphoserine and then reprobed for ectopic Strap using anti-HA. Strap was phosphorylated fivefold over S221A; n=2. Chk2, Checkpoint kinase 2; HA, haemagglutinin; Strap, stress responsive activator of p300; WT, wild type.
Chk2 regulates Strap protein stability
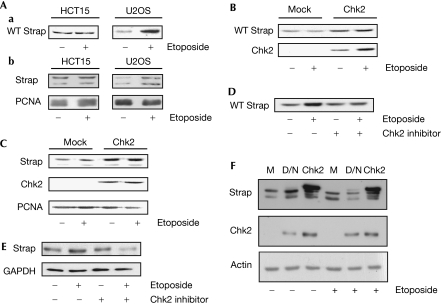
As Chk2 is a nuclear phosphokinase (Matsuoka et al, 1998), it might be responsible for the nuclear stabilization of Strap (Fig 1C). To establish the function of Chk2 in Strap control, we studied Strap stabilization in HCT15 cells, which lack Chk2 kinase activity (Grigorova et al, 2004). In contrast to the DNA damage-dependent stabilization in U2OS cells, both ectopic and endogenous Strap stabilization were compromised in HCT15 cells (Fig 4A). Furthermore, when Chk2 was reinstated in HCT15 cells by expressing ectopic Chk2, both ectopic and endogenous Strap were upregulated (Fig 4B,C). We verified the function of Chk2 in Strap stability control by using the Chk2 inhibitor, which resulted in poor stabilization of Strap under DNA-damaging conditions; both ectopic and endogenous Strap behaved in a similar manner (Fig 4D,E). Finally, although the effect of expressing ectopic wild-type Chk2 was to increase Strap levels, ectopic dominant-negative Chk2 (Chehab et al, 2000) decreased Strap levels under DNA-damaging conditions (Fig 4F). Taken together, these results argue that the phosphorylation of Strap by Chk2 is required for protein stabilization.
Figure 4.
Chk2 kinase phosphorylation controls Strap stabilization. (A) U2OS or HCT15 cells were transfected with Strap expression vector (500 ng). Cells were treated with vehicle or etoposide (+; 10 μM) for 16 h before collection. Cell extracts were loaded on the basis of β-galactosidase activity and Strap was detected using anti-HA (a). (b) The level of endogenous proteins in the same extracts. Strap underwent a fivefold increase in U2OS cells in (a), and a fourfold increase in (b), which was not apparent in HCT15 cells; n=4. (B) HCT15 cells were transfected with the Strap expression vector (500 ng) in the presence or absence of the Flag-Chk2 expression vector (500 ng). Cells were treated with vehicle or etoposide (+; 10 μM) for 16 h before collection. Cell extracts were loaded on the basis of β-galactosidase. Strap was detected using anti-HA and Chk2 with anti-Flag. Strap underwent 1.8-fold increase without etoposide treatment, and 2.3-fold increase under etoposide treatment on Chk2 expression; n=3. (C) HCT15 cells were transfected with the Flag-Chk2 expression vector (500 ng) as indicated. Endogenous Strap was detected using the Strap antibody and anti-Flag M2 to detect Chk2. PCNA was used as a loading control. Strap underwent 3.7-fold increase without etoposide treatment, and 5.6-fold increase with etoposide treatment, on Chk2 expression when normalized to the PCNA loading control; n=3. (D) U2OS cells transfected with HA-Strap WT (500 ng) were treated with etoposide (+; 10 μM) for 16 h in the presence or absence of Chk2 inhibitor (2 μM). Cell extracts were loaded on the basis of β-galactosidase activity and Strap was detected using anti-HA; n=3. (E) U2OS cells were treated with vehicle control or etoposide (+; 10 μM) for 16 h in the presence (for 2 h) or absence of Chk2 inhibitor (2 μM). Cell extracts were probed for endogenous Strap as described. GAPDH was used as a loading control; n=3. (F) U2OS cells were transfected with the Flag-tagged dominant-negative (D/N), Flag-tagged Chk2 or vector alone (M; 500 ng) as indicated and treated with etoposide (+; 10 μM) for 16 h before collection. Extracts were loaded by total protein content. Strap was detected using the anti-Strap, and dominant-negative Chk2 and Chk2 were detected using anti-Flag. β-Actin was used as a loading control; n=4. Chk2, Checkpoint kinase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HA, haemagglutinin; PCNA, proliferating cell nuclear antigen; Strap, stress responsive activator of p300; WT, wild type.
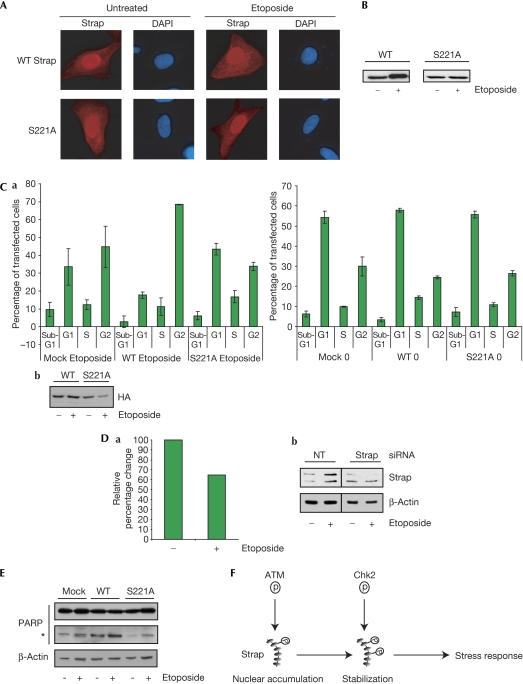
To address the function of Chk2 phosphorylation in Strap, and more generally the function of S221 in regulating the DNA damage response, we studied the properties of the Strap derivative defective in Chk2 phosphorylation, S221A. The intracellular distribution of S221A was very similar to wild-type Strap, being localized mainly to nuclei (Fig 5A); this was the expected result given the integrity of the S203 phosphorylation site and its function in nuclear localization (Fig 1; Demonacos et al, 2004). However, in contrast to wild-type Strap, under DNA-damaging conditions, S221A failed to show any significant increase in stability (Fig 5B). Furthermore, the ability of S221A to prompt cell-cycle arrest in response to DNA damage caused by etoposide treatment (which in U2OS (p53+/+) cells results in G2 arrest; supplementary Fig 1A online) was significantly impaired in comparison to wild-type Strap (Fig 5C; 69% and 33% for wild type and S221A, respectively). By contrast, in SAOS2 (p53−/−) cells, Strap had a negligible effect on the cell-cycle profile (supplementary Fig 1B online). Furthermore, the G2 arrest required for Strap activity was investigated by depleting endogenous Strap in etoposide-treated U2OS cells with siRNA, whereupon a reduced population of G2 cells was evident (∼40% reduction; Fig 5D; supplementary Fig 1C online). The induction of poly(ADP-ribose) polymerase cleavage, which occurs during the DNA damage response (Satoh & Lindahl, 1992), was also compromised in cells expressing S221A compared with wild-type Strap (Fig 5E). Phosphorylation of Strap at S221 is most likely, therefore, to augment the checkpoint response to DNA damage.
Figure 5.
Control of Strap stabilization. (A) U2OS cells were transfected with the indicated Strap expression vector (200 ng). Cells were treated with either vehicle or etoposide (10 μM) for 16 h before fixation and processing for immunofluorescence. Strap was detected using anti-HA and DAPI staining was used to visualize nuclei; n=5. (B) U2OS cells were transfected with the indicated Strap expression vector (WT and S221A; 500 ng) and treated with etoposide (+; 10 μM) for 16 h before collection. Cell extracts were loaded on the basis of β-galactosidase activity and Strap was detected using anti-HA. The fold increase in wild-type Strap levels on DNA damage was about twofold; n=4. (C) (a) U2OS cells were transfected with pBB14 (Us9-GFP; 500 ng) as an internal transfection marker and the appropriate expression vector (500 ng). Cells were treated with or without etoposide (10 μM for 16 h) as indicated and then collected for flow cytometry analysis as described. The graph represents cell-cycle profiles of transfected cells only. (b) Immunoblot from the cells used in the FACS profiles shown in (a). Cells were loaded on the basis of β-galactosidase activity and Strap was detected using anti-HA HA11. (D) (a) U2OS cells were transfected with Strap small interfering RNA (siRNA) or control non-targeting (NT) siRNA (25 nM) for 48 h and then treated with etoposide (+; 10 μM) for 16 h. Cells were then collected for flow cytometry analysis as described. The graph represents the percentage change in G2 of Strap siRNA relative to the control NT siRNA-treated cells. (b) Immunoblot from the cells used in the FACS profiles shown in (a). Extracts were loaded on total protein content. Strap was identified by immunoblotting and β-actin was used as a loading control. (E) U2OS cells were transfected with the indicated Strap expression vectors (WT and S221A) and vector alone (500 ng), and treated with etoposide (+; 10 μM) for 16 h before collection. Extracts were loaded on total protein content. PARP was identified by immunoblotting and β-actin was used as a loading control. The asterisk indicates the cleaved PARP polypeptide; n=3. (F) Model for the dual regulation of Strap by ATM and Chk2: phosphorylation of ATM results in increased nuclear presence of Strap, whereas subsequent phosphorylation by Chk2 augments Strap stabilization. ATM, ataxia telangiectasia mutated; Chk2, Checkpoint kinase 2; DAPI, 4,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; HA, haemagglutinin; PARP, poly(ADP-ribose) polymerase; Strap, stress responsive activator of p300; WT, wild type.
Discussion
Phosphorylation control of Strap
By analysing the properties of a panel of mutants and the effect of leptomycin B, it seems that Strap undergoes nuclear accumulation by virtue of a mechanism that influences nuclear export. This idea is supported by the cytoplasmic location of the S203A mutant that became nuclear when combined with ΔNES or expressed in the presence of leptomycin B, and the nuclear localization of S203A/ΔNES coincided with DNA damage-responsive protein levels. These results are consistent with a model in which the ATM phosphorylation influences nuclear export, thereby favouring nuclear accumulation (Fig 5F).
Regulated nuclear export of effector proteins targeted by the DNA damage response pathway is becoming increasingly recognized as an important mechanism of control. The activity of NES in Mdm2 is involved in exporting p53 to the cytoplasm (Freedman & Levine, 1998). In p53, two NES motifs have been described: one in the N-terminal region and the other in the oligomerization domain (Stommel et al, 1999). The N-terminal NES is regulated through DNA damage-dependent phosphorylation, which assists p53 nuclear accumulation by hindering nuclear export (Zhang & Xiong, 2001). Strap shows several similarities to the control of p53 because DNA damage-dependent phosphorylation by ATM kinase seems, as for p53, to hinder nuclear export.
The interplay between ATM and Chk2 kinase
Our studies highlight the different functions of ATM and Chk2 in controlling cofactor activity during the response of p53, and suggest that Strap activity is influenced through a two-step sequential phosphorylation mechanism (Fig 5F). The dependence of nuclear location on ATM phosphorylation, as well as stabilization of protein on the phosphorylation of Chk2 (which requires Strap nuclear localization), ensures that Strap is activated only once the DNA damage response is underway, when both crucial DNA damage-responsive protein kinases, ATM and Chk2, have been activated. It might also provide a mechanism through which the response of p53 can be fine-tuned during the DNA damage response; limiting the nuclear accumulation of Strap will, in turn, affect the level of p53 activity.
Methods
Plasmids and reagents. The Strap mutant derivative S221A was created using oligonucleotides designed in accordance with Stratagene's QuikChange® Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) using the following primer: S221A: GTGGACAGGAAAGCAGCTAGCAACCCTGACCTTCATCTC. The Strap mutant derivatives ΔNES and S203A/ΔNES were created by deleting the appropriate region from wild-type or S203A Strap in accordance with Stratagene's QuikChange Site-Directed Mutagenesis kit using the following primers: ΔNES: forward: GAAGCGAAGCACGTCTACTGTTTTCGAGAC; reverse: GTCTCGAAAACAGTAGACGTGCTTCGCTTC. The resulting constructs were all verified by sequencing. The Strap siRNA sequence was CAGAGAAAGUUGACAGAAAUU custom made from Dharmacon (Chicago, IL, USA). Leptomycin B was supplied by Sigma (Dorset, UK) and the Chk2 inhibitor (Sharma & Tepe, 2004) was from Merck (Nottingham, UK).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Fig 1
Supplementary Information
Acknowledgments
We thank the Medical Research Council, Cancer Research UK, Leukaemia Research Fund, Association of International Cancer Research and the European Union for supporting this study, and Rosemary Williams for help to prepare this paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn J, Urist M, Prives C (2004) The Chk2 protein kinase. DNA Repair 3: 1039–1047 [DOI] [PubMed] [Google Scholar]
- Bartek J, Falck J, Lukas J (2001) CHK2 kinase—a busy messenger. Nat Rev Mol Cell Biol 2: 877–886 [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD (2000) Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev 14: 278–288 [PMC free article] [PubMed] [Google Scholar]
- D'Andrea LD, Regan L (2003) TPR proteins: the versatile helix. Trends Biochem Sci 28: 655–662 [DOI] [PubMed] [Google Scholar]
- Demonacos C, Krstic-Demonacos M, La Thangue NB (2001) A TPR motif cofactor contributes to p300 activity in the p53 response. Mol Cell 8: 71–84 [DOI] [PubMed] [Google Scholar]
- Demonacos C, Krstic-Demonacos M, Smith L, Xu D, O'Connor DP, Jansson M, La Thangue NB (2004) A new effector pathway links ATM kinase with the DNA damage response. Nat Cell Biol 6: 968–976 [DOI] [PubMed] [Google Scholar]
- Freedman DA, Levine AJ (1998) Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol 18: 7288–7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Staines JM, Ozdag H, Caldas C, Edwards PA (2004) Possible causes of chromosome instability: comparison of chromosomal abnormalities in cancer cell lines with mutations in BRCA1, BRCA2, CHK2 and BUB1. Cytogenet Genome Res 104: 333–340 [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW (2000) DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282: 1893–1897 [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B, Bachelerie F, Dargemont C (1997) Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278: 141–144 [DOI] [PubMed] [Google Scholar]
- Satoh MS, Lindahl T (1992) Role of poly(ADP-ribose) formation in DNA repair. Nature 356: 356–358 [DOI] [PubMed] [Google Scholar]
- Sharma V, Tepe JJ (2004) Potent inhibition of checkpoint kinase activity by a hymenialdisine-derived indoloazepine. Bioorg Med Chem Lett 16: 4319–4321 [DOI] [PubMed] [Google Scholar]
- Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM (1999) A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J 18: 1660–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y (2001) A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292: 1910–1915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1
Supplementary Information