Abstract
Elevation of proinflammatory cytokines in the brain have potent effects on altering physiological, behavioral, and cognitive processes. The mechanism(s) by which brain cytokines are induced during a peripheral immune challenge remains unclear since microorganisms/cytokines do not cross the blood-brain barrier (BBB). Recent studies indicate that central β-adrenergic receptors (β-ADRs) may mediate brain interleukin-1beta (IL-1) production. This has direct implications for the production of brain cytokines during a peripheral immune response since peripheral pathogens and cytokines rapidly stimulate brainstem catecholamine neurons via peripheral nerves and circumventricular pathways. Studies here examine the role of central β-ADRs in regulating brain cytokine production following peripheral Escherichia coli (E.coli) challenge. Rats were centrally administered propranolol (β-ADR antagonist) or vehicle followed by peripheral E.coli or saline and sacrificed 6h later for measurement of cytokines. Pretreatment with propranolol completely blocked the induction of brain IL-1 following E.coli. Surprisingly, central propranolol also attenuated E.coli-induced peripheral cytokines. To examine whether the attenuated peripheral cytokine response following central propranolol administration was due leakage of propranolol into the general circulation and blockade of peripheral β-blockade, nadolol (β-ADR antagonist that does not cross the BBB) was administered peripherally prior to E.coli. Nadolol administration did not block central cytokine production following E.coli, but instead enhanced both peripheral and central proinflammatory cytokine production. Furthermore, central administration of isoproterenol (β-ADR agonist) results in a time-dependent increase in brain IL-1 production. These data demonstrate central β-ADRs may play a critical role to induce brain IL-1, while peripheral β-ADRs inhibit cytokine response to bacterial challenge.
Keywords: catecholamine, brain, E.coli, norepinephrine, propranolol, IL-1
Introduction
Inflammatory cytokines (i.e. IL-1β, TNF-α, IL-6), particularly IL-1β, are potent stimulators of brain-mediated sickness responses, which includes fever, decrease in food/water intake, decrease in exploration and social interaction, cognitive impairment, and activation of the sympathetic nervous system and hypothalamic-pituitary-adrenal axis (Dantzer, 2001; Kelley et al., 2003). During a peripheral immune challenge increased levels of inflammatory cytokines can be measured in the brain, likely contributing to the initiation of sickness responses (Laye et al., 2000). During a peripheral immune challenge, toll-like receptors (TLRs) expressed on peripheral cells (e.g. immune and endothelial) detect pathogens and initiate peripheral cytokine production, however, microorganisms and cytokines are too large to passively cross the blood-brain barrier (BBB) and directly stimulate cytokine production in the brain. Studies presented here investigate the possibility of alternative signaling pathways involved in the induction of brain proinflammatory cytokines following a peripheral immune challenge.
One potential pathway that has been suggested to be involved in stimulating inflammatory cytokines in the brain is the vagus-to-nucleus of the solitary tract (NTS) pathway. This neural pathway has been well documented to be involved in immune-to-brain communication. Stimulation of this pathway occurs when peripheral cytokine levels increase and bind to cytokine receptors expressed on vagal paraganglia (Goehler et al., 1997). This results in activation of vagal afferent neurons that project to the brainstem (Ek et al., 1998; Kurosawa et al., 1997; Niijima, 1996) and synapse on catecholamine neurons located in the NTS (Gaykema et al., 2007; Sumal et al., 1983). C-Fos mapping studies demonstrate that vagal afferents and neurons within the NTS rapidly become activated following peripheral immune stimuli (e.g. pathogens or cytokine administration) (Brady et al., 1994; Day and Akil, 1996; Elmquist et al., 1996; Ericsson et al., 1994). Subdiaphragmatic vagotomy blocks the induction of brain IL-1β mRNA (Hansen et al., 1998; Laye et al., 1995) and the initiation of fever, decreased social interaction, and attenuates adrenocorticotropic hormone release following peripheral cytokine or endotoxin administration (Bluthe et al., 1994; Gaykema et al., 1995; Hansen et al., 2001). Recent publications demonstrate that β-adrenergic receptors (β-ADRs) mediate the induction of brain IL-1β during stressor exposure, suggesting that brain catecholamines may play an important role in mediating brain cytokine production. In these studies, administration of the β-ADRs antagonist prior to tailshock exposure blocked the stress-induced production of brain IL-1β (Johnson et al., 2005), while administration of the norepinephrine reuptake inhibitor, desipramine, potentiated hypothalamic IL-1β production following footshock exposure (Blandino et al., 2006). Thus, stimulation of brainstem catecholaminergic neurons in the NTS during a peripheral immune challenge may mediate the induction of brain cytokines via activation of central β-ADRs.
Here, we present a series of studies that examined the hypothesis that stimulation of central β-ADRs mediate the induction of brain cytokines (IL-1, IL-6, TNF-α) following a peripheral bacterial challenge. Fisher 344, male rats were centrally injected with propranolol (a β-ADR antagonist) or vehicle prior to a peripheral Escherichia coli (E.coli) or saline challenge, and brain and peripheral cytokines were measured 6 h later. To determine if any of the effects observed following central β-blockade could be explained by leaking of the pharmacological agent to the periphery, additional rats were injected intraperitoneally (i.p.) with nadolol (a β-blocker that does not cross the BBB) or vehicle peripherally prior to challenge with E.coli or saline. Finally, to determine if activation of central β-ADRs alone are sufficient to induce brain IL-1β production, rats were centrally administered isoproterenol (a β-ADRs agonist) or vehicle and sacrificed after 30, 60, or 120 minutes for measurement of cytokines.
Materials and Methods
Subjects
Adult male viral-free Fischer 344 rats (245–300 gms; Harlan, Inc., Indianapolis, IN) were individually housed in Plexiglas cages (60 × 30 × 24 cm) with food and water available ad libidum. Animal colonies were maintained in a pathogen-free barrier facility with 12:12-h light-dark cycle (lights on 0730 h). Rats were given at least 1 wk to habituate to the colony facility before experimentation. Rats were handled for 3–4 days and weighed before each study began. Care and use of the animals was in accordance with protocols approved by the University of Colorado & Kent State University Institutional Animal Care and Use Committee and studies were designed to minimize pain and the number of animals used.
Adrenergic Drugs
All pharmacological agents were purchased from Sigma-Aldrich (St.Louis, MO) and dissolved in sterile, endotoxin-free saline. Propranolol, a β-adrenergic receptor antagonist, was dissolved at a concentration of 10 µg/µl and 100 µg administered per rat into the cisterna magna. Isoproterenol, a β-adrenergic receptor agonist, was dissolved at a concentration of 1 µg/µl and 10 µg administered per rat into the cisterna magna. Nadolol, a β-adrenergic receptor antagonist that does not cross the BBB, was dissolved at a concentration of 7.5 mg/ml and administered intraperitoneally (i.p.) at a dose of 7.5 mg/kg. All doses were chosen based on prior studies in our laboratory (Johnson et al., 2005) or doses reported in the literature (Soszynski et al., 1996).
Intra-cisterna magna Injections
Rats were briefly anesthetized with Isoflurane and a sterile 30 G needle connected with polyethylene tubing (PE-10) to a 50 µl Hamilton syringe was used to administer adrenergic pharmacological agents or vehicle (in a 10 µl volume) directly into the cisterna magna. Injections were made over a 60 sec time period. Animals were anesthetized for approximately 2–3 min. Intracisterna magna (i.c.m.) injections were made to avoid confounds of an inflammatory response induced by surgically implanting indwelling cannulae. Administration of radiolabelled inulin i.c.m. has been shown to spread throughout the brain (Proescholdt et al., 2000), and we have successfully administered pharmacological agents via i.c.m. injections previously to block physiological responses to laboratory stressors (Johnson et al., 2004).
Bacterial Growth
Escherichia coli (E.coli 0111:B4; ATCC 15746, American Type Culture Collection; Bethesda, MD) was re-hydrated and grown overnight in 30 ml of Brain-Heart Infusion (BHI; DIFCO Laboratories, Detroit, MI) at 37° C, 5% CO2. Bacterial cultures were then aliquoted into 1 ml BHI supplemented with 10% glycerol & frozen at −20° C. All experiments used bacteria from these stock cultures. One day prior to experimentation, stock cultures were thawed and cultured overnight in 40 ml of BHI (37° C, 5% CO2). Bacteria were quantified by extrapolating from previously determined growth curves. Cultures were centrifuged (10 min, 3000 rpm), supernatants discarded, and bacteria re-suspended in sterile PBS at a concentration of 1×109 Colony Forming Units (CFU) per ml (except in study 1 where various concentrations of E.coli were tested). Rats were injected i.p. with 250 µl E.coli (2.5×108 CFU).
Blood and Tissue Collection
Rats were sacrificed by decapitation and trunk blood, pituitary, liver, spleen, and brain tissues collected. Trunk blood was collected in 10 ml EDTA-vacutainer tubes (BD PharMingen Franklin Lakes, NJ) and stored on ice until completion of the study. Plasma was collected and stored at −80° C until time of assay. Brains were placed on a frosted glass plate placed on top of crushed ice and free-hand dissections of limbic areas involved in sickness responses (i.e. hypothalamus, hippocampus, prefrontal cortex, and amygdala) were made as previously described (Johnson et al., 2004). All tissues were placed in microfuge tubes and quickly frozen in liquid nitrogen. Tissue samples were stored at −80° C until the time of tissue processing.
Brain Tissue Processing
Each tissue was added to 0.25–1 ml of cold Eagles Minimum Essential Media containing 2% Aprotinin (Sigma, cat# A-6279). Tissue was dissociated using a sonic dismembrator (Fisher Scientific, Model 100). Sonicated samples were centrifuged at 10,000 rpm at 4°C for 10 min. Supernatants were removed and an ELISA was performed. Bradford protein assays were performed to determine total protein concentrations in tissue sonication samples and tissue cytokine content was presented as pg/mg protein to correct for differences in dissection size.
Measurement of Cytokines
Cytokines were measured from plasma and tissue samples using commercially available ELISAs for rat IL-1β, TNF-α, and IL-6 (R & D Systems, Minneapolis, MN). The ELISAs were run according to the manufacturer’s instructions except the standard curves were further diluted so that the lowest standard was 3.9 pg/ml for IL-1β, 6.25 pg/ml for TNF-α, and 15.6 pg/ml for IL-6, and the substrate incubation was lengthened from 30 min to 45 min. Peripheral tissue samples were diluted for measurement of IL-1β. The rat IL-1β kits have a detection limit of <3.9 pg/ml and the TNF-α and IL-6 kit have a detection limit of < 8.0 pg/ml.
Procedure
Study 1: Rats (n=4/group) were weighed immediately prior to i.p. challenge with saline or 2.5×106, 2.5×107, or 2.5×108 CFU E.coli. Rats were weighed daily to calculate changes in body weight post-E.coli challenge. Additional rats (n=4/group) were administered 2.5×108 CFU E.coli and sacrificed 2, 4, 6, or 8 h later for measurement of brain cytokines. Study 2: Rats (n=7-8/group) received either an i.c.m. injection of saline or 100 µg propranolol immediately followed by either an i.p. injection of saline or 2.5×108 CFU E.coli. Six hours later rats were decapitated and blood and tissues collected for measurement of cytokines. Study 3: Rats (n=8-9/group) received either an i.p. injection of saline or 7.5 mg/kg nadolol followed immediately by an i.p. injection of saline or 2.5×108 CFU E.coli. Six hours later rats were decapitated and blood and tissues collected for measurement of cytokines. Study 4: Rats (n=7/group) received an i.c.m. injection of either saline or 10 µg isoproterenol and decapitated 30, 60, or 120 min later for measurement of blood and tissue cytokines. To determine receptor specificity, additional rats (n=4) received an i.c.m. injection of both 10 µg isoproterenol and 100 µg propranolol and decapitated 60 min later for measurement of blood and tissue cytokines.
Statistics
Data from experiments examining the effects of central propranolol or peripheral nadolol on E.coli-induced cytokines were analyzed using a 2 × 2 ANOVA between immune stimuli (Saline vs. E.coli) and drug treatment (Vehicle vs. Drug). Data from the experiment examining the effects of central isoproterenol on cytokine production were analyzed using a 1 × 4 ANOVA across time (0, 30, 60, 120 min). T-test comparisons were made between isoproterenol-injected animals sacrificed 60 min later and animals injected with isoproterenol + propranolol and sacrificed 60 min later. In all cases p < 0.05 was used for the level of confidence for acceptance of significance to exclude the null hypothesis. When necessary post hoc analyses were done using a Fisher’s least significant difference test.
Results
Dose-Response & Time-Course of E.coli-Induced Sickness and Cytokine Response
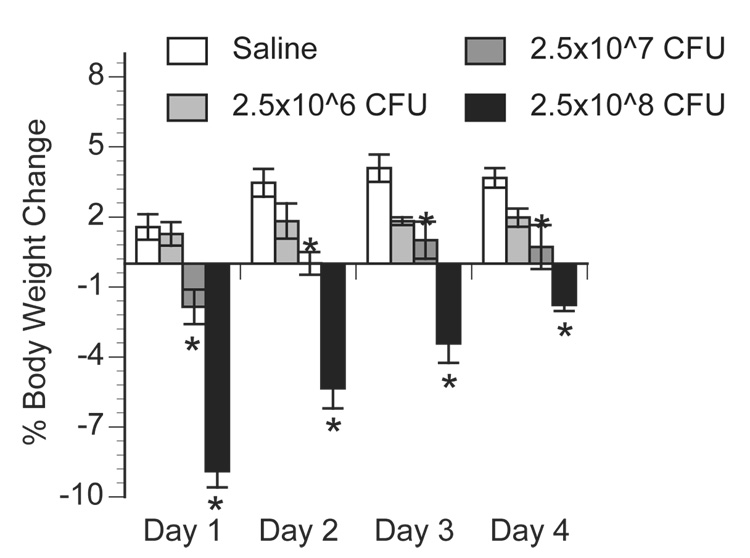
Study 1 aimed to determine a concentration of live E.coli that significantly and consistently elevates peripheral and central cytokines and stimulates sickness responses without resulting in death. Figure 1 presents changes in body weight across time following i.p. administration of saline, 2.5 × 106, 2.5 × 107, or 2.5 × 108 CFU E.coli. Body weight change did not differ between animals injected with saline and those challenged with the lowest concentration of E.coli (2.5×106 CFU), both groups of animals slowly gained weight across time. In contrast, animals challenged with 2.5×107 or 2.5×108 CFU E.coli significantly lost body weight, approximately 2% and 9% respectively, one day following challenge but then gained weight on each day thereafter. A repeated measures ANOVA revealed a significant effect of body weight change across days between groups [F(3, 27) = 13.47; p < 0.0001] and post-hoc analysis indicated no significant difference in body weight change between saline and 2.5×106 CFU E.coli groups (p = 0.088), but a significant difference between saline and 2.5×107 CFU E.coli (p = 0.002) and between saline and 2.5×108 CFU E.coli (p < 0.0001). For all subsequent studies, 2.5×108 CFU E.coli was used because it resulted in robust, acute loss of body weight, an indication of sickness, without resulting in signs of severe sepsis (i.e. organ failure or death).
Figure 1.
Percent body weight change following E.coli challenge. Rats were injected i.p. with saline, 2.5×10^6, 2.5×10^7, or 2.5×10^8 CFU E.coli and body weight recorded daily. Data are presented as a percent change from each individual animals’ body weight recorded immediately prior to injection compared to 24h (Day 1), 48h (Day 2), 72h (Day 3), and 96h (Day 4) after injection. Bars represent group averages +/− SEM (n=4/group). * represents p < 0.05 compared to saline group.
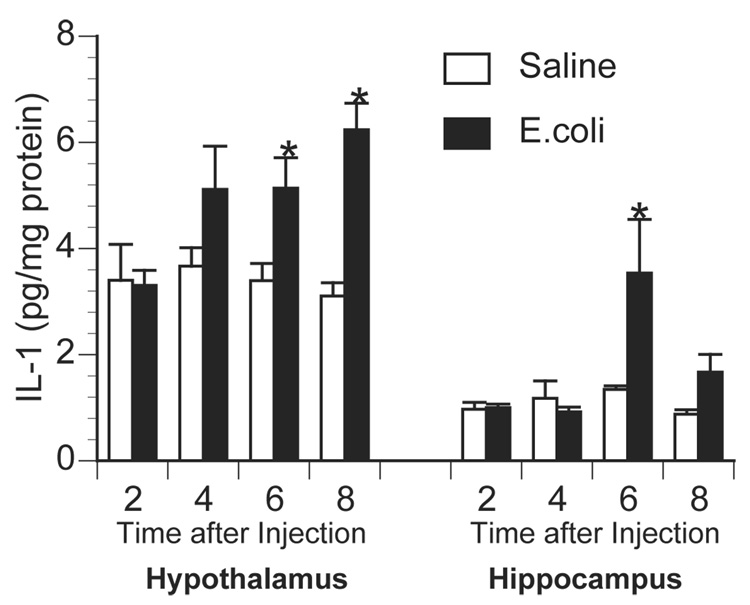
To determine the time course of central proinflammatory cytokine responses following E.coli challenge, animals were injected with saline or 2.5×108 CFU E.coli and sacrificed 2, 4, 6, or 8 h later. Saline-injected animals had extremely low concentrations of basal inflammatory cytokines, while E.coli-challenge resulted in a time-dependent elevation in brain IL-1β (Figure 2). Brain TNF-α and IL-6 protein levels remained near the detection limit of the assay (data not shown).
Figure 2.
Time course of brain IL-1 production following E.coli challenge. Rats were injected i.p. with saline or 2.5×10^8 CFU E.coli and sacrificed 2, 4, 6, or 8 h later for measurement of hypothalamic and hippocampal IL-1 by ELISA. Bars represent group averages +/− SEM (n=4/group). * represents p < 0.05 compared to saline-injected controls.
Effect of Central β-Adrenoceptor Blockade on E.coli-Induced Cytokines
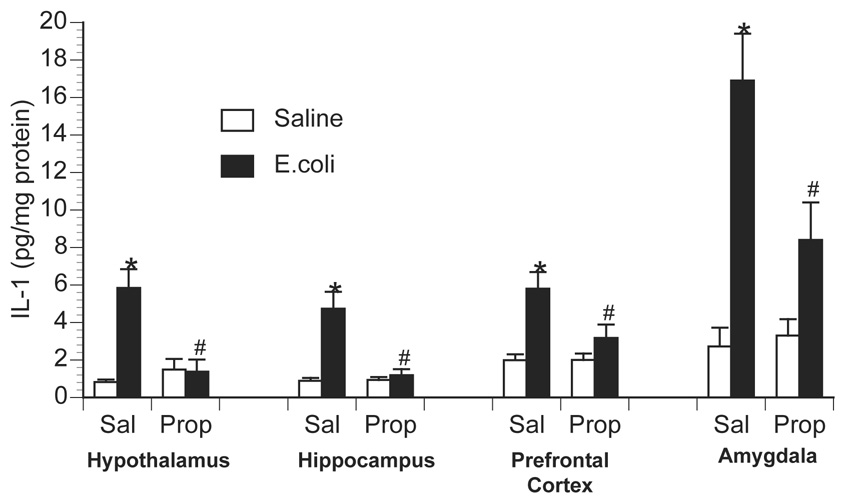
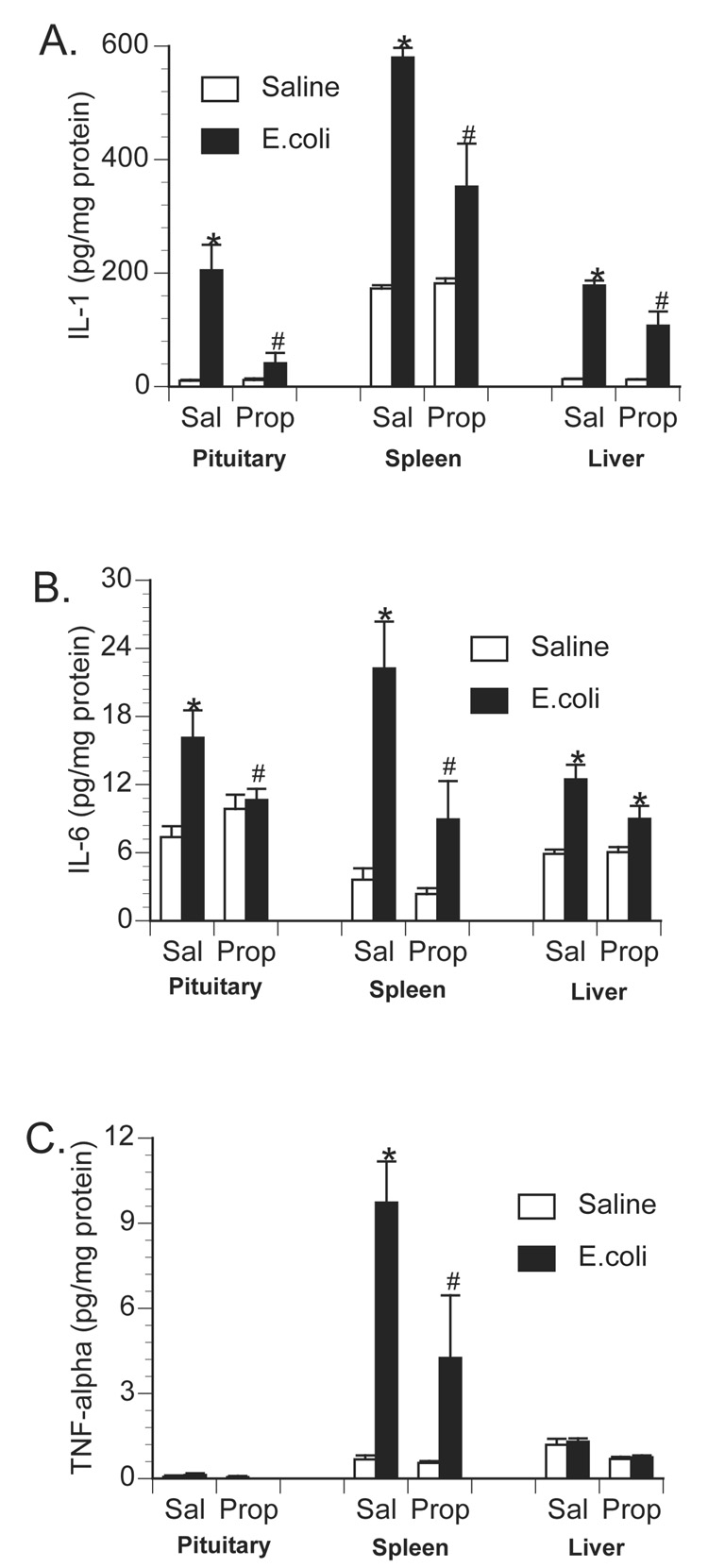
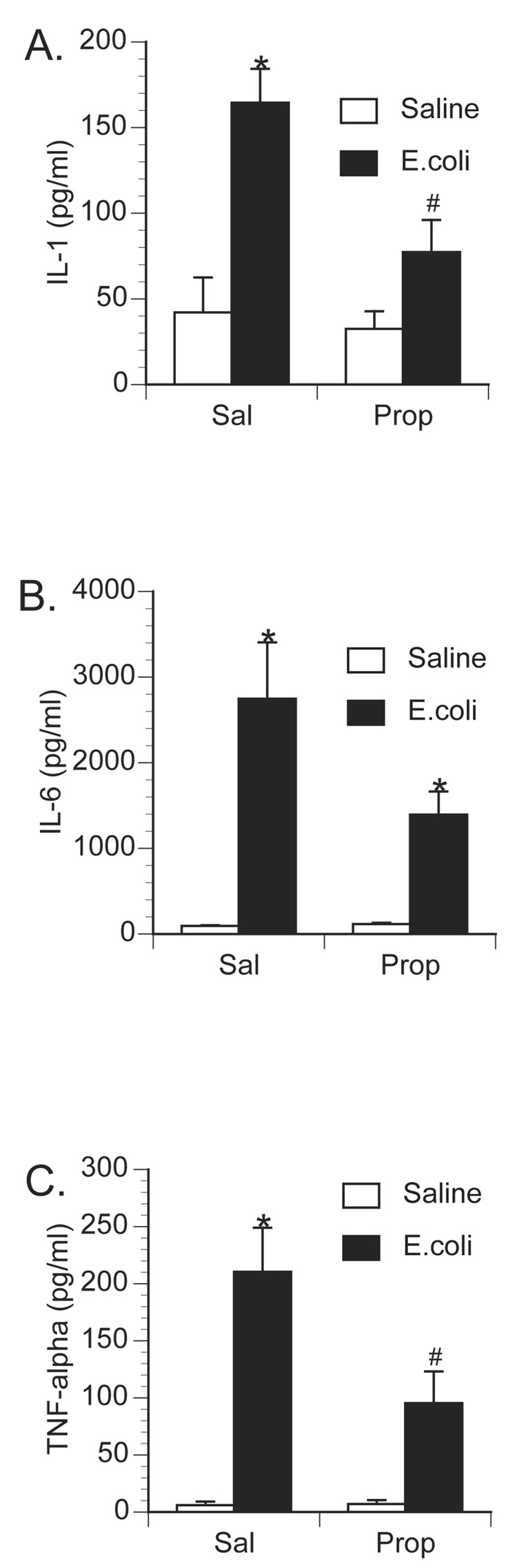
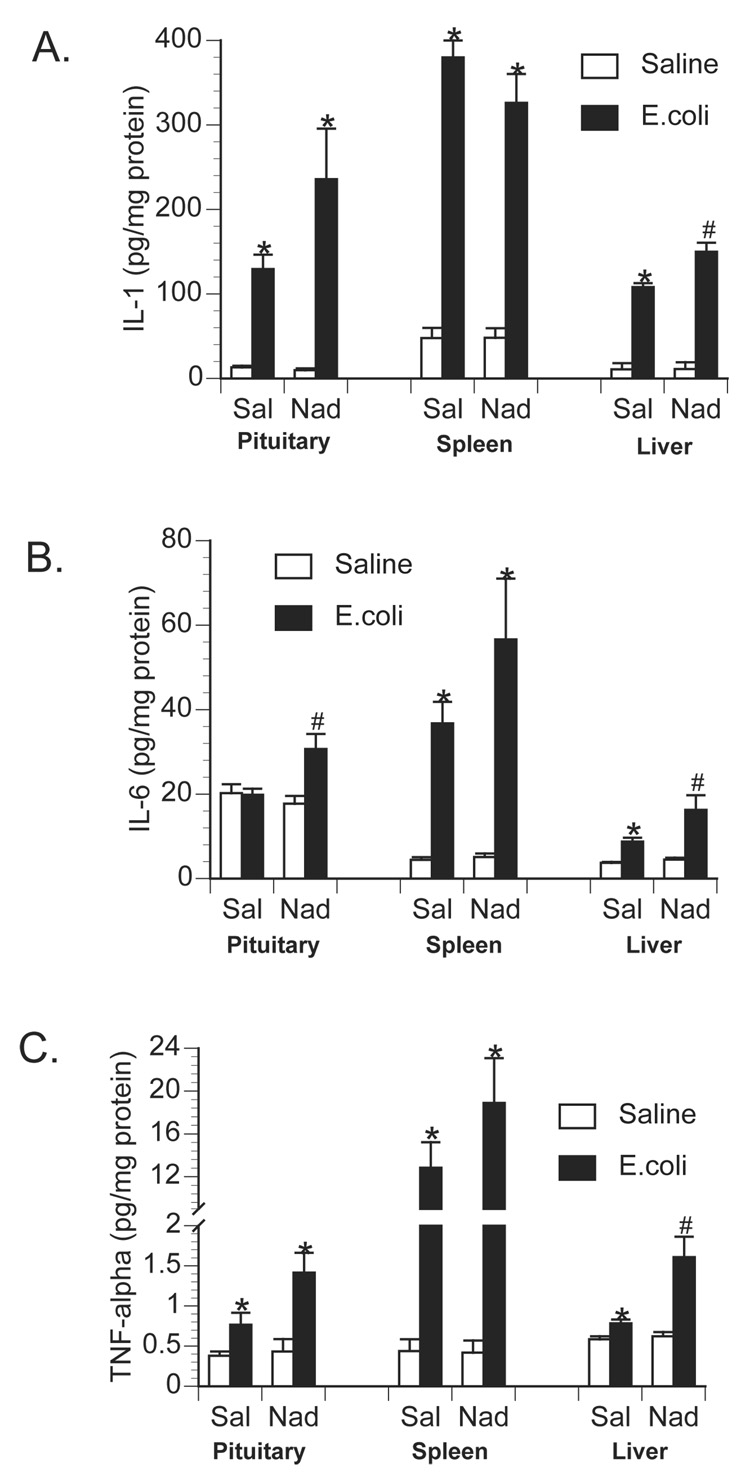
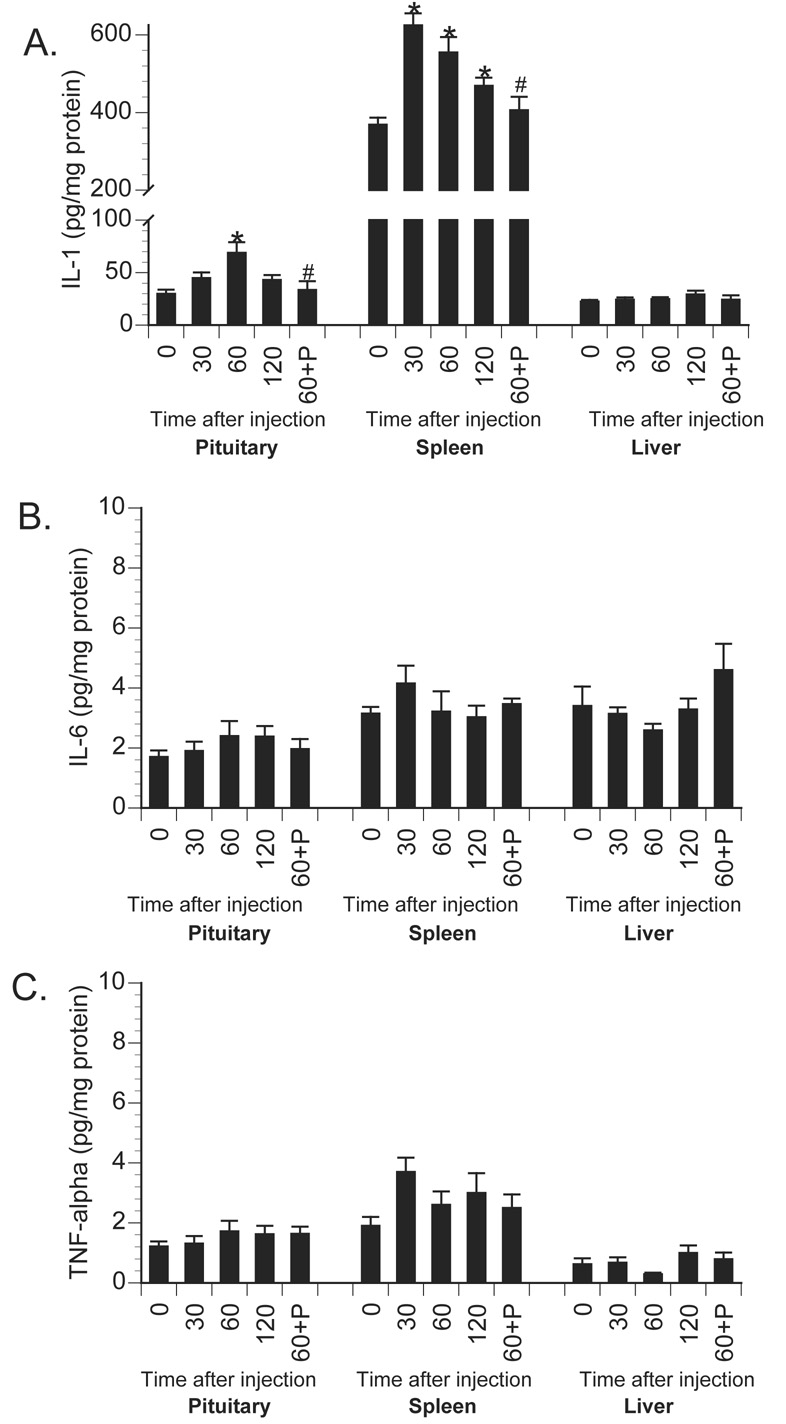
To examine the role of central β-adrenoceptors in the induction of brain proinflammatory cytokines during a peripheral immune response, rats were injected with either saline or the β-adrenoceptor antagonist, propranolol, into the cisterna magna (i.c.m.). This i.c.m. injection delivers drug directly into the brain cerebrospinal fluid. Immediately after i.c.m. injection, rats received an i.p. challenge of either saline or 2.5 × 108 CFU E.coli. Rats were decapitated 6 h later for measurement of brain and peripheral cytokines. As previously observed, E.coli challenge increased brain IL-1β levels 6 h following injection in control animals, however, prior central administration of propranolol blocked or greatly attenuated the E.coli-induced increase in brain IL-1β (Figure 3). A 2×2 ANOVA revealed a significant interaction between drug administration (i.c.m. saline vs. i.c.m. propranolol) and immune challenge (i.p. saline vs. i.p. E.coli) for IL-1β concentration in the hypothalamus [F(1,26) = 14.367; p < 0.001]; amygdala [F(1,26) = 5.957; p = 0.022]; prefrontal cortex [F(1,26) = 4.89; p = 0.036]; and hippocampus [F(1,26) = 13.49; p = 0.001]. Interestingly, central administration of propranolol attenuated a number of peripheral proinflammatory cytokine responses in both tissues (Figure 4) and plasma (Figure 5). TNF-α concentration was significantly reduced in the spleen [F(1,26) = 5.19; p = 0.031] and plasma [F(1,26) = 6.022; p = 0.02]. IL-6 concentration was significantly reduced in the spleen [F(1,26) = 5.1; p = 0.032] and pituitary [F(1,26) = 6.95; p = 0.014], and IL-6 was attenuated, although not significantly, in the liver [F(1,26) = 3.87; p = 0.060] and plasma [F(1,26) = 3.44; p = 0.075]. IL-1β concentration was significantly attenuated in the spleen [F(1,26) = 13.78; p = 0.001], liver [F(1,26) = 9.93; p = 0.004], pituitary [F(1,26) = 10.46; p = 0.003], and plasma [F(1,26) = 4.68; p = 0.039].
Figure 3.
Effect of centrally administered propranolol on brain IL-1 production following peripheral E.coli challenge. Rats were injected i.c.m. with either saline (Sal) or propranolol (Prop; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of IL-1 by ELISA. Bars represent group averages +/− SEM (n=6-8/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.c.m. saline.
Figure 4.
Effect of centrally administered propranolol on peripheral tissue proinflammatory cytokine production following peripheral E.coli challenge. Rats were injected i.c.m. with either saline (Sal) or propranolol (Prop; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of A) IL-1, B) IL-6, and C) TNF-α in pituitary, spleen and liver tissues. Bars represent group averages +/− SEM (n=6-8/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.c.m. saline.
Figure 5.
Effect of centrally administered propranolol on circulating proinflammatory cytokine production following peripheral E.coli challenge. Rats were injected i.c.m. with either saline (Sal) or propranolol (Prop; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of plasma A) IL-1, B) IL-6, and C) TNF-α. Bars represent group averages +/− SEM (n=6–8/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.c.m. saline.
Effect of Peripheral β-Adrenoceptor Blockade on E.coli-Induced Cytokines
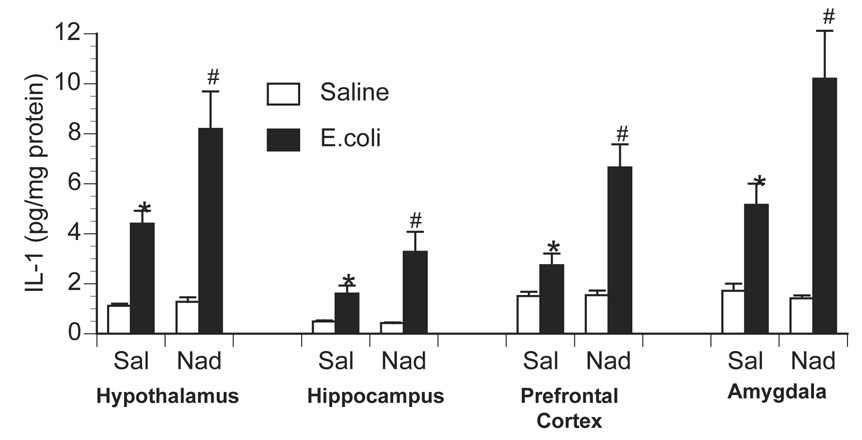
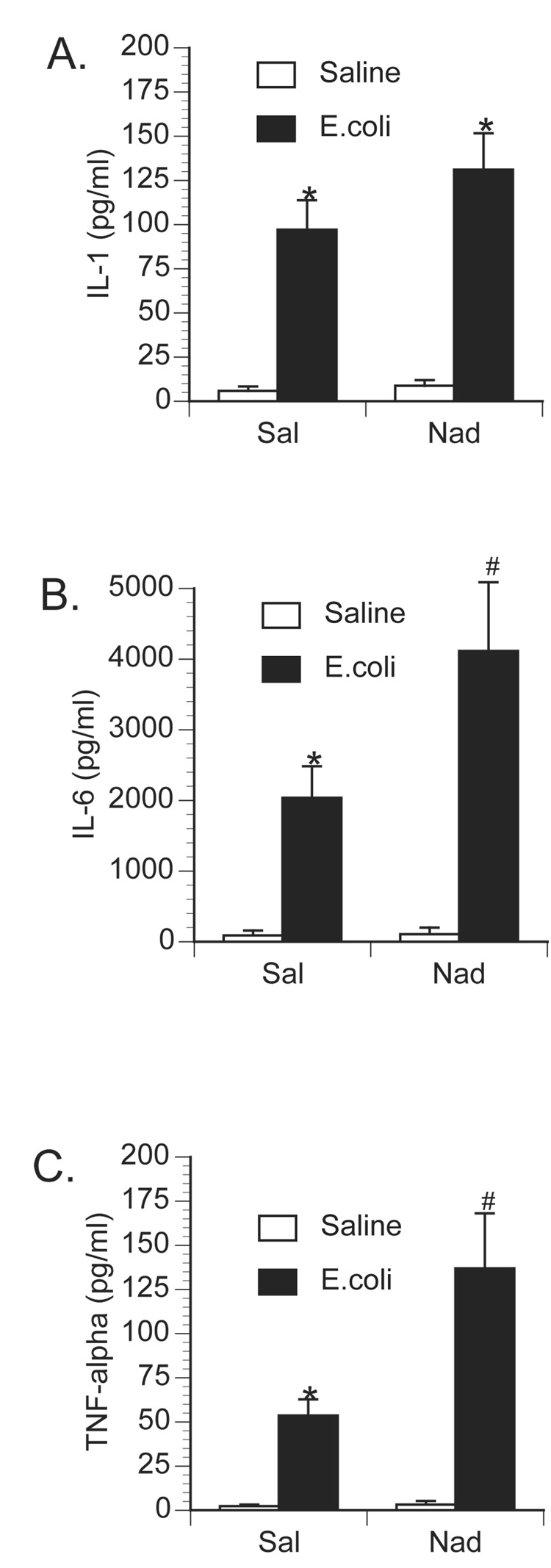
Substances administered directly into the brain may drain or may be transported to the periphery (Banks et al., 1989; Chen et al., 1997). To examine whether the attenuated E.coli-induced cytokine response observed following central propranolol administration was actually caused by peripheral β-blockade, additional animals were administered i.p. saline or nadolol (a β-adrenoceptor antagonist that does not cross the blood-brain barrier) followed by i.p. challenge with saline or 2.5 × 108 CFU E.coli. Again, control animals challenged with E.coli had a significant increase in both brain and peripheral proinflammatory cytokines. While prior peripheral administration of nadolol had no effect on basal cytokine levels, nadolol significantly enhanced both brain (Figure 6) and peripheral (Figure 7 & 8) cytokine concentration following E.coli-challenge. A 2×2 ANOVA revealed a significant interaction between drug administration (saline vs. nadolol) and immune challenge (saline vs. E.coli) on IL-1β concentration in the hypothalamus [F(1,30) = 5.80; p = 0.022], amygdala [F(1,30) = 7.21; p = 0.011], prefrontal cortex [F(1,30) = 14.75; p < 0.001], and hippocampus [F(1,30) = 3.96; p = 0.05]. In the periphery, significant interactions between drug administration and immune challenge were observed for TNF-α concentration in the liver [F(1,30) = 9.56; p = 0.004] and plasma [F(1,30) = 7.28; p = 0.011], IL-6 concentration in the liver [F(1,30) = 3.95; p = 0.05], pituitary [F(1,30) = 8.02; p = 0.008] and plasma [F(1,30) = 4.12; p = 0.05], and IL- 1β concentration in the liver [F(1,30) = 6.84; p = 0.014].
Figure 6.
Effect of peripherally administered nadolol on brain IL-1 production following peripheral E.coli challenge. Rats were injected i.p. with either saline (Sal) or nadolol (Nad; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of IL-1 by ELISA. Bars represent group averages +/− SEM (n=8–9/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.p. saline.
Figure 7.
Effect of peripherally administered nadolol on peripheral tissue proinflammatory cytokine production following peripheral E.coli challenge. Rats were injected i.p. with either saline (Sal) or nadolol (Nad; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of A) IL-1, B) IL-6, and C) TNF-α in pituitary, spleen and liver tissues. Bars represent group averages +/− SEM (n=8–9/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.p. saline.
Figure 8.
Effect of peripherally administered nadolol on circulating proinflammatory cytokine production following peripheral E.coli challenge. Rats were injected i.p. with either saline (Sal) or nadolol (Nad; β-antagonist) immediately prior to challenge with i.p. saline or E.coli. Animals were sacrificed 6 h later for measurement of plasma A) IL-1, B) IL-6, and C) TNF-α. Bars represent group averages +/− SEM (n=6–8/group). * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to E.coli group receiving i.p. saline.
Effect of Central β-Adrenoceptor Stimulation on Brain & Peripheral Cytokine Production
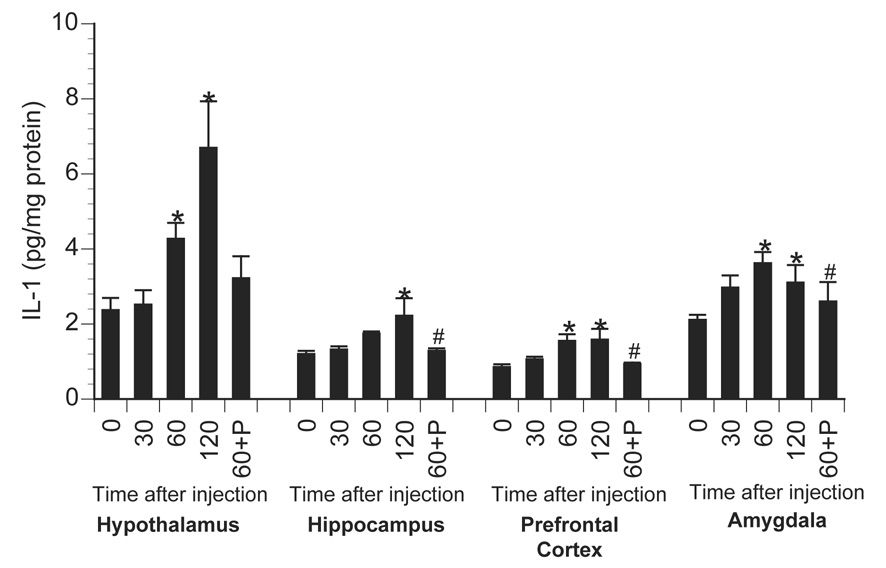
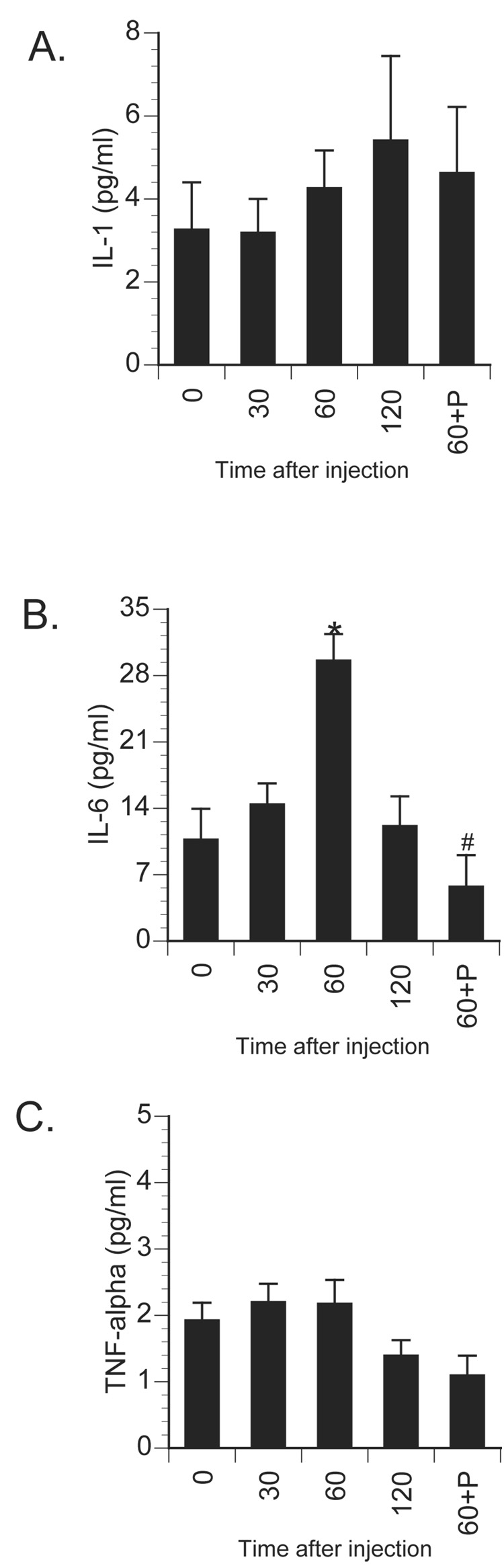
Further studies examined whether activation of central β-ADRs were themselves sufficient to increase brain IL-1β concentration. Here, animals were centrally administered saline or isoproterenol and sacrificed 30, 60, or 120 min later for measurement of central and peripheral cytokines. Saline injection failed to alter cytokine concentration so for graphing and statistical purposes these animals were averaged into a 0 min time-point. Central isoproterenol administration resulted in a time-dependent increase in brain IL-1β concentrations in all brain areas measured with peak responses observed between 60 and 120 min following administration (Figure 9). A 1×4 ANOVA revealed a significant increase in brain IL-1β across time in the hypothalamus [F(3,24) = 8.47; p < 0.001], amygdala [F(3,24) = 3.65; p = 0.027], prefrontal cortex [F(3,24) = 4.2; p = 0.016], and hippocampus [F(3,24) = 2.87; p = 0.05]. Figure 10 shows the effects of central β-ADR stimulation on the concentration of peripheral cytokines. Interestingly, activation of central β-ADRs elevated IL-1β in the pituitary [F(3,24) = 6.52; p = 0.002] and spleen [F(3,24) = 15.25; p < 0.001], but not in liver tissue (p = 0.20). There were no significant changes in levels of central (not shown) or peripheral tissue IL-6 or TNF-α levels following central isoproterenol administration. A different pattern was observed in the circulation where IL-6 levels increased 60 min following central isoproterenol administration [F(3,24) = 8.85; p < 0.001], but no change was observed in circulating IL-1β or TNF-α (Figure 11). To verify receptor specificity, additional animals were administered the β-adrenoceptor antagonist, propranolol, immediately prior to isoproterenol administration and sacrificed 60 min later. Prior administration of propranolol completely prevented the isoproterenol-induced increase of brain IL-1β in the amygdala [F(1,9) = 5.39; p = 0.048], prefrontal cortex [F(1,9) = 6.28; p = 0.033], and hippocampus [F(1,9) = 27.09; p < 0.001], and lowered IL-1β concentrations in the hypothalamus, though this difference did not reach significance (p = 0.16). Peripherally, prior administration of propranolol completely blocked the cytokine changes observed following central isoproterenol administration, including pituitary IL-1β [F(1,9) = 5.97; p = 0.04], spleen IL-1β [F(1,9) = 6.23; p = 0.034], and plasma IL-6 [F(1,9) = 28.17; p < 0.001].
Figure 9.
Effect of centrally administered isoproterenol on brain IL-1 production. Rats were injected i.c.m. with either saline or isoproterenol (β-agonist) and sacrificed 30, 60, or 120 min later (n=7/group). Values from saline-injected animals were averaged and presented as 0 min. Additional rats were injected i.c.m. with propranolol (β-antagonist) immediately prior to isoproterenol administration and sacrificed 60 min later (60+P); n=4/group). Brains were dissected and IL-1 measured using ELISA. Bars represent group averages +/− SEM. * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to isoproterenol-injected animals sacrificed at 60 min.
Figure 10.
Effect of centrally administered isoproterenol on peripheral tissue proinflammatory cytokine production. Rats were injected i.c.m. with either saline or isoproterenol (β-agonist) and sacrificed 30, 60, or 120 min later (n=7/group). Values from saline-injected animals were averaged and presented as 0 min. Additional rats were injected i.c.m. with propranolol (β-antagonist) immediately prior to isoproterenol administration and sacrificed 60 min later (60+P; n=4/group). Pituitary, spleen, and liver tissues were dissected and A) IL-1, B) IL-6, and C) TNF-α measured. Bars represent group averages +/− SEM. * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to isoproterenol-injected animals sacrificed at 60 min.
Figure 11.
Effect of centrally administered isoproterenol on circulating proinflammatory cytokine production. Rats were injected i.c.m. with either saline or isoproterenol (β-agonist) and sacrificed 30, 60, or 120 min later (n=7/group). Values from saline-injected animals were averaged and presented as 0 min. Additional rats were injected i.c.m. with propranolol (β-antagonist) immediately prior to isoproterenol administration and sacrificed 60 min later (60+P; n=4/group). Plasma was collected for measurement of A) IL-1, B) IL-6, and C) TNF-α. Bars represent group averages +/− SEM. * represents p < 0.05 compared to saline-injected controls. # represents p < 0.05 compared to isoproterenol-injected animals sacrificed at 60 min.
Discussion
The studies presented here examined the hypothesis that following peripheral immune challenge stimulation of central β-ADRs induces brain inflammatory cytokine production. We found that central administration of the β-ADR antagonist, propranolol, prevents the induction of brain IL-1β that normally occurs following peripheral E.coli challenge. It is unlikely that the effects of propranolol were due to leaking of propranolol into the periphery since peripheral administration of nadolol, a selective β-ADR antagonist that does not readily cross the blood-brain barrier, resulted in an enhancement, not suppression, of brain IL-1β production following E.coli challenge. In addition, direct stimulation of central β-ADRs via central administration of isoproterenol was sufficient to induce brain IL-1β in a time-dependent fashion. Taken together, these findings suggest that stimulation of central β-adrenoceptors are both necessary and sufficient to stimulate brain IL-1β production during a peripheral bacterial challenge.
The findings presented here support data demonstrating that stimulation of β-ADRs alone (i.e. in the absence of immune stimuli) stimulates cytokine production. Several laboratories have now demonstrated that β-ADR agonist stimulate the induction of both IL-1β and IL-6 mRNA in cultured macrophages and microglia (Hetier et al., 1991; Tan et al., 2007; Tomozawa et al., 1995). β-ADR-mediated proinflammatory cytokine induction appears to depend on the elevation of intracellular cyclic adenosine monophosphate (cAMP) (Tomozawa et al., 1995), but occurs via a PKA-independent pathway that involves extracellular signal regulated kinase (ERK) and p38 signaling (Tan et al., 2007). Here, we demonstrate that in vivo stimulation of central β-ADRs with isoproterenol increased brain IL-1β protein concentrations and this could be blocked by pre-treatment with propranolol. Furthermore, our data suggest that stimulation of brain β-ADRs may mediate the induction of central IL-1β during normal physiological processes such as response to peripheral E.coli challenge (data presented here) and exposure to psychological stressors (Johnson et al., 2005).
Interestingly, blockade of central β-ADRs altered the peripheral cytokine response following E.coli challenge as indicated by attenuated IL-1β, TNF-α, and IL-6 responses in circulation and in peripheral tissues (e.g. pituitary, spleen and liver). This is probably not due to centrally administered propranolol leaking out of the brain and blocking peripheral β-ADRs because blockade of peripheral β-ADRs resulted in enhanced, not suppressed, central cytokine responses following an E.coli challenge; most likely due to increased signaling of elevated peripheral inflammatory cytokines. These data suggest that activation of central β-ADR stimulate brain-mediated physiological responses that are necessary for maximum peripheral cytokine production. Conversely, stimulation of central β-ADR with isoproterenol not only induced IL-1β in the brain, but also resulted in induction of IL-1β in pituitary and spleen tissue and IL-6 in plasma. It is unclear why IL-1β was more responsive in tissues, while IL-6 was selectively elevated in blood. One likely mechanism by which central β-ADRs may alter peripheral cytokine responses is via affecting sympathetic nervous system activity. There is some evidence to support the notion that central β-ADRs may alter sympathetic nerve responses. For example, intracerebroventricular administration of β-ADR antagonists prevents the air jet stress-induced increase in renal sympathetic nerve activity (Koepke and DiBona, 1985, 1986). Thus, we propose that central administration of isoproterenol may induce spleen and pituitary IL-1 via stimulation of sympathetic nerves that results in the release of norepinephrine directly into peripheral tissues. The fact that liver IL-1β did not increase following central β-ADR stimulation would suggest that selective branches of sympathetic nerves may be stimulated following central isoproterenol. Unfortunately, this was an unexpected finding and blood samples in the studies presented here were not collected with antioxidant compounds so plasma catecholamines could not be measured to assess sympathetic nervous system activation. Because of the obvious bidirectional regulation of peripheral and central cytokines one must consider the possibility that central β-ADRs may also indirectly regulate brain cytokines via modulation of the sympathetic nervous system and its affects on peripheral cytokine levels.
Interestingly, it has been demonstrated that the sympathetic nervous system can suppress peripheral proinflammatory cytokine production during immune challenge (Elenkov et al., 2000), and this is at least partly due to β-ADR-mediated intracellular elevation of cAMP that inhibits NFκβ activation via a protein kinase A (PKA)-dependent pathway (Ollivier et al., 1996; Parry and Mackman, 1997). NFκβ is a very potent transcription factor that regulates inflammatory cytokine production in response to immune stimuli (i.e. TLR & cytokine signaling). The data presented here support the notion that stimulation of β-ADRs suppress immune-induced inflammatory cytokines since it was demonstrated that peripheral administration of nadolol enhanced circulating and peripheral tissue cytokine responses following E.coli challenge. Note, however, this does not contradict data that demonstrate stimulation of β-ADRs in the absence of immune challenge induce IL-1β & IL-6 production via a PKA-independent pathway, but indicate that β-ADR stimulation can have opposing effects on cytokine production depending on the activation state of the cell (i.e. presence or absence of antigenic stimulation). We suggest because E.coli are too large to cross the BBB that following peripheral E.coli challenge it results in the scenario where stimulation of β-ADRs on peripheral cells suppresses proinflammatory cytokine production, while stimulation of β-ADRs on central cells induces cytokine production.
Inflammatory cytokines, particularly IL-1β, are thought to contribute to the initiation of brain-mediate sickness responses following peripheral immune challenge (Cartmell et al., 1999), but the exact function of individual inflammatory cytokines remains unclear due to the pluripotent actions of inflammatory cytokines (Bluthe et al., 2000). It has been demonstrated that fever, one brain-mediated sickness response, is beneficial to the health/survival of an infected organism (Blatteis, 1986; Kluger et al., 1975), most likely because elevated core body temperature increases antimicrobial responses of immune cells (Jiang et al., 1999; Roberts, 1991; Sebag et al., 1977). Due to the enormous metabolic demands of elevating body temperature, it has been suggested that other brain-mediated sickness responses function to minimize other energy usages (e.g. decrease foraging for food, decreased exploration, increase sleep) and liberate energy stores (e.g. elevate glucocorticoid and catecholamine levels) (Hart, 1988; Maier and Watkins, 1998). One might hypothesize that inflammatory cytokines might only increase in brain areas that mediate specific sickness responses, but in the studies presented here IL-1β increased in all brain areas examined. The fact that data presented here indicate central catecholamine systems regulate brain IL-1β levels following peripheral immune challenge may explain the widespread increase in brain IL-1β, since noradrenergic neurons are known to have vast projections throughout the central nervous system; thus, activation of noradrenergic neurons during an immune challenge could globally elevate IL-1β throughout the brain. Several laboratories have demonstrated that IL-1β can enhance GABA activity thereby suppressing firing rates of neurons (Brambilla et al., 2007; Tabarean et al., 2006). In fact, it is by suppressing wake-active serotonergic dorsal raphe neurons and preoptic/anterior hypothalamus that some researchers have suggested brain IL-1 promotes NREM sleep (Tabarean et al., 2006) and changes in feeding and thermoregulation (Brambilla et al., 2007). In following the idea that many cytokine-induced sickness responses function to shift energy usage towards maintaining fever, we propose that widespread increases in brain IL-1β might act to reduce the overall metabolic demand of the central nervous system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks WA, Kastin AJ, Durham DA. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989;23:433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Fever: is it beneficial? Yale J Biol Med. 1986;59:107–116. [PMC free article] [PubMed] [Google Scholar]
- Bluthe R, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stennig B, Kelley K, Dantzer R. Lipopolysaccharide induces sickness behavior in rats by a vagal mediated mechanism. Comptes Rendus de l' academie des sciences. 1994;317:499–503. [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Brady LS, Lynn AB, Herkenham M, Gottesfeld Z. Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci. 1994;14:4951–4964. doi: 10.1523/JNEUROSCI.14-08-04951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla D, Franciosi S, Opp MR, Imeri L. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur J Neurosci. 2007;26:1862–1869. doi: 10.1111/j.1460-9568.2007.05796.x. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Rothwell NJ. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J Physiol. 1999;518(Pt 2):585–594. doi: 10.1111/j.1469-7793.1999.0585p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Castro WL, Chow HH, Reichlin S. Clearance of 125I-labeled interleukin-6 from brain into blood following intracerebroventricular injection in rats. Endocrinology. 1997;138:4830–4836. doi: 10.1210/endo.138.11.5533. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Chen CC, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: Evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130:130–145. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Dijkstra I, Tilders FJ. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136:4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]
- Goehler L, Relton J, Dripps D, Kiechle R, Tartaglia N, Maier S, Watkins L. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: A possible mechanism for immune-to-brain communication. Brain Research Bulletin. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Hansen MK, O'Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J Neurosci. 1998;18:2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hetier E, Ayala J, Bousseau A, Prochiantz A. Modulation of interleukin-1 and tumor necrosis factor expression by beta-adrenergic agonists in mouse ameboid microglial cells. Exp Brain Res. 1991;86:407–413. doi: 10.1007/BF00228965. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Detolla L, Singh IS, Gatdula L, Fitzgerald B, van Rooijen N, Cross AS, Hasday JD. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Watkins LR, Maier SF. The role of IL-1β in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Koepke JP, DiBona GF. Central beta-adrenergic receptors mediate renal nerve activity during stress in conscious spontaneously hypertensive rats. Hypertension. 1985;7:350–356. [PubMed] [Google Scholar]
- Koepke JP, DiBona GF. Central adrenergic receptor control of renal function in conscious hypertensive rats. Hypertension. 1986;8:133–141. doi: 10.1161/01.hyp.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Uvnas-Moberg K, Miyasaka K, Lundeberg T. Interleukin-1 increases activity of the gastric vagal afferent nerve partly via stimulation of type A CCK receptor in anesthetized rats. J Auton Nerv Syst. 1997;62:72–78. doi: 10.1016/s0165-1838(96)00111-7. [DOI] [PubMed] [Google Scholar]
- Laye S, Bluthe RM, Kent S, Combe C, Medina C, Parnet P, Kelley K, Dantzer R. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268:R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to- brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Niijima A. The afferent discharges from sensors for interleukin-1B in the hepatoportal system in the anesthetized rat. Journal of the Autonomic Nervous System. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- Proescholdt MG, Hutto B, Brady LS, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience. 2000;95:577–592. doi: 10.1016/s0306-4522(99)00417-0. [DOI] [PubMed] [Google Scholar]
- Roberts NJ., Jr Impact of temperature elevation on immunologic defenses. Rev Infect Dis. 1991;13:462–472. doi: 10.1093/clinids/13.3.462. [DOI] [PubMed] [Google Scholar]
- Sebag J, Reed W, Williams R. Effects of temperature on bacterial killing by serum and by polymorphonuclear leukocytes. Infection and Immunity. 1977;10:947–954. doi: 10.1128/iai.16.3.947-954.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soszynski D, Kozak W, Conn CA, Rudolph K, Kluger MJ. Beta-adrenoceptor antagonists suppress elevation in body temperature and increase in plasma IL-6 in rats exposed to open field. Neuroendocrinology. 1996;63:459–467. doi: 10.1159/000127072. [DOI] [PubMed] [Google Scholar]
- Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y, Yabuuchi K, Inoue T, Satoh M. Participation of cAMP and cAMP-dependent protein kinase in beta- adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci Res. 1995;22:399–409. doi: 10.1016/0168-0102(95)00922-g. [DOI] [PubMed] [Google Scholar]