Abstract
Integrin-mediated cell–ECM (extracellular matrix) adhesion is a fundamental process that controls cell behaviour. For correct cell–ECM adhesion, both the ligand-binding affinity and the spatial organization of integrins must be precisely controlled; how integrins are regulated, however, is not completely understood. Kindlins constitute a family of evolutionarily conserved cytoplasmic components of cell–ECM adhesions that bind to β-integrin cytoplasmic tails directly and cooperate with talin in integrin activation. In addition, kindlins interact with many components of cell–ECM adhesions—such as migfilin and integrin-linked kinase—to promote cytoskeletal reorganization. Loss of kindlins causes severe defects in integrin signalling, cell–ECM adhesion and cytoskeletal organization, resulting in early embryonic lethality (kindlin-2), postnatal lethality (kindlin-3) and Kindler syndrome (kindlin-1). It is therefore clear that kindlins, together with several other integrin-proximal proteins, are essential for integrin signalling and cell–ECM adhesion regulation.
Keywords: cell adhesion, extracellular matrix, integrin, kindlin
Glossary
CH-ILKBP calponin homology domain-containing integrin-linked kinase-binding protein
CHO Chinese hamster ovary
FERM band 4.1, ezrin, radixin and moesin
FERMT1 fermitin family homolog 1
ILK integrin-linked kinase
MIG-2 mitogen-inducible gene 2
PAT paralysed, arrested elongation at twofold
PH pleckstrin homology
PINCH particularly interesting new Cys–His protein
PTB phosphotyrosine binding
Talin-H N-terminal head of talin
TGF-β1 transforming growth factor-β1
UNC-112 uncoordinated protein 112
URP2 UNC-112 related protein 2
VASP vasodilator-stimulated phosphoprotein
Introduction
Cell–ECM (extracellular matrix) adhesion is essential for embryonic development, and for the maintenance of tissue integrity and function in adults. At the molecular level, cell–ECM adhesion is mediated primarily by integrins, which constitute a family of α/β heterodimeric transmembrane receptors (Hynes, 2002). Integrin-mediated cell–ECM adhesion can be regulated through two distinct mechanisms: integrin activation and cytoskeletal reorganization. The cytoplasmic tails of integrins are essential for their regulation and bind to several proteins. Talin—a protein that contains an amino-terminal FERM domain—was the first protein found to bind integrin cytoplasmic tails and is perhaps the best characterized (reviewed by Critchley, 2005; Critchley & Gingras, 2008). The FERM domain, which consists of three modules (F1, F2 and F3 subdomains) that form a clover-shaped structure (Pearson et al, 2000), was initially identified in band 4.1, and was subsequently found in many cytoskeletal and signalling proteins that associate with the plasma membrane. A strong and substantial body of evidence demonstrates that talin is essential for integrin regulation (Calderwood et al, 1999, 2002; Garcia-Alvarez et al, 2003; Nieswandt et al, 2007; Petrich et al, 2007a,b; Qin et al, 2004; Tadokoro et al, 2003; Vinogradova et al, 2002; Wegener et al, 2007). Nonetheless, substantial evidence from theoretical considerations (Iber & Campbell, 2006), mutational data (Ma et al, 2006) and human in vivo data (Chen et al, 1992, 1994) suggest that other crucial integrin regulators also exist. This hypothesis has been borne out by recent studies that demonstrate that kindlins are essential regulators of integrin activation and cytoskeletal reorganization.
Domain structure and molecular activities of kindlins
Kindlins constitute a family of evolutionarily conserved and structurally related multidomain proteins. Despite being encoded by different genes, the three mammalian kindlins—kindlin-1 (also known as kindlerin and FERMT1), kindlin-2 (also known as MIG-2) and kindlin-3 (also known as URP2)—exhibit identical domain architecture (Fig 1A) and high sequence similarities. Human kindlin-2 and kindlin-3 are approximately 65% and approximately 59% identical to human kindlin-1 at the amino-acid sequence level, respectively, and UNC-112—the Caenorhabditis elegans orthologue of kindlins—is approximately 41% identical to kindlin-2 at the amino-acid sequence level (Rogalski et al, 2000). A crucial structural feature of kindlins is that they contain a FERM domain (Fig 1A). Unlike talin and most other FERM-domain-containing proteins, which have FERM domains in the N-terminal region, the kindlin FERM domains are located at the carboxyl terminus. In addition, the kindlin FERM domains are split in the F2 subdomain by the insertion of a PH domain (Fig 1A). Among all FERM proteins, the kindlin FERM domains exhibit the highest sequence similarity with that of talin (>50%). Structural modelling suggests that the F3 subdomains of kindlin-1 and kindlin-2 fold into a canonical FERM PTB fold resembling that of talin (Fig 1B; Kloeker et al, 2004; Shi et al, 2007).
Figure 1.
Kindlin domain structure and binding partners. (A) Domain architecture of kindlins and talin. All members of the kindlin protein family show identical domain architecture (top). Arrows indicate the regions of kindlins that interact with β1-integrin and β3-integrin, ILK or migfilin. The interactions with ILK and migfilin are based mainly on the studies of kindlin-2 and UNC-112 (see text). (B) Overlay of the kindlin-2 F3 subdomain model structure (blue) and the talin F3 subdomain structure (green) in complex with the β1-integrin peptide (red) showing a conserved PTB fold. Panel reproduced with permission from Shi et al, 2007. ILK, integrin-linked kinase; PTB, phosphotyrosine binding; UNC-112, uncoordinated protein 112.
Kindlins do not contain catalytic domains and therefore their primary function is to mediate protein interactions. So far, four kindlin-binding proteins have been identified, all of which are components of cell–ECM adhesions: ILK (Mackinnon et al, 2002; Tu et al, 2003; Montanez et al, 2008), migfilin (Tu et al, 2003; Wu, 2005), and β1-integrin and β3-integrin (Kloeker et al, 2004; Shi et al, 2007; Montanez et al, 2008; Moser et al, 2008; Fig 1A). Consistent with the structural similarity between the talin and kindlin F3 subdomains (Fig 1B), binding studies using recombinant proteins and mutational analyses have shown that the F3 subdomains of kindlin-1, kindlin-2 and kindlin-3 can also interact directly with the β1-integrin and β3-integrin cytoplasmic tails (Kloeker et al, 2004; Shi et al, 2007; Montanez et al, 2008; Moser et al, 2008). Both kindlin-2 and UNC-112 can bind to ILK (Mackinnon et al, 2002; Tu et al, 2003; Montanez et al, 2008), although whether kindlin-1 and kindlin-3 can also interact with it remains to be determined. Kindlin-1 and kindlin-2 can also bind to migfilin (Tu et al, 2003; Wu, 2005; Lai-Cheong et al, 2008), but whether kindlin-3 can do so is still unknown.
Although some kindlins can probably localize to other subcellular sites—where they could interact with yet-to-be identified proteins—they are highly concentrated at sites of cell–ECM adhesion (Fig 2), where they mediate their primary function. A fraction of kindlin-1 is phosphorylated in keratinocytes (Herz et al, 2006); casein kinase-2—a serine–threonine kinase that is involved in cytoskeleton regulation—is at least partly responsible for this modification, as its inhibition leads to a reduction in kindlin-1 phosphorylation. These results suggest that kindlins could potentially act as targets of intracellular kinase signalling. It will therefore be interesting to characterize the sites and the precise function of kindlin phosphorylation in order to understand more thoroughly the processes that are regulated by these proteins.
Figure 2.
Subcellular localization of kindlin-1 and kindlin-2. (A) Human HaCaT keratinocytes stained with monoclonal antibodies that recognize kindlin-1. (B) The cells were dual-stained with rhodamine-phalloidin (red) and anti-kindlin-1 (green). Note that kindlin-1 was concentrated at focal adhesions where actin stress fibres were anchored (arrows). (C) Human HaCaT keratinocytes stained with monoclonal antibodies against kindlin-2. Images kindly provided by G. Owen.
Kindlins are crucial components of cell–ECM adhesions
Several lines of evidence have led to the identification of kindlins as crucial cell–ECM adhesion proteins. First, the characterization of UNC-112 in C. elegans shows that it localizes to integrin-rich muscle-attachment structures (Rogalski et al, 2000). Furthermore, the null mutation of UNC-112 results in an embryonic-lethal PAT phenotype resembling that induced by the lack of several other crucial focal-adhesion components, including PAT-2/α-integrin, PAT-3/β-integrin, PAT-4/ILK, PAT-6/actopaxin/CH-ILKBP/parvin and UNC-97/PINCH (Rogalski et al, 2000). UNC-112 interacts with PAT-4/ILK (Mackinnon et al, 2002), and its loss alters PAT-3/β-integrin spatial organization (Rogalski et al, 2000) and PAT-4/ILK (Mackinnon et al, 2002).
The second line of evidence comes from the characterization of kindlin-2 (Tu et al, 2003). Kindlin-2 localizes to cell–ECM-adhesion sites, and interacts directly with the focal-adhesion proteins ILK and migfilin; depletion of kindlin-2 or migfilin impairs cell spreading (Tu et al, 2003). These and other studies suggest that kindlin-2 and migfilin constitute an important connection between cell–ECM adhesions and the actin cytoskeleton (Wu, 2005; Zhang et al, 2006).
The third line of evidence comes from the studies on kindlin-1. The loss of kindlin-1 causes Kindler syndrome (Jobard et al, 2003; Siegel et al, 2003), which is a rare autosomal recessive skin-fragility disorder (Kindler, 1954). This finding is consistent with a role for kindlin-1 in cell–ECM adhesion, as skin-fragility disorders are frequently a consequence of compromised ECM adhesion of keratinocytes. Indeed, molecular analyses have shown that kindlin-1 localizes to focal adhesions in cultured keratinocytes (Kloeker et al, 2004; Siegel et al, 2003). Furthermore, it interacts directly with β1-integrin and β3-integrin cytoplasmic tails, and is required for normal keratinocyte spreading in culture (Kloeker et al, 2004).
Kindlins in cell–ECM adhesion assembly
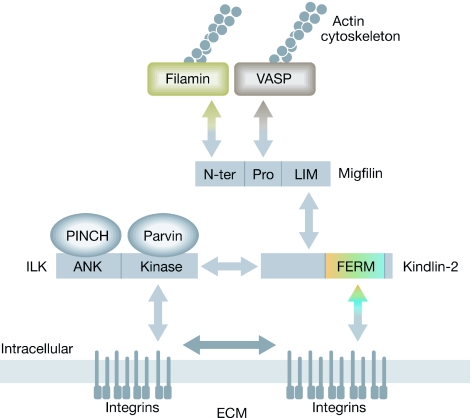
Kindlins are required for the correct assembly of integrin-rich cell–ECM adhesions. Kindlin-2 is localized to cell–ECM adhesions by its direct interaction with the cytoplasmic tails of β-integrin (Shi et al, 2007). In turn, kindlin-2 binds to migfilin and recruits it to these sites (Tu et al, 2003). The presence of migfilin in cell–ECM adhesions facilitates the accumulation of other migfilin-binding proteins such as VASP and filamin (Zhang et al, 2006). Therefore, kindlin-2 is immediately downstream of integrins in the hierarchy of cell–ECM-adhesion assembly (Fig 3). The localization of ILK to cell–ECM adhesions also requires kindlin-2 (Mackinnon et al, 2002; Montanez et al, 2008); however, unlike migfilin, which is not essential for kindlin-2 localization to cell–ECM adhesions (Tu et al, 2003), ILK is essential (Mackinnon et al, 2002). Therefore, the assembly of kindlin-2 and ILK into cell–ECM adhesions seems to be interdependent.
Figure 3.
Assembly of kindlin-2-containing cell–ECM adhesions. Model for the assembly of kindlin-2-containing cell–ECM adhesions, which occurs through multiple protein–protein interactions (double arrows). ILK is recruited to cell–ECM adhesions as part of a ternary protein complex (the PINCH–ILK–Parvin complex; Wu, 2004). The interaction between kindlin-2 and ILK (open double arrows) facilitates their localization to cell–ECM adhesions, where they contribute to integrin clustering (filled double arrow) and cytoskeletal organization. ECM, extracellular matrix; ILK, integrin-linked kinase; PINCH, particularly interesting new Cys–His protein.
The functional importance of kindlins in mediating reorganization of the cytoskeleton at cell–ECM adhesions was shown in mammalian cells. Expression of kindlin-2 in kindlin-2-deficient colon cancer cells was shown to promote focal-adhesion formation and to enhance cell–ECM adhesion through a mechanism dependent on cytoskeletal reorganization (Shi et al, 2007).
Kindlins in integrin activation
In addition to promoting cytoskeletal reorganization, kindlins act as crucial co-activators in integrin activation. Various structural (Vinogradova et al, 2002; Wegener et al, 2007), cellular (Kim et al, 2003), mutational (Han et al, 2006; Hughes et al, 1996) and biophysical (Haas & Plow, 1996) approaches have indicated that the cytoplasmic tails of α-integrin and β-integrin interact with one another to form a weak clasp. This clasp maintains the integrin in a resting state, such that the extracellular domains have low affinity for ligands, and its disruption triggers integrin activation, leading to a substantial increase in ligand affinity.
A function of kindlins in integrin activation was first observed in a study using CHO cells that express αIIbβ3-integrin (Shi et al, 2007). Kindlin-2 was shown to interact directly with the β3-integrin cytoplasmic tail, and its overexpression was shown to induce a modest activation of the αIIbβ3-integrin. Structural modelling identified Gln 614 and Trp 615, within the kindlin-2 PTB domain, as crucial residues at the integrin-binding interface. Substitution of these residues with two alanines abrogated integrin-binding and concomitantly abolished the ability of kindlin-2 to activate integrin (Shi et al, 2007). Therefore, kindlin-2 seems to regulate integrin activation through, at least in part, direct interaction with the integrin. These results provided the initial evidence for a role of kindlin binding in integrin activation.
The second breakthrough came when it was found that co-transfection with talin-H and kindlin-2 resulted in substantially greater integrin activation than that obtained with talin-H or kindlin-2 alone (Ma et al, 2008). Mutation of Gln 614 and Trp 615 on kindlin-2 abolished the direct interaction with integrin (Shi et al, 2007; Montanez et al, 2008) and resulted in a complete loss of co-activator activity (Ma et al, 2008), indicating that the capacity of kindlin-2 to activate αIIbβ3-integrin together with talin-H depends on its direct binding to residues in the C-terminal region of the β3 cytoplasmic tail. Despite the homology between the PTB domains of kindlins and talin (Fig 1B), they have been shown to bind to two distinct sites of the β3 cytoplasmic tail. The NPXY motif in the mid-segment of the β3 cytoplasmic tail—which includes Tyr 747 and is crucial for talin binding—is not required for kindlin-2 binding (Shi et al, 2007), whereas a proline substitution at Ser 752 impaired kindlin-2 binding (Ma et al, 2008; Shi et al, 2007). This substitution is found in a variant of Glanzmann thrombasthenia—a congenital bleeding disorder caused by a lack of functional αIIbβ3-integrin in platelets—which is known to suppress αIIbβ3-integrin activation without affecting talin binding, thereby emphasizing the importance of kindlins for correct integrin functionality.
The third breakthrough came from studies in kindlin-knockout mice and human primary cells. Moser and colleagues characterized platelet function in kindlin-3 knockout mice (Moser et al, 2008); platelet aggregation—a response dependent on the activation of αIIbβ3-integrin—was absent and platelet adhesion to immobilized ligands of the integrin was blunted. These mice also failed to form a thrombus in vivo. The same residues that were involved in kindlin-2 binding to the β3-integrin cytoplasmic domain were implicated in the binding of kindlin-3 (Moser et al, 2008). Therefore, kindlin-2 and kindlin-3 both have co-activator activity, and can probably cooperate with talin-H in a similar manner. In studies using human primary cells (Ma et al, 2008), knockdown of kindlin-2 markedly altered endothelial cell adhesion to fibrinogen and vitronectin. In addition, impairments in β1-integrin activation, basement-membrane organization and cell–ECM adhesion were observed in embryoid bodies derived from kindlin-2-null embryonic stem cells (Montanez et al, 2008). Taken together, these studies demonstrate a crucial role of kindlins in integrin regulation. The phenotypes observed in the absence of kindlins probably arise, at least in part, from a loss of the integrin co-activator activity of the kindlins.
Kindlins in development and disease
Kindlin-1 and kindlin-2 are widely expressed in murine and human tissues, including skin, heart, lung, liver, kidney, colon, prostate, ovary and pancreas (Siegel et al, 2003; Ussar et al, 2006); the expression patterns of kindlin-1 and kindlin-2, however, are not identical. For example, kindlin-1 is strongly expressed in the epidermis and only weakly expressed in the dermis, whereas the level of kindlin-2 in the dermis is higher than that in the epidermis (Ussar et al, 2006). Unlike kindlin-1 and kindlin-2, the expression of kindlin-3 is restricted to haematopoietic tissues, where it is the dominant form of kindlin expressed, although relatively low expression levels of kindlin-1 and kindlin-2 have also been detected (Ussar et al, 2006). These differential expression patterns might explain, at least in part, the distinctive phenotypes that result from the loss of different kindlins, which are discussed below.
In C. elegans, UNC-112 is essential for embryonic development (Rogalski et al, 2000). The phenotypes resulting from the loss of individual kindlins have also been characterized in vertebrates. Loss of kindlin-2 in mice results in peri-implantation embryonic lethality (Dowling et al, 2008; Montanez et al, 2008) and knockdown of kindlin-2 in zebrafish reveals a strong dependence of cardiac development and function on kindlin-2 (Dowling et al, 2008). Consistent with the restricted expression of kindlin-3 in haematopoietic tissues, mice lacking kindlin-3 show severe osteopetrosis, haemorrhage and defects in the erythrocyte membrane skeleton, and die within one week of birth (Kruger et al, 2008; Moser et al, 2008).
Loss of kindlin-1 in humans causes Kindler syndrome (Jobard et al, 2003; Siegel et al, 2003). The characteristic clinical features of this disease include congenital skin blistering and poikiloderma with extensive atrophy (Fig 4; Kindler, 1954). Most reported Kindler syndrome patients also have oral symptoms such as restricted mouth opening, gingivitis, gingival atrophy and aggressive periodontal disease (Wiebe et al, 2008). Kindlin-1 deficiency does not seem to affect many tissues beyond the skin and oral mucosa, with the exception of the colon (Kern et al, 2007). Unlike in the skin, two forms of kindlin-1—arising from alternative splicing—can be detected in the colon (Siegel et al, 2003). However, the histological manifestations of colon blisters in Kindler syndrome are similar to those of the skin and oral mucosa, presenting epithelial detachment from the underlying tissue.
Figure 4.
Clinical manifestations of skin and oral mucosal lesions in Kindler syndrome. (A) A 3-year-old male Kindler syndrome patient with healing ulcers (arrows). (B, C) A 13-year-old male Kindler syndrome patient with acute skin blister (B, arrow) and oral mucosal lesions (C, arrows indicate red, swollen and spontaneously bleeding gingiva). Photographs courtesy of C. Wiebe.
The most characteristic histological abnormality found in the skin and oral mucosa of Kindler syndrome patients is the reduplication of the lamina densa (Shimizu et al, 1997; Wiebe & Larjava, 1999). Breaks in the basement membrane are often also present, but the localization of most basement membrane proteins is normal (Wiebe & Larjava, 1999). Another notable histological feature is an abnormal deposition of type VII collagen (Wiebe & Larjava, 1999). Kindlin-1-deficient keratinocytes show reduced cell adhesion, migration and proliferation, and increased apoptosis (Herz et al, 2006; Kloeker et al, 2004); the periodontal disease phenotype is associated with a reduced ability of the epithelial cells to attach and seal the gingiva to the tooth (Wiebe et al, 2008). Given the crucial roles of integrins in cell–ECM adhesion, proliferation, survival, cytoskeleton organization and ECM deposition, many of the defects observed in the absence of kindlins could arise from abnormal integrin regulation.
Although the loss of kindlins is often deleterious, an aberrantly high level of kindlin-1 might also be harmful. Kindlin-1, but not kindlin-2, is overexpressed in colorectal and lung tumours (Weinstein et al, 2003). The expression of kindlin-1 is upregulated by TGF-β1 (Kloeker et al, 2004), which is known to increase the motility of tumour cells. This raises the interesting possibility that kindlin-1 overexpression mediates the increase in motility, thereby contributing to tumour invasion and cancer progression. By contrast, overexpression of kindlin-2 suppresses mesenchymal cancer cell invasion (Shi & Wu, 2008). Therefore, although kindlin-1 and kindlin-2 share common activities, their functions might not be entirely redundant.
Conclusions and future directions
Although kindlins have been studied for only a few years, they have been firmly established as an important family of integrin regulators. Two mechanisms whereby kindlins regulate integrins have emerged. First, kindlins bind to the β-integrin cytoplasmic domains, cooperate with talin and consequently activate integrins. Second, kindlins interact not only with integrins, but also with other components of cell–ECM adhesions—such as migfilin and ILK—thereby regulating cytoskeletal reorganization and strengthening cell–ECM adhesion. These findings put kindlins, together with several other integrin-proximal proteins, at the centre stage of integrin signalling and cell–ECM adhesion regulation. There are currently several unanswered questions regarding kindlin function and regulation (Sidebar A). Addressing these questions will not only provide new mechanistic insights into integrin regulation, but also could lead to a better understanding of the molecular basis underlying the pathogenesis and progression of several human diseases, including cancer and cardiovascular disease.
Sidebar A | In need of answers.
How do kindlins cooperate with talin to elicit integrin activation?
What other proteins interact with kindlins besides integrin, migfilin and integrin-linked kinase?
Are there unique activities associated with different members of the kindlin family?
What are the functions of the splice variants of kindlins?
Do kindlins function outside of cell–extracellular matrix adhesion?
How are the expression and activities of kindlins regulated?
Can kindlins act as targets for therapeutic intervention of human diseases such as cancer and cardiovascular diseases?

Hannu Larjava

Edward F. Plow

Chuanyue Wu
Acknowledgments
We thank C. Wiebe for providing the images of patients with Kindler syndrome and G. Owen for the immunostaining images. This work was supported by National Institutes of Health grants DE016099 (to H.L.), HL073311 (to E.F.P.), and GM65188 and DK54639 (to C.W.).
References
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH (1999) The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem 274: 28071–28074 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Yan B, de Pereda JM, Alvarez BG, Fujioka Y, Liddington RC, Ginsberg MH (2002) The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem 277: 21749–21758 [DOI] [PubMed] [Google Scholar]
- Chen Y, Djaffar I, Pidard D, Steiner B, Cieutat A, Caen JP, Rosa J (1992) Ser-752→Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci USA 89: 10169–10173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, O'Toole TE, Ylanne J, Rosa JP, Ginsberg MH (1994) A point mutation in the integrin beta 3 cytoplasmic domain (S752→P) impairs bidirectional signaling through alpha IIb beta 3 (platelet glycoprotein IIb-IIIa). Blood 84: 1857–1865 [PubMed] [Google Scholar]
- Critchley DR (2005) Genetic, biochemical and structural approaches to talin function. Biochem Soc Trans 33: 1308–1312 [DOI] [PubMed] [Google Scholar]
- Critchley DR, Gingras AR (2008) Talin at a glance. J Cell Sci 121: 1345–1347 [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Gibbs E, Russell M, Goldman D, Minarcik J, Golden JA, Feldman EL (2008) Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res 102: 423–431 [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC (2003) Structural determinants of integrin recognition by talin. Mol Cell 11: 49–58 [DOI] [PubMed] [Google Scholar]
- Haas TA, Plow EF (1996) The cytoplasmic domain of alpha IIb beta 3. A ternary complex of the integrin alpha and beta subunits and a divalent cation. J Biol Chem 271: 6017–6026 [DOI] [PubMed] [Google Scholar]
- Han J et al. (2006) Reconstructing and deconstructing agonist-induced activation of integrin alpha IIb beta 3. Curr Biol 16: 1796–1806 [DOI] [PubMed] [Google Scholar]
- Herz C, Aumailley M, Schulte C, Schlotzer-Schrehardt U, Bruckner-Tuderman L, Has C (2006) Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem 281: 36082–36090 [DOI] [PubMed] [Google Scholar]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH (1996) Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem 271: 6571–6574 [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Iber D, Campbell ID (2006) Integrin activation—the importance of a positive feedback. Bull Math Biol 68: 945–956 [DOI] [PubMed] [Google Scholar]
- Jobard F, Bouadjar B, Caux F, Hadj-Rabia S, Has C, Matsuda F, Weissenbach J, Lathrop M, Prud'homme JF, Fischer J (2003) Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet 12: 925–935 [DOI] [PubMed] [Google Scholar]
- Kern JS et al. (2007) Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol 213: 462–470 [DOI] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301: 1720–1725 [DOI] [PubMed] [Google Scholar]
- Kindler T (1954) Congenital poikiloderma with traumatic bulla formation and progressive cutaneous atrophy. Br J Dermatol 66: 104–111 [DOI] [PubMed] [Google Scholar]
- Kloeker S, Major MB, Calderwood DA, Ginsberg MH, Jones DA, Beckerle MC (2004) The Kindler syndrome protein is regulated by transforming growth factor-beta and involved in integrin-mediated adhesion. J Biol Chem 279: 6824–6833 [DOI] [PubMed] [Google Scholar]
- Kruger M et al. (2008) SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 134: 353–364 [DOI] [PubMed] [Google Scholar]
- Lai-Cheong JE, Ussar S, Arita K, Hart IR, McGrath JA (2008) Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol 128: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YQ, Yang J, Pesho MM, Vinogradova O, Qin J, Plow EF (2006) Regulation of integrin alpha IIb beta 3 activation by distinct regions of its cytoplasmic tails. Biochem 45: 6656–6662 [DOI] [PubMed] [Google Scholar]
- Ma YQ, Qin J, Wu C, Plow EF (2008) Kindlin-2 (Mig-2): a co-activator of beta 3 integrins. J Cell Biol 181: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 12: 787–797 [DOI] [PubMed] [Google Scholar]
- Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev 22: 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14: 325–330 [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R (2007) Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med 204: 3113–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MA, Reczek D, Bretscher A, Karplus PA (2000) Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101: 259–270 [DOI] [PubMed] [Google Scholar]
- Petrich BG, Fogelstrand P, Partridge AW, Yousefi N, Ablooglu AJ, Shattil SJ, Ginsberg MH (2007a) The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest 117: 2250–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich BG et al. (2007b) Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med 204: 3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Vinogradova O, Plow EF (2004) Integrin bidirectional signaling: a molecular view. PLoS Biol 2: e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG (2000) The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol 150: 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wu C (2008) A suppressive role of mitogen inducible gene-2 in mesenchymal cancer cell invasion. Mol Cancer Res 6: 715–724 [DOI] [PubMed] [Google Scholar]
- Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem 282: 20455–20466 [DOI] [PubMed] [Google Scholar]
- Shimizu H et al. (1997) Immunohistochemical, ultrastructural, and molecular features of Kindler syndrome distinguish it from dystrophic epidermolysis bullosa. Arch Dermatol 133: 1111–1117 [PubMed] [Google Scholar]
- Siegel DH et al. (2003) Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet 73: 174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA (2003) Talin binding to integrin beta tails: a final common step in integrin activation. Science 302: 103–106 [DOI] [PubMed] [Google Scholar]
- Tu Y, Wu S, Shi X, Chen K, Wu C (2003) Migfilin and mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113: 37–47 [DOI] [PubMed] [Google Scholar]
- Ussar S, Wang HV, Linder S, Fassler R, Moser M (2006) The Kindlins: subcellular localization and expression during murine development. Exp Cell Res 312: 3142–3151 [DOI] [PubMed] [Google Scholar]
- Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin J (2002) A structural mechanism of integrin alpha IIb beta 3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110: 587–597 [DOI] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID (2007) Structural basis of integrin activation by talin. Cell 128: 171–182 [DOI] [PubMed] [Google Scholar]
- Weinstein EJ, Bourner M, Head R, Zakeri H, Bauer C, Mazzarella R (2003) URP1: a member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Biochim Biophys Acta 1637: 207–216 [DOI] [PubMed] [Google Scholar]
- Wiebe CB, Larjava HS (1999) Abnormal deposition of type VII collagen in Kindler syndrome. Arch Dermatol Res 291: 6–13 [DOI] [PubMed] [Google Scholar]
- Wiebe CB, Petricca G, Hakkinen L, Jiang G, Wu C, Larjava HS (2008) Kindler syndrome and periodontal disease: review of the literature and a 12-year follow-up case. J Periodontol 79: 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C (2004) The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta (Mol Cell Res) 1692: 55–62 [DOI] [PubMed] [Google Scholar]
- Wu C (2005) Migfilin and its binding partners: from cell biology to human diseases. J Cell Sci 118: 659–664 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tu Y, Gkretsi V, Wu C (2006) Migfilin interacts with vasodilator-stimulated phosphoprotein (VASP) and regulates VASP localization to cell-matrix adhesions and migration. J Biol Chem 281: 12397–12407 [DOI] [PubMed] [Google Scholar]