Abstract
Kennedy Disease (KD, or spinal and bulbar muscular atrophy) is caused by a CAG/polyglutamine expansion in the androgen receptor (AR) gene. Both motoneurons and muscles are affected by KD, but where mutant ARs act to initiate this disease is not clear. We discuss recent insights into this disease with two main themes. 1) KD is androgen-dependent, suggesting that blocking androgen action may be an effective treatment. 2) Androgens may trigger KD by acting in muscles, which indirectly affects the motoneurons, suggesting that blocking AR function in muscles may rescue motoneurons from disease and provide an effective treatment. Future research will provide a better understanding of how androgens trigger KD and the relative contributions of motoneurons versus muscles in this disease.
Introduction
Many disorders exhibit striking sex-biases in the human population, with some more common in males and showing an X-linked pattern of inheritance. One such disease affecting only men is spinal and bulbar muscular atrophy or Kennedy disease (KD), a so-called “lower motoneuron disease” characterized by an adult-onset, slowly progressing loss of motor function. In 1991, Kenneth Fischbeck and colleagues made the landmark discovery that individuals with KD have an expansion of a polymorphic CAG/glutamine tract in the first exon of the androgen receptor (AR) gene (normal range 9 – 37 CAG repeats; disease > 40 CAG repeats). [1]. The 100% concordance between disease occurrence and these expanded CAG alleles provided strong evidence that the cause of KD had been identified. Since then, comparable CAG repeat expansions in the coding regions of unrelated genes have been linked to eight other neurodegenerative diseases (Huntington disease, dentatorubropallidoluysian atrophy, and spinocerebellar ataxias 1, 2, 3, 6, 7, and 17). These nine disorders are commonly referred to as “polyglutamine (polyQ) diseases”, emphasizing the disease-causing role of the expanded glutamine tract in each mutant protein [2].
Identifying these mutations has allowed development of animal and cell models in which the human disease gene is expressed. These models provide powerful inroads to our understanding of disease-causing mechanisms, and have provided compelling evidence that elongated polyQ tracts lead to protein misfolding, a critical step in disease pathogenesis. We begin by outlining key features of KD in humans, since the first question asked of any new animal model is how well it mimics the human disease. We then discuss new insights based on these models and the therapeutic approaches they suggest.
Characteristics of SBMA in humans: Symptoms and underlying cellular pathology
Muscle weakness and reduced androgen sensitivity in men are core features of Kennedy disease (KD)
First described by William Kennedy and colleagues in 1968 [3], a core feature of KD is a progressive weakness and wasting of muscles, which interferes with the performance of routine motor tasks such as walking, lifting and climbing stairs. Although symptoms of KD typically lead to diagnosis in mid-life (between 30 – 50 years), men with KD often report experiencing frequent muscle twitching and/or cramping for a decade or more prior to diagnosis, suggesting that the disease process begins well before reaching the threshold of clinical muscle weakness. In addition to affecting limb muscles, bulbar symptoms of KD include difficulty swallowing, chewing and enunciating clearly. While individuals with longer CAG repeats tend to develop the disease earlier, there is marked variation in disease onset even among related individuals with the same CAG repeat size [4,5]. Female carriers with one or two copies of the KD allele commonly experience subclinical symptoms (e.g., muscle twitching) without significant loss in muscle strength [5,6]. Another core feature of KD is reduced androgen-sensitivity, such as gynecomastia and testicular atrophy [3,5,7,8], suggesting a partial loss of normal AR function in individuals with KD. However, it is not this loss of normal function but rather a gain of new and toxic functions conferred by the expanded glutamine tract that causes the neuromuscular pathology of KD, since humans inheriting completely dysfunctional AR do not appear prone to such weakness. Finally, blood creatine kinase levels are consistently elevated in KD patients, suggesting that muscle damage is significant in this disease.
Neurophysiological measures indicate loss of motor and sensory axons and compensatory sprouting in KD
Nerve conduction and electromyographic studies suggest that the muscle atrophy and weakness in KD are caused by a progressive loss of motor axons, resulting in denervation and atrophy of muscle fibers [5,8]. High amplitude motor unit potentials are also detected, suggesting that as muscle fibers are denervated, nearby surviving motor axons sprout to re-innervate them. Such studies also indicate a robust loss of sensory axons in men with KD. Which sensory neurons are affected and the degree of loss within different populations are not well documented, but the consistent absence of tendon reflexes in men with KD suggests that the sensory neurons most affected are the large caliber 1A afferents that relay information to the motoneurons about muscle stretch. Thus, it is likely that losses in both motor and sensory neurons contribute to the loss of motor function in KD.
Histopathological correlates confirm loss of motor and sensory neurons in KD
Direct examination of tissues from KD patients reveals a significant loss of motor axons accompanied by a marked and selective depletion of motoneuronal cell bodies in the spinal cord and brain stem [3,8,9]. Moreover, large diameter sensory axons appear selectively depleted from the dorsal roots and the sensory-rich peripheral sural nerve, consistent with the idea that 1A afferents supplying muscle spindles may be selectively lost in this disease. Muscle biopsies, however, suggest that two separate processes may be occurring: (1) ongoing denervation and sprouting indicated by muscle fiber atrophy and fiber-type grouping, and (2) primary myopathy indicated by pathology (e.g., fiber splitting, centralized nuclei, fiber degeneration) typical of diseases that originate in muscle [10]. Another hallmark of KD is an accumulation of mutant AR into insoluble aggregates or inclusions in neuronal and nonneuronal cells [11,12]. While the significance of these microscopically visible aggregates is not clear [12,13**], experimental evidence indicates that toxicity is mediated by soluble forms of the misfolded, mutant protein [14*].
Clinical reports describing KD raise questions
What factors besides CAG repeat size determine disease onset and progression?
Although individuals with longer CAG repeats tend to develop the disease at an earlier age, disease onset can vary by a decade or more for a given CAG repeat size, even within a family [1,5]. Moreover, females having one or both copies of the KD allele show only mild sub-clinical symptoms and are not deemed as having KD [5,6]. These observations suggest that factors other than the expanded CAG repeat affect expression of this disease.
Is KD a disease of motoneurons, muscles or both? In other words, where does the mutant AR act to trigger KD?
Calling KD a “motoneuron disease” carries with it the implicit assumption that the mutant AR acts in motoneurons to cause their death, resulting in denervation-induced atrophy of skeletal muscles and loss of motor function. However, muscles affected by KD undergo deterioration that is not solely attributable to denervation. In particular, men with KD generally show significantly elevated levels of creatine kinase, questioning whether KD involves primary myopathic processes, akin to the muscular dystrophies.
Experimental models of KD provide some answers and hope for treatment
Six different mouse models of KD have been described that express full length AR and largely recapitulate the disease phenotype, with the first models described in 2002. Five are transgenic (Tg) models [15–19] and one is a knock-in (KI) model [20]. Only one Tg model expresses experimental AR in a cell-specific manner [19], thus allowing investigators for the first time to address directly questions about which cells AR acts in to cause KD.
KD phenotype in mice
Despite various promoters and CAG repeat lengths, the different mouse models show remarkably similar phenotypes, with each exhibiting core features of KD (Supp Table 1). For example, the KD phenotype in mice shows a pronounced sex bias, with experimental males affected more than females, reminiscent of KD in humans. Symptomatic male mice show a marked loss of muscle strength, particularly in their hindlimbs, which impairs their ability to walk normally. Affected males are also typically smaller than controls and are kyphotic (showing a dorsal curvature of the spine), aspects of the KD phenotype that appear unique to rodents. The age of onset of KD symptoms can vary widely among these mouse models, from 3 weeks to 6 months, and likely reflects differences in both the size of the CAG repeat and the level of AR expression, but the rise in circulating androgens at puberty probably plays a role in all the models.
KD models establish that testicular androgens are a key factor triggering disease
That only men develop KD suggested a role for androgens in this disease, but direct support for this idea came only recently from cell and animal models. For example, castrating KD male mice, either at puberty or as adults, can rescue them from disease [15,16,19,20]. Moreover, testosterone (T) treatment has been shown in some models to “turn on” the disease in asymptomatic mutant females, inducing a KD phenotype comparable to that of gonadally intact mutant males [16**,19].
Finding that KD is triggered by androgens represents a significant step in understanding disease pathogenesis, and demonstrates that an expanded polyQ AR is not sufficient to trigger disease but that male levels of androgens are also required. This explains why females, even when homozygous for expanded CAG AR alleles, do not develop the disease. Moreover, differences in androgen titers may account for some of the variability in the age of onset between individuals that have comparable CAG expansions in their AR gene. Finally and most significantly, the apparent androgen-dependence of KD indicates that giving androgens to men with the disease to combat muscle atrophy is contraindicated. Although there are few documented cases of how exogenous androgens affect men with KD, one recent report that androgen treatment worsened symptoms in a patient with KD [21] indicates that the KD phenotype in humans also depends on androgens. A clear implication of these data is that agents that interfere with androgen action may reverse KD symptoms in men (discussed in more detail below).
The mechanism by which androgens stimulate toxicity is of great interest. Changes to AR induced by androgens include translocation from the cytoplasm to the nucleus, altered post-translational modifications, and less stable association with molecular chaperones including Hsp70 and Hsp90. Evidence implicates each of these in contributing to pathogenesis [2]. The nucleus appears to be a critical site for triggering KD pathology [22**]. Whether androgen-driven transcriptional activation by the AR is also a requisite step in the pathogenic pathway is less clear, given that AR antagonists fail to block androgens’ disease-promoting effects in some models despite their ability to block normal transcriptional function via classical androgen responsive elements [22,23]. It is possible that nuclear translocation of AR without activation is sufficient to induce toxicity, or that transcriptional activity of AR is also required, but is mediated via nonclassical regulatory elements, akin to tamoxifen-bound estrogen receptors acting at AP1 sites [24]. That the length of the polyQ tract affects cofactor recruitment is consistent with this view [25]. Clearly, considerably more work needs to be done to gain a full understanding of androgens’ role in triggering KD.
Evidence from models shifts the focus from cell death to cell dysfunction as the proximate cause of KD
Motoneuronal cell death has long been assumed to critically mediate the loss of motor function in KD, but data from KD models suggest otherwise. While two of the six KD mouse models show the expected motoneuronal loss [17,18], four do not [15,16,19,20]. Of these, all four models show an androgen-dependent loss of motor function without any apparent loss of motoneurons, with two providing evidence of a distal axonopathy [19,20]. Given that castration can rescue KD male mice from the disease well after its onset [15,19], cell death is likely a late event in KD and cell dysfunction may be at the root of this disorder.
Axonal trafficking defects have been implicated in many neurodegenerative diseases, including KD. In KD Tg mice, abnormal accumulations of neurofilament occur in distal motor axons before the onset of muscle weakness [26**]. Perhaps more tantalizing is finding the same abnormal accumulation of neurofilament in muscle of a KD patient. Katsuno and colleagues also show more directly that axonal trafficking is impaired, demonstrating androgen-dependent deficits in retrograde labeling of motoneurons and dynactin 1 expression in motoneurons. Dynactin 1 is part of the motor protein complex involved in retrograde trafficking in axons. Alterations in anterograde and/or retrograde axonal transport are also suggested by findings in other KD models [15,27,28]. Thus, an androgen-dependent failure in the trafficking of needed cargo along axons, either anterogradely to motor synapses or retrogradely to motoneuronal cell bodies, may be an early and pivotal event triggering the loss of motor function in KD.
Androgens may act in muscles to trigger KD
One great challenge of any chemical intervention is to eliminate the disease-causing action of an endogenous molecule while preserving its health-promoting actions. Thus, for KD, ideal treatments will likely be aimed at reducing mutant AR action in the cells where the disease begins while sparing the beneficial effects of AR in other tissues, such as those that support reproduction. Knowing where mutant ARs act to trigger KD would greatly facilitate the development of such treatments.
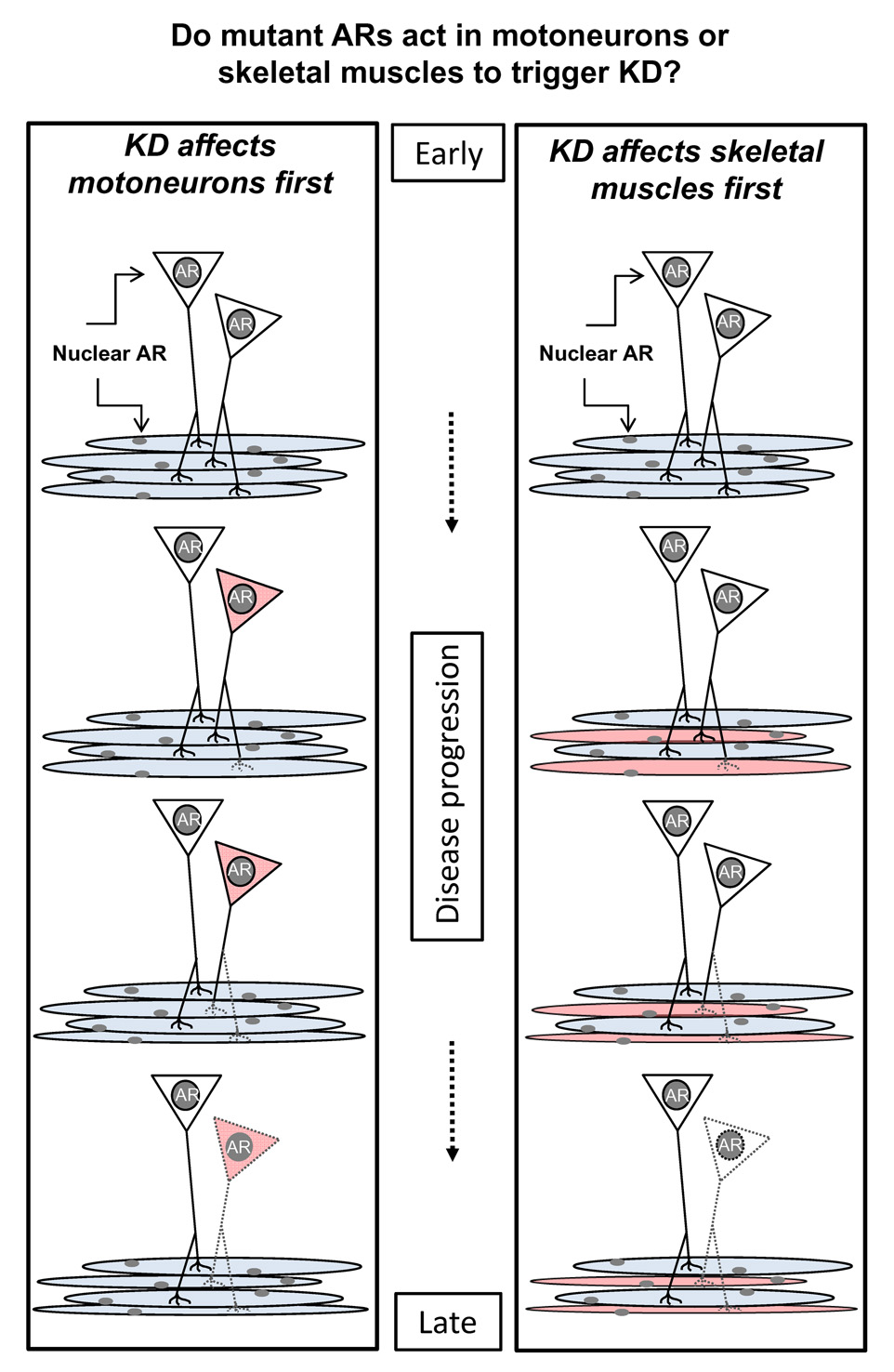
ARs are expressed by many cell types and tissues, including motoneurons and skeletal muscles [29]. Thus, mutant ARs might act in motoneurons, skeletal muscles or both to cause KD (Fig 1). However, it has long been assumed that ARs act in motoneurons to cause the disease, an idea driven by the fact that men with KD suffer what appears to be “neuogenic atrophy” of skeletal muscles caused by a loss of motoneurons. However, there is little direct evidence to support the idea that mutant ARs act only in motoneurons to initiate disease.
Figure 1.
Schematic showing two possible sites (motoneurons or muscle fibers) where mutant ARs might act to trigger Kennedy Disease (KD). Because motoneurons and muscle fibers each express ARs, each represents candidate sites for triggering KD. Mutant ARs could cause the same distal axonopathy (depicted by dashed lines for some motor axons and their terminals) by directly impairing the function of motoneurons or muscle fibers. In one case, distal axonopathy is caused by altered anterograde signaling in the affected motoneuron (shown in red in left panel) while in the other case, axonopathy is induced indirectly via changes in retrograde signals from the affected muscle fibers (shown in red in right panel). Based on several mouse models of KD, axonopathy occurs early in the disease process and may involve a perturbation in motoneuronal axonal transport. As this disease progresses, such axonopathy leads to functionally denervated muscle fibers, triggering muscle atrophy and motoneuronal cell death, two key pathological features of KD. It is also possible that AR acts in both cell types to induce the full complement of disease symptoms underlying KD and that AR action at both sites are critically involved. Studies are needed to fully discern the role of ARs in motoneurons and muscle fibers in causing KD.
One Tg model of KD expresses wildtype AR only in skeletal muscle fibers, but nonetheless shows an androgen-dependent motor phenotype, including the loss of motor axons akin to other mouse models of KD [19**]. That over-expression of the wildtype AR with 22 CAG repeats triggers disease similar to the expanded polyQ AR suggests that it is mediating toxicity by similar mechanisms, and raises the possibility that over-expression of AR in muscle could lead to human disease. Importantly, analysis of these Tg mice led to the conclusion that AR may act in muscle rather than motoneurons to trigger disease. Given that expression of a growth factor, vascular endothelial growth factor, is also reduced in muscles of these mice, the disease process may entail a loss of trophic support to motonerons by muscles. A similar loss of trophic factor expression has been documented in skeletal muscle from a KI mouse model of KD [20**]. While it will be important to test the role of ARs in muscle in other models of KD, these results provide the first direct demonstration that ARs acting in skeletal muscle fibers can trigger a KD phenotype and indirectly cause disease in the motoneurons, including axonal loss and defects in axonal transport (Kemp and Jordan, unpublished data). Such a scenario is in line with the well established role of muscles in maintaining motoneurons [30], and suggests that therapeutics preventing AR action in muscle may rescue both individuals and their motoneurons from KD.
Molecular targets for therapy emerge from the study of KD models
Hormone dependence
Since the KD phenotype is androgen-dependent, decreasing androgen levels should reduce disease severity. This notion was confirmed by treating KD Tg mice with leuprorelin, a luteinizing hormone-releasing hormone agonist that reduces serum testosterone levels [23**]. Clinical trials to treat KD patients with anti-androgens have been initiated in the United States and Japan, where modest results were reported after a short trial with leuprorelin [26]. The efficacy of dutasteride, an inhibitor of 5 alpha-reductase, the enzyme that converts testosterone to the more potent dihydrotestosterone, has not yet been reported.
Androgen receptor degradation
The misfolded, polyQ AR protein is the proximal mediator of toxicity in KD, and as such, therapeutic strategies that promote its degradation have attracted considerable attention. ARs associate with the molecular chaperones hsp90 and hsp70, components of the cellular defense against unfolded proteins and possible therapeutic targets. Geldenamycin and its derivatives that inhibit hsp90 impair ligand-dependent trafficking of the receptor, promote its degradation [31] and improve the phenotype of KD Tg mice [32]. Over-expression of hsp70 [33] or the chaperone-dependent E3 ubiquitin ligase CHIP (carboxy terminus of Hsc70-interacting protein) [34] similarly promote AR degradation to yield phenotypic improvement in KD Tg mice. Additional strategies to promote AR degradation include novel small molecules that disrupt the interaction of the receptor with co-regulators [35], and activation of protein turnover through autophagy [36].
Histone acetylation
Changes in gene expression and chromatin modification accompany cellular dysfunction in KD. These changes may be secondary responses to injury or manifestations of a primary pathway leading to toxicity. In either case, treating cellular [37] and mouse models [38] with histone deacetylase (HDAC) inhibitors, such as sodium butyrate, has proven effective in ameliorating the disease phenotype. Notably, broad spectrum HDAC inhibitors may also influence movement along microtubules that is influenced by HDAC6, a cytoplasmic HDAC. Since this movement machinery is needed for protein degradation, the mechanisms underlying HDACs’ therapeutic effects warrant further study.
Emerging targets
While small molecules offer great promise for treating KD, other strategies are under active investigation. Gene silencing by RNA interference can decrease production of toxic proteins and has proven to be effective in animal models of other polyQ diseases. Similarly, replacement of dysfunctional or dead neurons by stem cells is an attractive strategy whose potential remains to be fully explored.
Conclusion
Recent work in KD mouse models has identified a host of molecular pathways that modulate toxicity. Furthermore, the cell types mediating pathogenesis are being defined to include skeletal muscle fibers in addition to motoneurons. Given that skeletal muscles likely contribute to the disease phenotype and may mediate early pathogenic events, they offer a therapeutic target outside the blood brain barrier that is much more accessible to treatment than motoneurons. It is likely that a combination of targets and treatment strategies will prove to be the most effective therapeutic approach for KD and other chronic neurodegenerative disorders.
Supplementary Material
Table 1.
Characteristics and outcomes of six different mouse models of KD that express a full length AR having variable CAG repeat lengths and controlled by different promoters. Disease characteristics are noted for only males unless specified.
| citation describing model | Model type (Tg or KIa)/# CAGs/promote r | Sex bias? | Onset of motor symptom s | Motor symptoms | Disease-related changes in motoneurons/motor axons | Disease-related changes in muscle | sensory loss? | Depends on androgens? |
|---|---|---|---|---|---|---|---|---|
| (McManamny, Chy et al. 2002) | Tg /120/CMV | Disease severity: Tg♂= Tg♀ Onset age: Tg♂< Tg♀ (by wks) |
~3 wks | Clasping ↓cage activity ↓time on hang test |
↓# MNsb; ↓ MN size |
mfc type grouping & atrophy ↓ # of mfs |
No data | No data |
| (Katsuno, Adachi et al. 2002; Katsuno, Adachi et al. 2006) | Tg/97/ chicken β-actin promoter with CMV enhancer |
Disease severity: Tg♂ > Tg♀ Onset age: Tg♂ < Tg♀ (by wks) |
~8 wks | ↓ cage activity ↓ time on rotarod Impaired gait |
marginal loss of MNs ↓ MN size ↓ motor axon size ↓ retrograde axonal transport Accumulation of NFe in distal axons |
mf grouped atrophy small angulated mfs ↑ variability of mf size |
DRGs intact | castration prevents KD symptoms in Tg ♂s; Td induces KD symptoms in Tg ♀s; deficts in axonal transport depend on androgens |
| (Chevalier- Larsen, O'Brien et al. 2004) | Tg/112/prion | Disease severity: Tg♂ > Tg♀ Onset age: Tg♂ < Tg♀ (by mos) |
~6 wks | Clasping ↓ time on rotarod ↓ open field activity ↓ grip strength impaired gait |
altered NF stainingf (no change in size or # of MNs); | none | No data | Castration of Tg ♂s reverses deficits in motor function & MN NF staining |
| (Sopher, Thomas et al. 2004) | Tg/100/ human AR regulatory elements | Disease severity: Tg ♂ > Tg♀ Onset age: Tg♂ < Tg♀ (by mos) |
~6 mos | ↓time on hang test ↓stride length Impaired gait hindlimb weakness & paralysis |
MNs appear smaller, less densely stained and fewer in # ↓ expression of VEGFg in spinal cord |
hindlimb muscle atrophy ↑ mf staining for NADHh & NCAMi fiber type grouping |
No data | No data |
| (Yu, Dadgar et al. 2006) | KI/113/mouse AR regulatory elements | Disease severity: Tg ♂ > Tg♀ Onset age: Tg♂ < Tg♀ (by mos) |
~ 10 wks | ↓ grip strength | No evidence of cell death based on glial staining Abnormal EMG recordings indicative of neurogenic and primary myopathic processes |
↑ levels of AChRαj and myogenin mRNA ↓ levels of GDNFk and NT-4l mRNA |
No data | Castration of Tg ♂s partially rescues KI males from muscle weakness and prevents early death |
| (Monks, Johansen et al. 2007) | Tg/22/human skeletal actin α | Only Tg ♂ show a motor phenotype | From earliest age examined (peripubertally) | ↓time on hang test ↓ stride length Impaired gait ↓ time on rotarod |
↓motor axons Normal # of MN cell bodies |
↓ # of mfs Centralized nuclei split muscle fibers Fiber atrophy ↑ NADH staining ↑ levels of AChRα and myogenin mRNA ↓ level of VEGF mRNA |
No data | Castration of Tg males reverses disease symptoms T treatment of asymptomatic Tg females induces disease |
Tg = transgenic; KI = knock-in
MN = motoneuron cell bodies
mf = muscle fiber
T = testosterone
NF = neurofilament
altered NF staining = ↓ # of MNs that stain for unphosphorylated neurofilament- heavy chain
VEGF = vascular endothelial growth factor
NADH = nicotinamide adenine dinucleotide
NCAM = neural cell adhesion molecule
AChRα = acetylcholine receptor α
GDNF = glial-derived neurotrophic factor
NT-4 = neurotrophin-4
Acknowledgements
We thank Marc Breedlove for helpful comments on earlier versions of this manuscript. This work was supported by National Institute of Health grants NS045195 (CLJ) and NS055746 (APL), and a McKnight Neuroscience of Brain Disorders Award (APL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 2.Orr HT, Zoghbi HY. Trinucleotide Repeat Disorders. Annu Rev Neurosci. 2007 doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- 4.La Spada AR, Roling DB, Harding AE, Warner CL, Spiegel R, Hausmanowa-Petrusewicz I, Yee WC, Fischbeck KH. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992;2:301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- 5.Mariotti C, Castellotti B, Pareyson D, Testa D, Eoli M, Antozzi C, Silani V, Marconi R, Tezzon F, Siciliano G, et al. Phenotypic manifestations associated with CAG-repeat expansion in the androgen receptor gene in male patients and heterozygous females: a clinical and molecular study of 30 families. Neuromuscul Disord. 2000;10:391–397. doi: 10.1016/s0960-8966(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology. 2002;59:770–772. doi: 10.1212/wnl.59.5.770. [DOI] [PubMed] [Google Scholar]
- 7.Guidetti D, Vescovini E, Motti L, Ghidoni E, Gemignani F, Marbini A, Patrosso MC, Ferlini A, Solime F. X-linked bulbar and spinal muscular atrophy, or Kennedy disease: clinical, neurophysiological, neuropathological, neuropsychological and molecular study of a large family. J Neurol Sci. 1996;135:140–148. doi: 10.1016/0022-510x(95)00283-8. [DOI] [PubMed] [Google Scholar]
- 8.Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain. 1989;112(Pt 1):209–232. doi: 10.1093/brain/112.1.209. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima T, Seko K, Hirose K, Mannen T, Yoshimura S, Arima R, Nagashima K, Morimatsu Y. Familial bulbo-spinal muscular atrophy associated with testicular atrophy and sensory neuropathy (Kennedy-Alter-Sung syndrome). Autopsy case report of two brothers. J Neurol Sci. 1988;87:141–152. doi: 10.1016/0022-510x(88)90240-7. [DOI] [PubMed] [Google Scholar]
- 10.Soraru G, D'Ascenzo C, Polo A, Palmieri A, Baggio L, Vergani L, Gellera C, Moretto G, Pegoraro E, Angelini C. Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J Neurol Sci. 2008;264:100–105. doi: 10.1016/j.jns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Miwa S, Kobayashi Y, Merry DE, Yamamoto M, Tanaka F, Doyu M, Hashizume Y, Fischbeck KH, Sobue G. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol. 1998;44:249–254. doi: 10.1002/ana.410440216. [DOI] [PubMed] [Google Scholar]
- 12.Adachi H, Katsuno M, Minamiyama M, Waza M, Sang C, Nakagomi Y, Kobayashi Y, Tanaka F, Doyu M, Inukai A, et al. Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain. 2005;128:659–670. doi: 10.1093/brain/awh381. [DOI] [PubMed] [Google Scholar]
- 13**.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. These authors follow the fate of individual neurons that express mutant huntingtin protein. Contrary to expectation, they find that the formation of inclusion bodies predicts better survival of neurons then when inclusion bodies never form. Because the level of soluble protein within such neurons is lower once inclusion bodies form, they conclude that inclusion bodies may serve to protect neurons from the toxic effects of soluble mutant protein.
- 14*.Li M, Chevalier-Larsen ES, Merry DE, Diamond MI. Soluble androgen receptor Oligomers underlie pathology in a mouse model of spinobulbar muscular atrophy. J Biol Chem. 2007;282:3157–3164. doi: 10.1074/jbc.M609972200. These authors describe a series of experiments using a variety of methods including immunoprecipitation, Westerns and atomic force microscopy to show that soluble oligomers form in an androgen-dependent manner well before disease onset and that such oligomers can form proto-fibrillar structures which may underlie cell dysfunction in KD.
- 15.Chevalier-Larsen ES, O'Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, Merry DE. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24:4778–4786. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sang C, Kobayashi Y, Doyu M, Sobue G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–854. doi: 10.1016/s0896-6273(02)00834-6. These authors present data demonstrating for the first time that the KD phenotype in a mouse model is androgen-dependent. They show that castration of Tg males prevents the disease whereas testosterone treatment of Tg females “turns on” the disease.
- 17.McManamny P, Chy HS, Finkelstein DI, Craythorn RG, Crack PJ, Kola I, Cheema SS, Horne MK, Wreford NG, O'Bryan MK, et al. A mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2002;11:2103–2111. doi: 10.1093/hmg/11.18.2103. [DOI] [PubMed] [Google Scholar]
- 18.Sopher BL, Thomas PS, Jr, LaFevre-Bernt MA, Holm IE, Wilke SA, Ware CB, Jin LW, Libby RT, Ellerby LM, La Spada AR. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41:687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- 19**.Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci U S A. 2007;104:18259–18264. doi: 10.1073/pnas.0705501104. These investigators describe a Tg mouse model that expresses normal AR exclusively in skeletal muscle fibers. Unexpectedly, such mice show an androgen-dependent loss of motor function comparable to that shown by other KD mouse models. The authors conclude that AR may act in muscle fibers to trigger KD.
- 20**.Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP. Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest. 2006;116:2663–2672. doi: 10.1172/JCI28773. This report describes a knock-in mouse model of KD, in which gene targeting was used to introduce 113 CAG repeats into the AR gene. Mutant males exhibited androgen-dependent weakness associated with the early onset of myogenic and neuropathic changes in muscle and loss of trophic factor production. These pathologic changes preceded the occurrence of motoneuron loss, and suggested that disease arising in muscle significantly contributes to the KD phenotype.
- 21.Kinirons P, Rouleau GA. Administration of testosterone results in reversible deterioration in Kennedy's disease. J Neurol Neurosurg Psychiatry. 2008;79:106–107. doi: 10.1136/jnnp.2006.101899. [DOI] [PubMed] [Google Scholar]
- 22**.Takeyama K, Ito S, Yamamoto A, Tanimoto H, Furutani T, Kanuka H, Miura M, Tabata T, Kato S. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron. 2002;35:855–864. doi: 10.1016/s0896-6273(02)00875-9. These authors show in a drosophila model that the polyglutamine expanded AR causes neurodegeneration in an androgen-dependent manner but that the effect of androgen can be blocked by preventing AR from entering the nucleus. These results suggest that the role of androgens in AR toxicity is to promote translocation of AR to the nucleus where toxicity of AR is then realized.
- 23**.Katsuno M, Adachi H, Doyu M, Minamiyama M, Sang C, Kobayashi Y, Inukai A, Sobue G. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003;9:768–773. doi: 10.1038/nm878. Using a Tg mouse model of KD, this report established that anti-androgen therapy could ameliorate the disease phenotype. The authors showed that treatment with Leuprorelin, an LHRH agonist, reduced testosterone levels and decreased the behavioral and histological manifestations of the disease. This study proved the scientific basis for two clinical trials of KD with anti-androgens.
- 24.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 25.Hsiao PW, Lin DL, Nakao R, Chang C. The linkage of Kennedy's neuron disease to ARA24, the first identified androgen receptor polyglutamine region-associated coactivator. J Biol Chem. 1999;274:20229–20234. doi: 10.1074/jbc.274.29.20229. [DOI] [PubMed] [Google Scholar]
- 26**.Katsuno M, Adachi H, Minamiyama M, Waza M, Tokui K, Banno H, Suzuki K, Onoda Y, Tanaka F, Doyu M, et al. Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration. J Neurosci. 2006;26:12106–12117. doi: 10.1523/JNEUROSCI.3032-06.2006. Investigating a Tg mouse model of KD, these authors present convergent evidence based on a number of different methodologies (e.g., retrograde labeling of motoneurons, quantitative RT-PCR, and immunocytochemistry) suggesting that androgens induce a loss of motor function by perturbing axonal trafficking, and present evidence that such axonopathy may also occur in humans with KD.
- 27.Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- 28.Szebenyi G, Morfini GA, Babcock A, Gould M, Selkoe K, Stenoien DL, Young M, Faber PW, MacDonald ME, McPhaul MJ, et al. Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron. 2003;40:41–52. doi: 10.1016/s0896-6273(03)00569-5. [DOI] [PubMed] [Google Scholar]
- 29.Jordan CL, Doncarlos L. Androgens in health and disease: an overview. Horm Behav. 2008;53:589–595. doi: 10.1016/j.yhbeh.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 31.Thomas M, Harrell JM, Morishima Y, Peng HM, Pratt WB, Lieberman AP. Pharmacologic and genetic inhibition of hsp90-dependent trafficking reduces aggregation and promotes degradation of the expanded glutamine androgen receptor without stress protein induction. Hum Mol Genet. 2006;15:1876–1883. doi: 10.1093/hmg/ddl110. [DOI] [PubMed] [Google Scholar]
- 32.Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- 33.Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, et al. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci. 2003;23:2203–2211. doi: 10.1523/JNEUROSCI.23-06-02203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi H, Waza M, Tokui K, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J Neurosci. 2007;27:5115–5126. doi: 10.1523/JNEUROSCI.1242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 36.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 37.McCampbell A, Taye AA, Whitty L, Penney E, Steffan JS, Fischbeck KH. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc Natl Acad Sci U S A. 2001;98:15179–15184. doi: 10.1073/pnas.261400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamiyama M, Katsuno M, Adachi H, Waza M, Sang C, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Sobue G. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2004;13:1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.