Summary
New methods to distinguish between nontypeable Haemophilus influenzae and nonhemolytic Haemophilus haemolyticus were compared. The results of iga variable region hybridization to dotblots and library-on-a-slide microarrays were more similar to a “gold standard” multigene phylogenetic tree than iga conserved region hybridization or P6 7F3 epitope immunoblots.
Keywords: Haemophilus haemolyticus, Nontypeable Haemophilus influenza, Haemophilus classification, P6 7F3 epitope, immunoglobulin A1 protease
Nontypeable Haemophilus influenzae (NTHi) are gram-negative coccobacilli that asymptomatically colonize the nasopharynx, but cause respiratory infections such as bronchitis, otitis media, and sinusitis. Standard tests to identify H. influenzae in clinical specimens, X- and V- factor dependence (Kilian, 2005), do not distinguish between H. influenzae and a related, non-pathogenic species, Haemophilus haemolyticus. Such distinction has relied on the ability of H. haemolyticus to lyse horse red blood cells, which H. influenzae cannot do. However, this phenotype may be lost during passage (Kilian, 1976). Worse, it was reported recently that some strains of even low passage H. haemolyticus do not hemolyze, making this test unreliable (Murphy et al., 2007). In fact, between 4% and 27% of throat culture strains originally designated H. influenzae appear to be H. haemolyticus (Juliao et al., 2007, Mukundan et al., 2007, Murphy et al., 2007), a concern for research using throat strains. Because H. haemolyticus does not cause disease or live in normally sterile sites (Murphy et al., 2007, Xie et al., 2006), most clinical samples do not need to be tested for the presence of H. haemolyticus and may be tested for H. influenzae in the traditional manner, by X and V factor dependence (McCrea et al., 2008).
To better distinguish between H. influenzae and H. haemolyticus in studies of H. influenzae pharyngeal colonization, McCrea et al. (2008) used 88 strains of H. influenzae, 33 strains of hemolytic H. haemolyticus, 76 strains of nonhemolytic H. haemolyticus and five outgroup taxa to reconstruct a minimum evolution (ME) phylogenetic tree based on the concatenated sequences of five genes: adk, pgi, recA, infB, and 16S rRNA. Known H. influenzae and H. haemolyticus are found in separate clusters and biochemical tests correlate well with the tree structure. While such a tree serves as a “gold standard” to distinguish H. influenzae from nonhemolytic H. haemolyticus, classifying new strains by placing them in the tree involves sequencing portions of five genes and significant computational time. Trees reconstructed using single genes were not, by themselves, able to separate the two species.
Rapid methods for research laboratories to distinguish between H. influenzae and nonhemolytic H. haemolyticus have been suggested. The iga gene encoding the enzyme immunoglobulin A1 protease (Kilian, 2005) and the 7F3 epitope of P6 are present in H. influenzae but not H. haemolyticus (Murphy et al., 2007). In this study we compared the classification of putative H. influenzae strains using 1) the ME tree based on DNA sequences (McCrea et al., 2008), 2) DNA hybridization based ‘library-on-a-slide’ microarrays probed with conserved and variable regions of iga, 3) genomic dotblot hybridization with conserved and variable regions of iga, and 4) P6 7F3 epitope immunoblots.
The library-on-a-slide microarray (Zhang et al., 2004) consisted of the genomic DNA of 393 Haemophilus strains from three collections isolated from the throats of children in day care centers (Farjo et al., 2004, Granoff et al., 1979, St Sauver et al., 2000), 50 of which were also included in the ME tree. DNA was extracted by sonication (Zhang et al., 2005) and spotted on a microarray slide. For use as probes, the conserved β-core region of iga (Kilian, 2005) was amplified from H. influenzae strain 26225 using primers (igaBF1 5’-TGAATAACGAGGGGCAATATAAC-3’ and igaBR2 5’-TCACCGCACTTAATCACTGAAT-3’) corresponding to bases 4124–4978 in strain HK368 (GenBank accession M87492). Also, the variable region of iga was amplified from H. influenzae type b strain Eagan using primers (igaLF1 5’-GTTCCACCACCTGCGCCTGCTAC-3’ and igaLR2 5’-GTTATATTGCCCCTCGTTATTCAT-3’) corresponding to bases 3334–4146 in HK368 (Vitovski et al., 2002). The variable and conserved region fragments were fluorescein labeled as described (Kong et al., 2006). Duplicate microarrays were spotted, probed, and washed as described (Kong et al., 2006), with these exceptions: hybridization and washes were at 65 °C, PerfectHyb Plus (Sigma-Aldrich, St. Louis, MO) was used as a prehybridization and hybridization buffer, and the DNA concentration-control probe was a mixture of seven H. influenzae MLST gene fragments (http://Haemophilus.mlst.net/) and the coding region of pepN, in equal concentrations. Arrays hybridized with the conserved iga region, used a digoxigenin (DIG) labeled (DIG High Prime, Roche, Indianapolis) concentration-control probe, and then slides were destained and stripped by washing successively with 100% ethanol, 4M NaOH, 2 X SSC twice, and rehybridized with a fluorescein-labeled conserved region iga probe. Arrays hybridized with the variable iga region used a fluorescein-labeled concentration-control probe or a fluorescein-labeled iga variable region probe.
The Spotfinder v.3.1.1 program (http://www.tm4.org/spotfinder.html) determined the intensity of hybridization to each spot (settings: histogram, mask size 10, spot size 16, top background cut off 5%). MIDAS v.2.19 (http://www.tm4.org/midas.html) was used to remove poorly hybridized spots and to control for the concentration of genomic DNA in each spot by comparing the iga probing intensity with the concentration-control intensity.
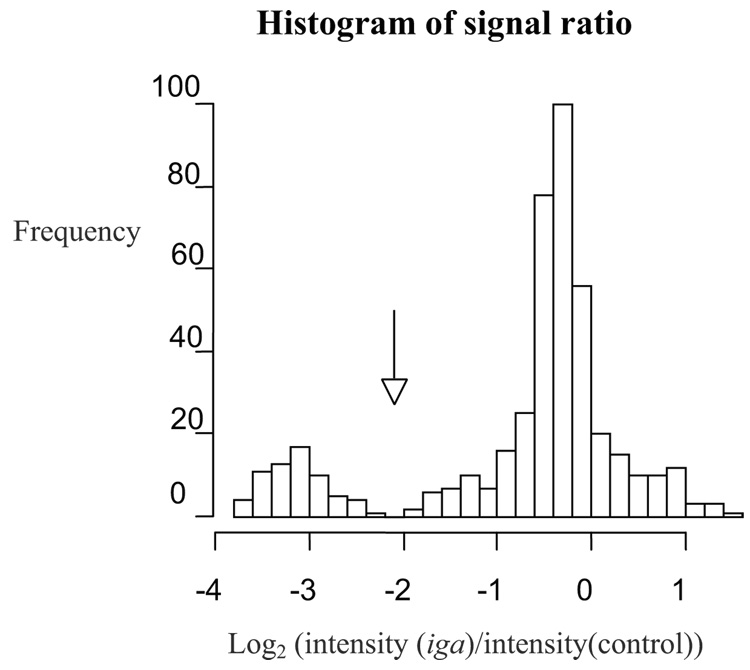
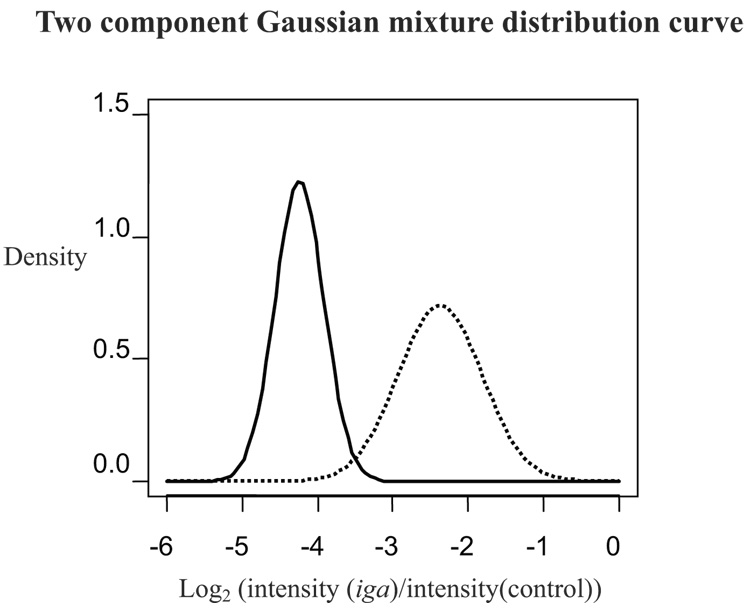
Programs written in the statistical software “R” (http://www.r-project.org/) graphed the frequency of the log transformed iga to concentration-control signal ratio (log2 (I(iga)/I (control)) where I = intensity calculated by Spotfinder) (Figure 1). Given that some strains contain the gene and some do not, thus the bimodal nature of the graphs, positive and negative spots clearly formed two different normal distributions. In cases in which two distributions were clearly separated (as in Figure 1), spots were considered positive for the probe if they were within the right-most peak (higher ratio) and negative if they were within the peak to the left (lower ratio). An obvious separation, however, between the positive and negative peaks for the two slides hybridized with the iga variable region probe was not seen. For these two slides, an alternate quantitative method was used (Figure 2). A two-component Gaussian mixture model was fitted using the EM algorithm (Dempster et al., 1977) to classify the observed intensities into positive or negative spots. This procedure was performed using an R program and is identical to the approach developed by Fraley and Raftery (2003) implemented in an R software package MCLUST (http://www.stat.washington.edu/mclust). A >50% probability was used as the cut off between positive and negative hybridization results. Although the absolute intensities of the spots varied, each strain was consistently positive or negative on duplicate slides.
Figure 1.
Histogram used to determine cut off between positive and negative strains for iga-conserved probe on microarray. Strains positive for the probe are to the right of arrow, negative to the left. Intensities calculated using Spotfinder.
Figure 2.
Modeled intensities were used when histogram was unclear. The solid line indicates modeled curve for strains negative for the iga-variable probe, the dotted line for positive strains. A signal at the intersection of the two lines has a 50% probability of being positive. Strains with signals to the right of the intersection were considered positive, to the left negative.
Variable region iga dotblot analysis and P6 immunoblots were previously published (McCrea et al., 2008), for 197 strains that were also included in the ME tree. Conserved region iga dotblot analyses were performed as described (Xie et al., 2006) for 118 strains also in the ME tree. The conserved region iga β-core domain-specific probe was amplified from the H. influenzae strain Eagan using primers described above.
The ability of the three nonphylogenetic techniques to correctly identify the strains, defined as H. haemolyticus and H. influenzae based on the ME tree, is shown on Table 1. The iga variable region probe used in the dotblot hybridization assay, amplified from the H. influenzae strain Rd, was the best approximation to the ME tree, correctly categorizing 100% of the strains analyzed by both methods (McCrea et al., 2008). The microarray hybridization technique using a similar iga variable region probe, amplified from the H. influenzae strain Eagan, correctly categorized 96% of the strains. Using the microarray technique, 331 (84%) of the 393 total strains, including strains not used in the ME tree, were positive for the conserved region iga probe. When probed with the variable region of iga, 298 (76%) were positive (Table 2). Cross-species conservation of the conserved region of iga may account for the greater number of H. haemolyticus strains that hybridized to it.
Table 1.
Methods to distinguish H. influenzae from H. haemolyticus, as defined by five-gene ME tree clustersa
| A: Microarray hybridization using Iga probes | ||
|---|---|---|
| Hhb cluster N = 25 strains |
Hic cluster N = 25 strains |
|
| Variable iga +d | 0 | 23e |
| Variable iga −f | 25 | 2 |
| Conserved iga + | 8 | 23 |
| Conserved iga − | 17 | 2 |
| B: Dotblot hybridization using Iga probes | ||
|---|---|---|
| Hh cluster | Hi cluster | |
| Variable iga +* | 0 (N = 109 strains) | 88 (N = 88 strains) |
| Variable iga −* | 109 | 0 |
| Conserved iga + | 11 (N = 42 strains) | 74 (N = 76 strains) |
| Conserved iga − | 31 | 2 |
| C: Immunoblot using 7F3 antibody of P6* | ||
|---|---|---|
| Hh cluster N = 109 strains |
Hi cluster N = 88 strains |
|
| P6 + | 13 | 85 |
| P6 − | 96 | 3 |
Comparisons are to clusters of strains in the "gold standard " tree (McCrea et al., 2008).
Hh designates Haemophilus haemolyticus.
Hi designates H. influenzae.
+ indicates positive hybridization.
Bold numbers indicate correctly categorized strains.
− indicates no hybridization.
from published data (McCrea et al., 2008)
Table 2.
Statistics comparing five-gene tree results to other methods
| % Strains Misassigneda | % + Predictive Valueb | % − Predictive Valuec | % Sensitivityd | % Specificitye | |
|---|---|---|---|---|---|
| P6 7F3 antibody* | 8.1 | 86.7 | 97 | 96.6 | 88.1 |
| Iga conserved dotblot | 11 | 87 | 94 | 97 | 74 |
| Iga variable* dotblot | 0 | 100 | 100 | 100 | 100 |
| Iga variable microarray | 4 | 100 | 92.6 | 92 | 100 |
| Iga conserved microarray | 20 | 74.2 | 89.5 | 92 | 68 |
Misassigned strains: H. haemolyticus positive for iga or p6 and H. influenzae negative for iga or P6.
Positive predictive value: correctly identified positives divided by all strains that were positive for that probe.
Negative predictive value: correctly identified negatives divided by all strains that were negative for that probe.
Sensitivity: positive Hi strains divided by all Hi strains.
Specificity: negative Hh strains divided by all Hh strains.
from published data, (McCrea et al., 2008).
Of rapid methods tested, dotblots probed with the iga variable region correlated best with the results of the multigene phylogenetic analysis (McCrea et al., 2008). However, the same region of iga, used as a microarray probe, was nearly as accurate, lower in cost, and less time-consuming per strain because more strains could be analyzed at once. The cost of consumable reagents to analyze one isolate using the phylogenetic analysis is about 30% less than the cost for one microarray slide that contains hundreds of isolates (data not shown). The hands-on time required to examine hundreds of strains on an automated microarray is only about three times that of running one sample through the phylogenetic analysis (unpublished observation), and the number of trees to be analyzed increases rapidly with the number of strains (Felsenstein, 1978). Because nasopharyngeal colonization is frequently polyclonal (Farjo et al., 2004, Mukundan et al., 2007, St Sauver et al., 2000), our laboratory now analyzes up to 30 H. influenzae-like colonies per throat swab to attempt to sample most of the strains. Even small studies quickly accumulate many isolates. For studies analyzing a large number of isolates, high throughput methods such as microarrays are time efficient and cost effective.
The choice between phylogenetic analysis and microarray analysis to identify a strain as H. influenzae or H. haemolyticus depends, in part, on the questions asked in designing the study at hand. A representation of the history of the relationships between strains is a by-product of the phylogenetic method, which would be useful for future genetic comparisons and evolutionary studies. Additional identical microarray slides may be printed for little additional cost, which would be useful for future studies of variation in genetic regions between strains. If only a few strains need to be analyzed, we would recommend retrieving the sequences used by McCrea et. al. (2008) from Genbank, sequencing the five genes in the new strains, and building a phylogenetic tree. The cost/time/future-usefulness decision will be different for each research laboratory.
Acknowledgments
Funding was provided by grants from the National Institute of Deafness and Other Communication Disorders (DC 05840) and the National Heart, Lung, and Blood Institute (HL 083893) awarded to J.R.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J. Roy. Stat. Soc. B. 1977;39:1–38. [Google Scholar]
- Farjo RS, Foxman B, Patel MJ, Zhang L, Pettigrew MM, McCoy SI, Marrs CF, Gilsdorf JR. Diversity and sharing of Haemophilus influenzae strains colonizing healthy children attending day-care centers. Pediatr. Infect. Dis. J. 2004;23:41–46. doi: 10.1097/01.inf.0000106981.89572.d1. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. The number of evolutionary trees. Syst. Zool. 1978;27:27–33. [Google Scholar]
- Fraley C, Raftery AE. Enhanced model-based clustering, density estimation, and discriminant analysis software. MCLUST. J. Classif. 2003;20:263–286. [Google Scholar]
- Granoff DM, Gilsdorf J, Gessert C, Basden M. Haemophilus influenzae type B disease in a day care center: eradication of carrier state by rifampin. Pediatrics. 1979;63:397–401. [PubMed] [Google Scholar]
- Juliao PC, Marrs CF, Xie JP, Gilsdorf JR. Histidine auxotrophy in commensal and disease-causing nontypeable Haemophilus influenzae. J. Bacteriol. 2007;189:4994–5001. doi: 10.1128/JB.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Genus III. Haemophilus Winslow, Broadhurst, Buchanan, Krumwiede, Rogers and Smith1917, 561AL. In: Garrity GM, editor. Bergey's Manual of Systematic Bacteriology. New York, NY: Springer-Verlag; 2005. pp. 883–904. [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J. Gen. Microbiol. 1976;93:9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kong Y, Cave MD, Zhang L, Foxman B, Marrs CF, Bates JH, Yang ZH. Population-based study of deletions in five different genomic regions of Mycobacterium tuberculosis and possible clinical relevance of the deletions. J. Clin. Microbiol. 2006;44:3940–3946. doi: 10.1128/JCM.01146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea KW, Xie J, LaCross N, Patel M, Mukundan D, Murphy TF, Marrs CF, Gilsdorf JR. Relationships of nontypeable Haemophilus influenzae strains to hemolytic and nonhemolytic Haemophilus haemolyticus strains. J. Clin. Microbiol. 2008:406–416. doi: 10.1128/JCM.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan D, Ecevit Z, Patel M, Marrs CF, Gilsdorf JR. Pharyngeal colonization dynamics of Haemophilus influenzae and Haemophilus haemolyticus in healthy adult carriers. J. Clin. Microbiol. 2007;45:3207–3217. doi: 10.1128/JCM.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J. Infect. Dis. 2007;195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- St Sauver J, Marrs CF, Foxman B, Somsel P, Madera R, Gilsdorf JR. Risk factors for otitis media and carriage of multiple strains of Haemophilus influenzae and Streptococcus pneumoniae. Emerg. Infect. Dis. 2000;6:622–630. doi: 10.3201/eid0606.000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitovski S, Dunkin KT, Howard AJ, Sayers JR. Nontypeable Haemophilus influenzae in carriage and disease: a difference in IgA1 protease activity levels. JAMA. 2002;287:1699–1705. doi: 10.1001/jama.287.13.1699. [DOI] [PubMed] [Google Scholar]
- Xie J, Juliao PC, Gilsdorf JR, Ghosh D, Patel M, Marrs CF. Identification of new genetic regions more prevalent in nontypeable Haemophilus influenzae otitis media strains than in throat strains. J. Clin. Microbiol. 2006;44:4316–4325. doi: 10.1128/JCM.01331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Foxman B, Gilsdorf JR, Marrs CF. Bacterial genomic DNA isolation using sonication for microarray analysis. BioTechniques. 2005;39:640–644. doi: 10.2144/000112038. [DOI] [PubMed] [Google Scholar]
- Zhang L, Srinivasan U, Marrs CF, Ghosh D, Gilsdorf JR, Foxman B. Library on a slide for bacterial comparative genomics. BMC Microbiol. 2004:4–12. doi: 10.1186/1471-2180-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]