Abstract
Study Objectives:
The trigeminal nuclear complex (V) contains cholinergic neurons and includes the principal sensory trigeminal nucleus (PSTN) which receives sensory input from the face and jaw, and the trigeminal motor nucleus (MoV) which innervates the muscles of mastication. Pain associated with pathologies of V is often managed with opioids but no studies have characterized the effect of opioids on acetylcholine (ACh) release in PSTN and MoV. Opioids can increase or decrease ACh release in brainstem nuclei. Therefore, the present experiments tested the 2-tailed hypothesis that microdialysis delivery of opioids to the PSTN and MoV significantly alters ACh release.
Design:
Using a within-subjects design and isoflurane-anesthetized Wistar rats (n = 53), ACh release in PSTN during microdialysis with Ringer's solution (control) was compared to ACh release during dialysis delivery of the sodium channel blocker tetrodotoxin, muscarinic agonist bethanechol, opioid agonist morphine, mu opioid agonist DAMGO, antagonists for mu (naloxone) and kappa (nor-binaltorphimine; nor-BNI) opioid receptors, and GABAA antagonist bicuculline.
Measurements and Results:
Tetrodotoxin decreased ACh, confirming action potential-dependent ACh release. Bethanechol and morphine caused a concentration-dependent increase in PSTN ACh release. The morphine-induced increase in ACh release was blocked by nor-BNI but not by naloxone. Bicuculline delivered to the PSTN also increased ACh release. ACh release in the MoV was increased by morphine, and this increase was not blocked by naloxone or nor-BNI.
Conclusions:
These data comprise the first direct measures of ACh release in PSTN and MoV and suggest synaptic disinhibition as one possible mechanism by which morphine increases ACh release in the trigeminal nuclei.
Citation:
Zhu Z; Bowman HR; Baghdoyan HA; Lydic R. Morphine increases acetylcholine release in the trigeminal nuclear complex. SLEEP 2008;31(12):1629–1637.
Keywords: Orofacial sensory processing, upper airway, opioid receptors, GABAergic disinhibition
THE PRINCIPAL SENSORY TRIGEMINAL NUCLEUS (PSTN) IS A COMPONENT OF THE TRIGEMINAL NUCLEAR COMPLEX THAT CONVEYS INFORMATION ABOUT oral and facial sensations.1,2 Some patients with obstructive sleep apnea have abnormal orofacial sensory processing, which may contribute to development of apneas and hypopneas.3 The tooth-grinding movements of nocturnal bruxism are generated by the trigeminal motor nucleus (MoV) and create jaw pressures that are compatible with sleep only if sensory processing is somehow diminished.4,5
Sleep is disrupted by pain, and understanding neurotransmission in PSTN and MoV is relevant for clinical management of orofacial pain. For example, temporomandibular joint disorder is characterized by chronic myofacial and jaw pain, restricted range of jaw motion or jaw locking, and popping jaw sounds. The sharp, recurring pain associated with trigeminal neuralgia is also a challenge for pain management. Cancer of the head and neck is the fifth most common type of cancer,6 and cancer pain is commonly managed with opioids. Unwanted opioid side effects, including sleep disruption,7 emphasize the need for developing alternative or adjunctive drugs for pain management.8,9
Available evidence indicates a complex relationship between acetylcholine (ACh), opioids, and sensorimotor processing by the trigeminal nuclear complex. For example, recordings of trigeminal sensory neurons across states of rapid eye movement (REM) sleep, non-REM (NREM) sleep, and wakefulness have revealed suppression of sensory processing during REM sleep.10 Furthermore, orofacial sensory processing also varies as a function of sensory modality.11 There are reciprocal projections between the trigeminal motoneurons and regions of the pontine reticular formation12 where ACh increases during REM sleep.13,14 Interestingly, ACh activates some and inhibits other trigeminal sensory neurons.15 These complexities, and the fact that no previous studies have measured ACh release in PSTN and MoV, encouraged the present study testing the hypothesis that opioids alter ACh release in the trigeminal nuclear complex. The results revealed that morphine increased ACh release in both PSTN and MoV. Portions of these data have been presented in abstract form.16,17
METHODS
Animals, Chemicals, and Microdialysis Probes
All experiments followed the Public Health Service Policy on Humane Care and Use of Laboratory Animals (NIH Publication 80-23, 1996) and were approved by the University of Michigan Committee on Use and Care of Animals. Adult male Wistar rats (mean weight = 292 g; n = 53) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in a temperature- and humidity-controlled room on a 12-h light/dark cycle and had free access to food and water.
Isoflurane was supplied by Hospira, Inc., (Lake Forest, IL). Sigma-Aldrich (St. Louis, MO) was the source of chemicals used for mobile phase, Ringer's solution, ACh, and histology. Naloxone hydrochloride, carbamyl-β-methylcholine chloride (bethanechol), and [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin acetate salt (DAMGO) also were obtained from Sigma-Aldrich. Morphine sulfate was supplied by Hawkins Chemical, Inc. (Minneapolis, MN). Nor-binaltorphimine (nor-BNI) and bicuculline came from Tocris Bioscience (Ellisville, MO), and tetrodotoxin was purchased from Calbiochem (La Jolla, CA). All drug solutions used for microdialysis were made immediately prior to use.
Microdialysis probes (CMA/11) were purchased from CMA Microdialysis (North Chelmsford, MA). The cuprophane membrane was 1 mm long, 0.24 mm in diameter, and had a molecular weight cutoff of 6000 Dalton. Dialysis probes were perfused continuously (2 μL/min) with Ringer's solution (147 μM NaCl, 2.4 μM CaCl2, 4.0 μM KCl, 10 μM neostigmine, pH 5.8–6.2) using a CMA/400 syringe pump. Before and after each dialysis experiment, in vitro recovery of ACh by the dialysis probe was quantified.18 If recovery changed significantly in the same direction as the drug-induced change in ACh, the data were discarded. This procedure ensured that ACh release data were not confounded by alterations in dialysis probe function. ACh recovery by probes used for these studies averaged between 5.4% and 5.5%.
Procedures, Experiment Design, and Quantification of ACh
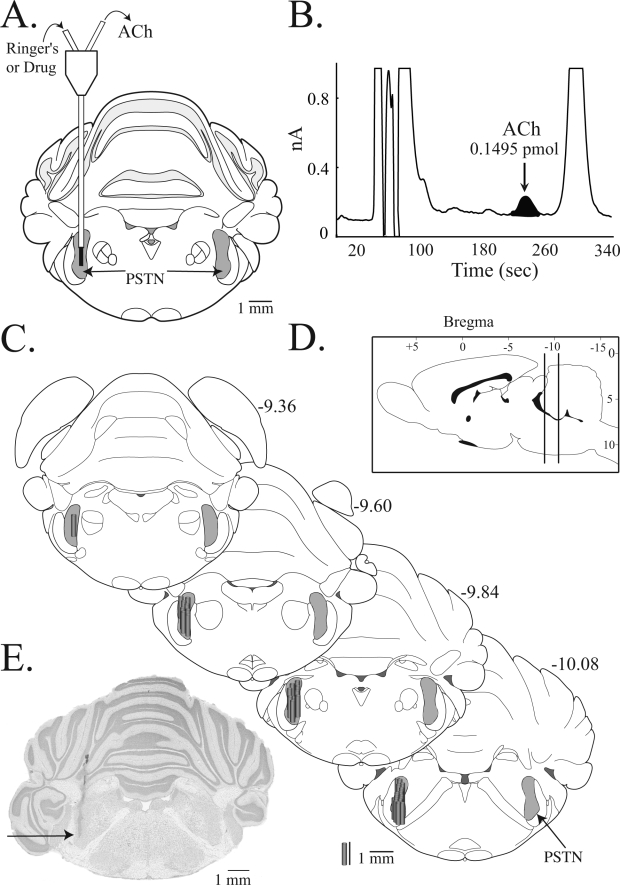
As has been previously described,18 rats were anesthetized in a plexiglass chamber using isoflurane delivered in 100% oxygen. Following the loss of righting response, rats were placed in a small-animal stereotaxic frame (David Kopf Instruments, Tujunga, CA), and isoflurane was delivered via a Kopf rat anesthesia mask. A Cardiocap/5 spectrometer (Datex-Ohmeda, Madison, WI) provided a continuous measure of delivered isoflurane concentration, which was maintained at 1.5%. A rectal thermometer, a T/Pump Heat Therapy System (Gaymar, TP-500 model, Orchard Park, NY), and a circulating water blanket were used to hold core body temperature at 37°C. A midline scalp incision exposed the skull and a craniotomy provided access to the underlying brainstem. For PSTN experiments, microdialysis probes were aimed 9.84 mm posterior to bregma, 2.95 mm lateral to the midline, and 9.10 mm ventral to bregma19 (Figure 1A). The stereotaxic coordinates used to aim the dialysis probe for the MoV were 9.5 mm posterior to bregma, 1.6 mm lateral to midline, and 8.7 mm ventral to bregma.19
Figure 1.
Acetylcholine (ACh) chromatography and localization of principal sensory trigeminal nucleus (PSTN) microdialysis sites. A. This schematic coronal diagram of the rat brainstem19 was modified to illustrate a dialysis probe placed in the PSTN (shaded area). The dialysis membrane (black) is drawn to scale. Probes were perfused with Ringer's solution (control) or drug solution through the inlet tubing, and samples were collected from the outlet tubing for quantification of ACh. B. A typical chromatogram illustrates elution of an ACh peak (arrow) and the excellent signal-to-noise ratio. The area under the peak is proportional to the amount of ACh in the sample. C. All 33 PSTN microdialysis sites are plotted as cylinders on this series of coronal diagrams modified from a rat brain atlas.19 Numbers at the top right of each coronal diagram indicate mm posterior to bregma. Gray cylinders representing microdialysis probe membranes are drawn to scale. D. Sagittal drawing of the rat brain with vertical lines denoting the approximate anterior-to-posterior extent of the PSTN dialysis sites. E. A cresyl violet-stained coronal section with a black arrow pointing to the deepest part of the microdialysis site. Similar cresyl violet-stained sections were evaluated to create the summary shown in part C.
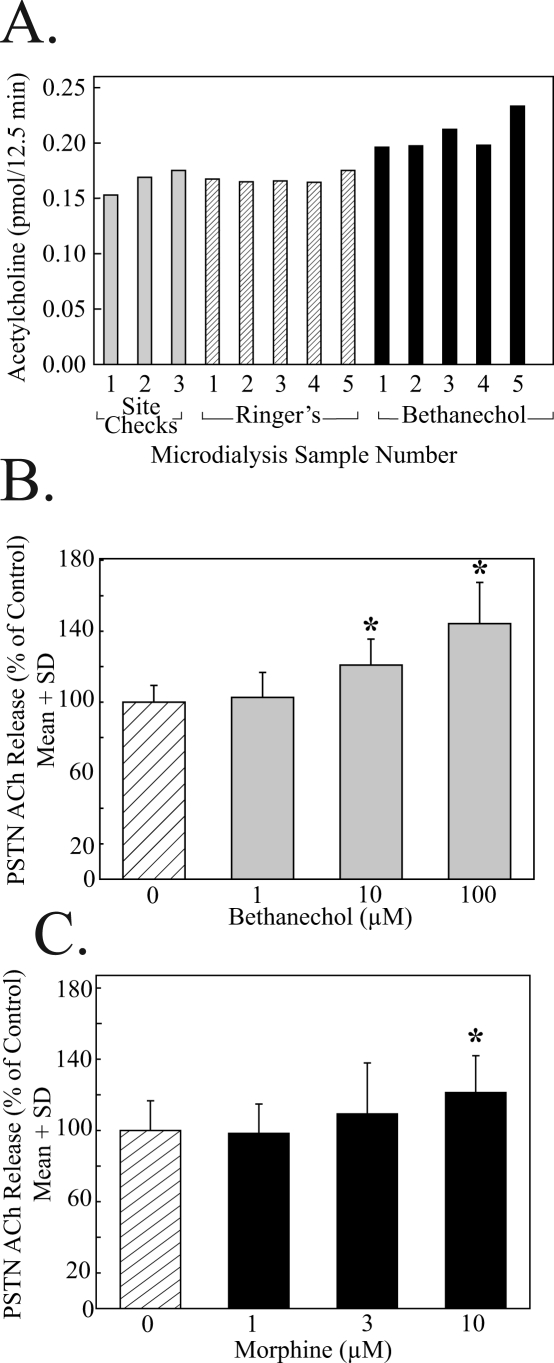
Each rat was used for 1 experiment in a within-subjects design. Only 1 drug combination or 1 concentration of a single drug was tested per experiment. Figure 2A illustrates ACh measurement from a representative experiment. The present and previous data18 demonstrate that ACh measures (25 μL/12.5 min) were stable within about 40 minutes after the dialysis probe was placed in the brainstem. These initial 3 samples (Figure 2A, site checks) were used to confirm that ACh could be measured from the brain region into which the dialysis probe had been stereotaxically positioned. Continuing the 2 μL/min microdialysis rate, we obtained 5 samples during dialysis with Ringer's solution (control) followed by collection of 5 samples during dialysis drug delivery (Fig 2A, Bethanecol).
Figure 2.
Acetylcholine (ACh) release in the principal sensory trigeminal nucleus (PSTN) was increased during dialysis delivery of bethanechol or morphine. A. Time course of a single, representative, microdialysis experiment quantifying ACh release in PSTN during dialysis with Ringer's solution (control) followed by dialysis delivery of the muscarinic cholinergic agonist bethanechol. One rat was used for each experiment, and, for a given experiment, the 5 ACh values associated with the Ringer's solution condition were averaged together to provide mean control ACh levels. Similarly, the 5 ACh values measured during dialysis drug delivery were averaged together to obtain mean druginduced ACh levels. Multiple animals were tested for each drug concentration. These data were averaged together, and inferential statistics were used to evaluate the hypothesis that ACh release varied as a function of drug concentration. B. Group data showing that bethanechol caused a significant (*) concentration-dependent increase in PSTN ACh release. Each concentration of bethanechol was delivered to the PSTN of 3 rats. C. Dialysis delivery of morphine to the PSTN significantly (*) increased ACh release. Each concentration of morphine was tested using 3 animals.
For the present experiments, the PSTN was dialyzed with Ringer's solution (control) followed by Ringer's solution containing the sodium channel blocker tetrodotoxin (1 μM), the muscarinic receptor agonist bethanechol (1, 10, and 100 μM), the opioid receptor agonist morphine (1, 3, and 10 μM), morphine (10 μM) in combination with the opioid receptor antagonist naloxone (1 μM), morphine (10 μM) coadministered with the kappa opioid receptor antagonist nor-BNI (1 μM), the mu opioid receptor agonist DAMGO (10 μM), or the gamma aminobutyric acid (GABA) type A receptor antagonist bicuculline (300 μM). For the MoV experiments, samples were obtained during dialysis with Ringer's solution (control) followed by dialysis with Ringer's solution containing morphine (10 μM), morphine (10 μM) in combination with either 1 μM or 10 μM naloxone, morphine (10 μM) coadministered with nor-BNI (1 μM), or nor-BNI alone (1 μM).
ACh in microdialysis samples was quantified by high performance liquid chromatography with electrochemical detection (HPLC-ECD), and these procedures have been described.18,20–22 Figure 1B shows a typical HPLC chromatogram generated from a sample collected during dialysis of the PSTN with Ringer's solution (control).
Data Analyses
The anatomic location of each dialysis probe was identified histologically to ensure that all measures of ACh were obtained from either the PSTN or MoV. Two to 3 days after the ACh measures were obtained, the rats were deeply anesthetized with isoflurane and decapitated. The brains were removed and frozen for histologic processing. The brainstem was cut into 40 micron coronal sections using a Leica CM3050S cryostat (Leica Microsystems, Nussloch, Germany). Brain sections were serially collected, mounted on glass slides, and stained with cresyl violet. Each slide was examined microscopically and compared to a rat brain atlas.19 This made it possible to identify the glial scar localizing the dialysis site (Figure 1E) relative to the boundaries of the PSTN (Figure 1A) and MoV (Figure 4A). The summaries of microdialysis sites (Figures 1 and 4) illustrate that stereotaxic placement of the dialysis probe was considered a success only if at least 50% of the final 1 mm of the glial scar was within the boundaries of the PSTN or MoV. Neurochemical data from experiments that did not satisfy this criterion were not included in this report.
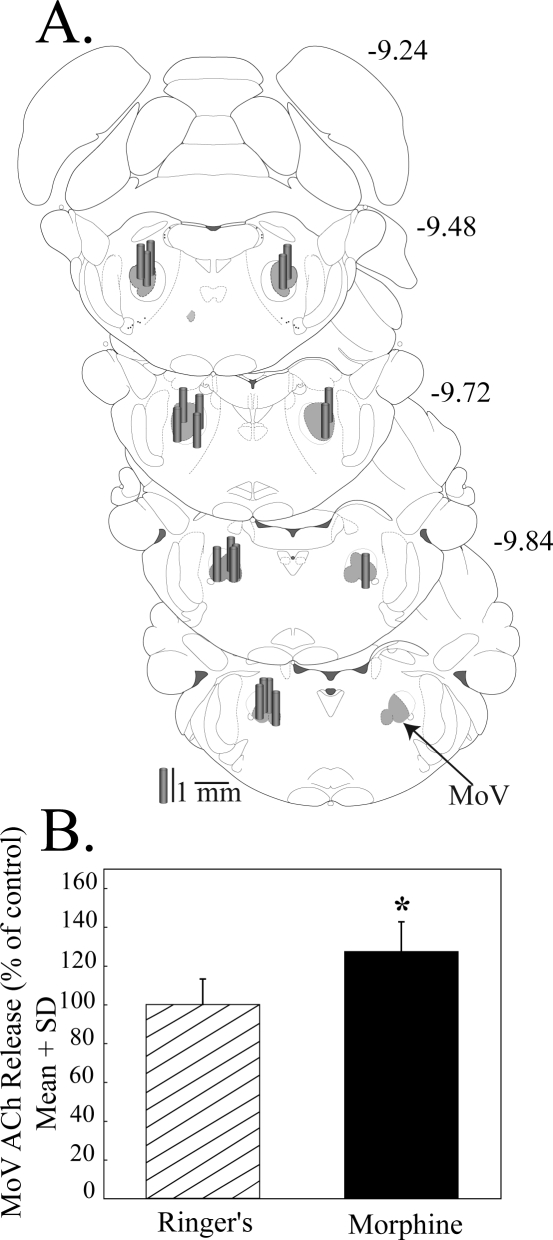
Figure 4.
Microdialysis delivery of morphine to the trigeminal motor nucleus (MoV) increased acetylcholine (ACh) release. A. Coronal brainstem diagrams modified from a rat brain atlas19 are used to illustrate dialysis probe placement in MoV (shaded area). Numbers at the top right of each diagram indicate mm posterior to bregma. Dialysis probe membranes are drawn to scale as gray cylinders. B. Morphine increased ACh release compared to Ringer's solution (control). ACh was measured in 5 animals.
The effects of drugs delivered by microdialysis to PSTN or MoV on local ACh release were analyzed using descriptive and inferential statistics (GBStat™ v.6.5.6, Dynamic Microsystems, Inc., Silver Spring, MD) and Statistical Analysis System v9.1.3, (SAS Institute, Inc., Cary, NC). One-way analysis of variance (ANOVA) for repeated measures and Dunnett's posthoc multiple comparisons test or Student's t-test were used to determine whether administered drugs altered ACh release. For the baseline condition (Ringer's solution) of a given experiment, the mean ACh value during dialysis with Ringer's solution minus the ACh value from each dialysis sample, divided by the mean, provided an index of sample variability. The ACh release data are plotted as mean ± SD, and a P-value of ≤ 0.05 was considered statistically significant.
RESULTS
Only ACh measures obtained from dialysis probes that were histologically confirmed to have been placed in the PSTN or MoV were included in the results reported below. Figure 1C plots the location of the PSTN dialysis sites. Average ± SD stereotaxic coordinates for the PSTN sites were 9.88 ± 0.21 mm posterior to bregma, 2.97 ± 0.15 mm from the midline, and 9.06 ± 0.14 mm below the skull surface. The mean ± SD stereotaxic coordinates for MoV sites (Figure 4A) were 9.55 ± 0.23 mm posterior to bregma, 2.02 ± 0.23 mm from the midline, and 8.58 ± 0.28 mm below the skull surface.
Tetrodotoxin Delivered to the PSTN Significantly Decreased PSTN ACh Release and Bethanechol Caused a Concentration-dependent Increase in PSTN ACh Release
To evaluate whether PSTN ACh measured by microdialysis reflects synaptic release, initial experiments delivered tetrodotoxin to the PSTN while measuring ACh. Tetrodotoxin significantly (t = 5.2; df = 4; P < 0.01) decreased (66%) ACh in PSTN, indicating action potential-dependent release (data not shown).
PSTN contains cholinergic terminals15 as well as muscarinic receptors,23 and the second set of experiments was designed to quantify the effects of a cholinergic agonist on PSTN ACh release. Microdialysis delivery of the muscarinic agonist bethanechol to the PSTN caused a statistically significant (F = 40.8; df = 3, 86; P < 0.0001) concentration-dependent increase in PSTN ACh release (Figure 2B). Dunnett's analysis indicated a statistically significant (P < 0.01) increase in ACh caused by dialysis with 10 μM and 100 μM bethanechol, compared with control (0 μM bethanechol).
Microdialysis Delivery of Morphine to PSTN Increased ACh Release in PSTN
Dialysis administration of morphine to the PSTN caused a statistically significant (F = 5.2; df = 3, 86; P = 0.002) concentration-dependent increase in PSTN ACh release (Figure 2C). Dunnett's test revealed that dialysis with 10 μM of morphine significantly (P < 0.01) increased ACh release over control (0 μM morphine) levels.
The opioid receptor antagonists naloxone and nor-BNI each were administered to the PSTN in combination with morphine in an effort to identify the opioid receptor subtype by which morphine increased PSTN ACh release. Figure 3A illustrates that dialysis with morphine (10 μM) plus naloxone (1 μM) did not block the morphine-induced increase in PSTN ACh release. In contrast, dialysis with morphine (10 μM) plus the kappa opioid antagonist nor-BNI (1 μM) did block the morphine-induced increase in PSTN ACh release (Figure 3A). As an additional test of the possibility that the morphine-induced increase in PSTN ACh release was not mediated by mu opioid receptors, the selective mu opioid receptor agonist DAMGO (10 μM) was administered by dialysis into the PSTN. DAMGO did not alter PSTN ACh release (data not shown).
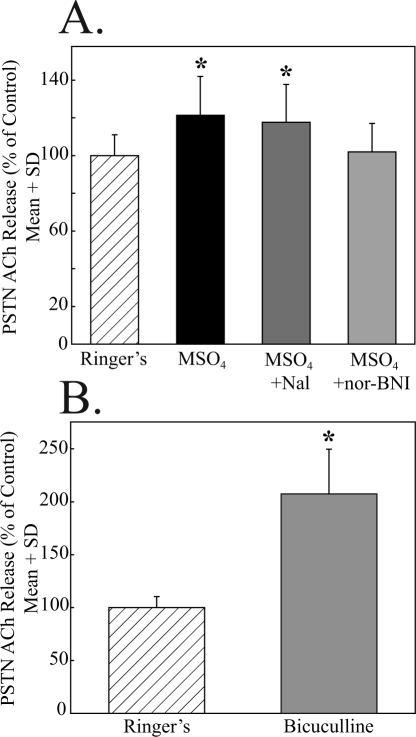
Figure 3.
Non-mu opioid and GABAA receptors modulate acetylcholine (ACh) release in the principal sensory trigeminal nucleus (PSTN). A. The significant (*) increase in PSTN ACh release caused by morphine (MSO4) was not blocked by the opioid antagonist naloxone (MSO4 + Nal) but was blocked by coadministration of the kappa opioid antagonist nor-binaltorphimine (MSO4 + norBNI). B. Relative to control (dialysis with Ringer's solution), the GABAA receptor antagonist bicuculline significantly (*) increased ACh release in the PSTN. The data in A and B were obtained from 12 rats, and each drug condition summarizes results from 3 rats.
The finding that PSTN ACh release was increased by bethanechol and morphine (Figure 2) raised the question of whether muscarinic and opioid receptor activation increased ACh release via disinhibition. Opioids have been shown to increase dopamine release in some brain regions by inhibiting the output of inhibitory GABAergic interneurons.24 The potential role of GABAergic disinhibition in PSTN was investigated by dialysis delivery of the GABAA receptor antagonist bicuculline. Figure 3B shows that ACh release in PSTN was significantly (t = 2.96; df = 4; P < 0.01) increased (108%) by local administration of bicuculline.
Microdialysis Delivery of Morphine to MoV Increased ACh Release in MoV
Figure 4B shows that, as in PSTN, dialysis with morphine (10 μM) caused a statistically significant (27%) increase in MoV ACh release (t = 2.3, df = 48, P = 0.03). Naloxone (1 μM) plus morphine (10 μM) did not block the increase in MoV ACh release (data not shown). Increasing the concentration of coadministered naloxone to 10 μM failed to block the morphine-induced increase in MoV ACh release (data not shown). Unlike the effects in PSTN, coadministration of the kappa antagonist nor-BNI (1 μM) plus morphine (10 μM) into MoV of 3 animals caused an average 79% increase in ACh release. Additional experiments (n = 4) revealed that dialysis with 1 μM nor-BNI alone caused a statistically significant (61%) increase in MoV ACh release, compared to Ringer's control (t = 3.95, df = 6, P = 0.001).
DISCUSSION
These experiments provide the first microdialysis measures of ACh release in the PSTN and MoV of Wistar rat. After a brief consideration of methodologic limitations, the results are discussed in relation to the effects of opioids on cholinergic neurotransmission within the trigeminal nuclear complex.
Methodologic Considerations and Limitations
In vivo microdialysis has revolutionized measurement of endogenous neurotransmitters.25,26 When neurotransmitter measures are combined with dialysis drug delivery, concentration-response and antagonist-blocking experiments can unmask presynaptic and postsynaptic receptor actions and can even identify the receptor subtypes mediating those actions.20,27–29 Ef-forts to characterize drug effects on endogenous neurotransmitters using in vivo microdialysis also must confront a number of methodologic limitations. As noted elsewhere,18,22,30,31 dialysis delivery of morphine concentrations greater than 10 μM interferes with electrochemical detection of ACh. Therefore, the present studies of ACh release in PSTN and MoV had an upper concentration limit of 10 μM morphine. Another limitation of microdialysis, compared with microinjection, is that the actual nanogram amount of drug delivered to the brain by microdialysis can only be estimated. As noted in the Methods section, CMA/11 microdialysis probes have a 6000 Dalton cutoff that limits both analyte recovery and drug delivery. Percentage probe recovery was calculated for every dialysis probe used in this study and probe recoveries averaged about 5%. In addition to limiting ACh recovery, this fact means that only about 5% of the drug concentrations used to perfuse the dialysis probe were delivered to the brain.25 The present study was limited to a focus on ACh, and it should be clear that the chemical identity of PSTN and MoV neurons is heterogeneous and complex.32–34 In addition, the trigeminal nuclear complex is comprised of many subdivisions,35,36 and the spatial resolution of all microdialysis studies is limited by dialysis probe size.25 For all microdialysis studies, the small size of the brainstem nuclei relative to the size of currently available microdialysis probes is a limitation. Consistent with other studies,18,37 when histologic analyses revealed that less than 50% of the dialysis probe had been positioned within the PSTN or MoV (Figures 1 and 4), the ACh data were not included in the analyses.
ACh Release was Decreased by Tetrodotoxin and Increased by Bethanechol
Tetrodotoxin is a sodium channel blocker38 that eliminates the propagation of action potentials. Thus, the decreased ACh caused by tetrodotoxin indicates that ACh measured by microdialysis reflected action potential-dependent release, rather than changes in ACh turnover. This finding is consistent with results of previous studies demonstrating tetrodotoxin-confirmed ACh release in pontine reticular formation regions adjacent to trigeminal nuclear complex.21
Electrophysiologic data demonstrate that cholinergic receptor agonists and antagonists alter the excitability of PSTN neurons.15 Using a brain slice preparation, current-voltage data were obtained from PSTN neurons before and after bath application of the mixed (nicotinic and muscarinic) cholinergic agonist carbachol.15 Carbachol evoked changes in current flow from PSTN neurons that were either inward (depolarizing), outward (hyperpolarizing), or biphasic (hyperpolarizing followed by depolarizing).15 These studies next sought to determine which subtype of muscarinic receptor caused the carbachol-evoked changes in current. There are 5 subtypes of muscarinic cholinergic receptors (M1 thru M5), and a challenge for efforts to specify the functional role of these receptors is the present lack of antagonists that are highly selective for only a single muscarinic receptor subtype.27,39 By systematically combining a cholinergic agonist with a range of cholinergic antagonists, one can make inferences regarding muscarinic receptor subtype mediation of the agonist-evoked response. The antagonist blocking experiments supported the conclusion that M1 receptors mediate the depolarizing currents and that M2 receptors mediate the hyperpolarizing currents recorded from PSTN neurons.15 These elegant experiments focusing on individual neurons clearly demonstrate the complex synaptic organization within the PSTN.
In contrast to the study of single neurons, the limited spatial resolution of microdialysis25,26 makes it an ideal technique for evaluating the overall effect of a drug on a population of neurons. Therefore, the present study was designed to determine whether dialysis delivery of the purely muscarinic receptor agonist bethanechol to PSTN alters ACh release in the PSTN. Bethanechol caused a concentration-dependent increase in PSTN ACh release (Figure 2A). Muscarinic receptors are present in trigeminal nucleus,23 and immunostaining studies have identified cholinergic terminals in PSTN and MoV.15 PSTN contains M1 and M2 subtypes of muscarinic receptors,15 and activation of M2 receptors by the mixed cholinergic agonist carbachol hyperpolarizes PSTN neurons.15 If GABAergic neurons in the PSTN express M2 muscarinic receptors, agonist binding to these receptors would cause hyperpolarization, resulting in removal of GABAergic inhibition. Previous studies in rat have shown that muscarinic receptors can inhibit release of GABA.40 The results shown in Figure 2A support and extend results obtained from brain slice preparations15 by showing that dialysis delivery of the muscarinic agonist bethanechol to the PSTN increases the release of endogenous ACh within PSTN. The finding that bethanechol enhances ACh release in PSTN has functional implications for the neurobiology of sleep and breathing. Microinjection of bethanechol into adjacent regions of the pontine reticular formation causes a greater than 360% increase in the REM sleep-like state and a 37% decrease in minute ventilation.41 This is consistent with evidence that the excitability of some trigeminal neurons is depressed10,11,42 dur-ing REM sleep, a time when cholinergic tone in the pontine reticular formation is high.43
Morphine Increased PSTN ACh Release by Actions at Non-Mu Opioid Receptors
The antinociceptive actions of opioids result from binding to presynaptic and postsynaptic receptors and decreasing excitability of neurons comprising pain pathways.44 Morphine can exert analgesic effects by binding to mu opioid receptors. However, morphine also binds in a concentration-dependent manner to other opioid receptor subtypes.45 An additional complexity for efforts to elucidate opioid effects on cholinergic neurotransmission is that multiple receptor systems modulate morphine-induced alterations in ACh release.46 In the present study, the morphine-induced increase in ACh release within PSTN and MoV was not blocked by naloxone (Figure 3A). The morphine-induced increase in PSTN ACh release was blocked by the kappa opioid receptor antagonist nor-BNI.
By what mechanism might morphine cause an increase in ACh release in both PSTN and MoV? Morphine can increase neurotransmitter release by a disinhibitory process that involves hyperpolarizing inhibitory interneurons.24 Anatomic data demonstrate that opioid and GABAA receptors are coexpressed in the mesencephalic trigeminal nucleus of rat brain.47 The finding that dialysis delivery of the GABAA receptor antagonist bicuculline increased ACh release (Figure 3B), considered together with the morphine-induced increase in ACh release in PSTN and MoV, suggests but does not prove GABAergic modulation of ACh release via disinhibition. This possibility is supported by immunohistochemical data that provide conclusive evidence for the presence of GABAergic interneurons in MoV.48 The finding that nor-BNI blocked the morphine-induced increase in PSTN ACh release suggests the possibility that morphine exerts its effects by activating kappa opioid receptors localized to GABAergic neurons or terminals within PSTN. The present data cannot explain the inability of naloxone or nor-BNI to block the morphine-induced increase in MoV ACh release. The results are novel, however, in demonstrating differences between two functionally interacting nuclei within the trigeminal nuclear complex.
Summary and Potential Clinical Relevance
Simultaneous ACh measurement and dialysis drug delivery to the trigeminal nuclear complex produced three novel findings. (1) The ACh measures represent action potential-dependent release, and dialysis delivery of the muscarinic agonist bethanechol to the PSTN caused a concentration-dependent increase in ACh release within the PSTN. (2) Dialysis delivery of morphine to the PSTN caused a concentration-dependent, naloxone-resistant increase in ACh release that was blocked by nor-BNI. The same concentration of morphine that increased ACh release in PSTN also increased ACh release in MoV. The morphine-induced increase in MoV ACh release was not blocked by naloxone or nor-BNI. (3) The GABAA receptor antagonist bicuculline increased ACh release in PSTN, suggesting that the morphine-induced increase in ACh release may be mediated, in part, by disinhibiting GABAergic input.
Trigeminal neurochemical transmission is functionally relevant for sleep because trigeminal sensorimotor processing modulates breathing and because sleep is disrupted by both orofacial pain and opioid treatment of pain. State-dependent hypotonia of tongue and upper airway muscles associated with sleep and opioid use can contribute to oropharyngeal obstruction. Clinical evidence that abnormal orofacial sensory processing can exacerbate apneas3 fits well with data showing that PSTN neurons project to the hypoglossal nucleus.49 The present finding that morphine increases ACh release in PSTN and MoV is consistent with evidence that morphine also increases ACh release in hypoglossal nuclei.18 Thus, morphine significantly enhances cholinergic neurotransmission in two cranial nerve nuclei that regulate upper airway patency.
Opioids are extensively and safely used for pain management yet there is an unmet need for developing opioids that are selective for receptor subtypes and that lack unwanted side effects.9 Sleep disruption is a major complaint of patients experiencing pain50 and opioids significantly disrupt normal sleep.7 Clinically relevant doses of opioids increase light NREM sleep (stage 2), decrease deep sleep (stage 4 NREM), and decrease REM sleep.51–56 Opioids also blunt wakefulness and slow the cortical electroencephalogram.57–61 Sleep loss and restriction reduce emotional and physical well-being62 and intensify pain.63–67 Thus, pain and opioids disrupt sleep, and sleep disruption enhances perception of painful stimuli. The need for a mechanistic understanding of opioid action is further emphasized by evidence that only 40% of the more than 50 million Americans who have chronic pain can obtain adequate relief68 and by the 21 million people in the US who, each year, have operations that require management of acute pain.69
The potential clinical relevance of trigeminal cholinergic neurotransmission also is emphasized by many lines of evidence suggesting cholinergic drugs as candidates for adjunctive treatment of pain. Data obtained from clinical70–74 and preclinical75–82 studies concur that cholinomimetics can decrease nociception. Muscarinic cholinergic receptors have been localized to PSTN83–85 and the adjacent oral and caudal pontine reticular nuclei84–86 where ACh release is increased during REM sleep.14 REM sleep is characterized by primary afferent depolarization and depressed neuronal excitability involving presynaptic and postsynaptic inhibition in the trigeminal nuclear complex.2 Application of muscarinic receptor antagonists to PSTN neurons alters the excitability of PSTN neurons.15 Thus, the data obtained from a brain slice preparation15 are supported by the present finding that dialysis delivery of bethanechol to the PSTN increased ACh release (Figure 2A). The additional discovery that morphine increased ACh release in PSTN (Figure 2B) suggests that some of the antinociceptive actions of morphine on orofacial pain may be cholinergically mediated. This possibility is testable, and the present data encourage future studies designed to determine whether supraspinal cholinergic neurotransmission can modulate nociceptive processing by the trigeminal nuclear complex.
ACKNOWLEDGMENTS
This work was supported by NIH grants HL57120, HL40881, MH45361, HL65272, and the Department of Anesthesiology. We thank S. Jiang and M.A. Norat for expert assistance and K. Welch from the University of Michigan Center for Statistical Consultation and Research.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Lydic has received research support from Sepracor. Dr. Baghdoyan has received research support from Sepracor. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sessle BJ, Greenwood LF. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: I. Responses to innocuous and noxious stimuli. Brain Res. 1976;117:211–26. doi: 10.1016/0006-8993(76)90731-9. [DOI] [PubMed] [Google Scholar]
- 2.Soja PJ. Modulation of prethalamic sensory inflow during sleep versus wakefulness. In: Lavigne G, Choinière M, Sessle BJ, Soja PJ, editors. Sleep and Pain. Seattle, WA: International Association for the Study of Pain Press; 2007. pp. 45–76. [Google Scholar]
- 3.Guilleminault C, Huang YS, Kirisoglu C, Chan A. Is obstructive sleep apnea syndrome a neurological disorder? A continuous positive airway pressure follow-up study. Ann Neurol. 2005;58:880–7. doi: 10.1002/ana.20654. [DOI] [PubMed] [Google Scholar]
- 4.Bader G, Lavigne G. Sleep bruxism; an overview of an oromandibular sleep movement disorder. Sleep Med Rev. 2000;4:27–43. doi: 10.1053/smrv.1999.0070. [DOI] [PubMed] [Google Scholar]
- 5.Lavigne GF, Guitard F, Rompre PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237–44. doi: 10.1046/j.1365-2869.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 6.Mignogna MD, Fedele S, Lo Russo L. The World Cancer Report and the burden of oral cancer. Eur J Cancer Prev. 2004;13:139–42. doi: 10.1097/00008469-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lydic R, Baghdoyan HA. Neurochemical mechanisms mediating opioid-induced REM sleep disruption. In: Lavigne G, Choinière M, Sessle BJ, Soja PJ, editors. Sleep and Pain. Seattle, WA: International Association for the Study of Pain Press; 2007. pp. 99–122. [Google Scholar]
- 8.Berde CB, Brennan TJ, Raja SN. Opioids: more to learn, improvements to be made. Anesthesiology. 2003;98:1309–12. doi: 10.1097/00000542-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006;147:S153–S62. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns BE, Fragoso MC, Soja PJ. Activity of rostral trigeminal sensory neurons in the cat during wakefulness and sleep. J Neurophysiol. 1995;73:2486–98. doi: 10.1152/jn.1995.73.6.2486. [DOI] [PubMed] [Google Scholar]
- 11.Cairns BE, McErlane SA, Fragoso MC, Jia WG, Soja PJ. Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep. J Neurosci. 1996b;16:8149–59. doi: 10.1523/JNEUROSCI.16-24-08149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Bo G, Lund JP, Verdier D, Kolta A. Inputs to nucleus pontis caudalis from adjacent trigeminal areas. Eur J Neurosci. 2005;22:1987–96. doi: 10.1111/j.1460-9568.2005.04371.x. [DOI] [PubMed] [Google Scholar]
- 13.Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–85. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–R54. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- 15.Kohlmeier KA, Soja PJ, Kristensen MP. Disparate cholinergic currents in rat principal trigeminal sensory nucleus neurons mediated by M1 and M2 receptors: a possible mechanism for selective gating of afferent sensory neurotransmission. Eur J Neurosci. 2006;23:3245–58. doi: 10.1111/j.1460-9568.2006.04875.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Baghdoyan HA, Lydic R. Morphine increases principal sensory trigeminal nucleus (PSTN) acetylcholine (ACh) release in anesthetized Wistar rat via GABAergic disinhibition. Sleep. 2007;30:005. (Abstract Suppl) [Google Scholar]
- 17.Bowman HR, Zhu Z, Baghdoyan HA, Lydic R. Acetylcholine release in rat trigeminal motor nucleus is increased by microdialysis delivery of morphine. FASEB J. 2008;22:LB148. [Google Scholar]
- 18.Skulsky E, Osman NI, Baghdoyan HA, Lydic R. Microdialysis delivery of morphine to the hypoglossal nucleus of Wistar rat increases hypoglossal acetylcholine release. Sleep. 2007;30:562–9. doi: 10.1093/sleep/30.5.566. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. 4th ed. New York, NY: Academic Press; 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 20.Douglas CL, Baghdoyan HA, Lydic R. M2 muscarinic autoreceptors modulate acetylcholine release in prefrontal cortex of C57BL/6J mouse. J Pharmacol Exp Ther. 2001;299:960–6. [PubMed] [Google Scholar]
- 21.Coleman CG, Lydic R, Baghdoyan HA. Acetylcholine release in the pontine reticular formation of C57BL/6J mouse is modulated by non-M1 muscarinic receptors. Neuroscience. 2004;126:831–8. doi: 10.1016/j.neuroscience.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Wamsley JK, Zarbin MA, Kuhar MJ. Distribution of muscarinic cholinergic high and low affinity agonist binding sites: a light microscopic autoradiographic study. Brain Res Bull. 1984;12:233–43. doi: 10.1016/0361-9230(84)90051-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarizing local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters using microdialysis sampling. Anal Chem. 2006;78:1391–9. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 26.Westerink BHC, Cremers TIFH. San Diego, CA: Academic Press; 2007. Handbook of Microdialysis: Methods, Applications and Clinical Aspects; pp. 1–697. [Google Scholar]
- 27.Baghdoyan HA, Fleegal MA, Lydic R. M2 muscarinic autoreceptors regulate acetylcholine release in the pontine reticular formation. J Pharmacol Exp Ther. 1998;286:1446–52. [PubMed] [Google Scholar]
- 28.Douglas CL, Baghdoyan HA, Lydic R. Postsynaptic muscarinic M1 receptors activate prefrontal cortical EEG of C57BL/6J mouse. J Neurophysiol. 2002;88:3003–9. doi: 10.1152/jn.00318.2002. [DOI] [PubMed] [Google Scholar]
- 29.Coleman CG, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem. 2006;96:1750–9. doi: 10.1111/j.1471-4159.2006.03700.x. [DOI] [PubMed] [Google Scholar]
- 30.Mortazavi S, Thompson J, Baghdoyan HA, Lydic R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology. 1999;90:1070–7. doi: 10.1097/00000542-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Bernard R, Lydic R, Baghdoyan HA. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate G protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–71. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- 32.Waite PME, Tracey DJ. Trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. San Diego, CA: Academic Press; 1995. pp. 705–24. [Google Scholar]
- 33.Avendaño C, Machín R, Bermejo PE, Lagares A. Neuron numbers in trigeminal nuclei of the rat: A GABAand glycine-immunocytochemical and stereological analysis. J Comp Neurol. 2005;493:538–53. doi: 10.1002/cne.20778. [DOI] [PubMed] [Google Scholar]
- 34.Lewinter RD, Scherrer G, Basbaum AI. Dense transient receptor potential cation channel, vanilloid family, type 2 (TRPV2) immunoreactivity defines a subset of motoneurons in dorsal lateral nucleus of the spinal cord, the nucleus ambiguus and the trigeminal motor nucleus in rat. Neuroscience. 2008;151:164–73. doi: 10.1016/j.neuroscience.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cropper EC, Eisenman JS, Azmitia EC. 5-HT-Immunoreactive fibers in the trigeminal nuclear complex. Exp Brain Res. 1984;5:515–22. doi: 10.1007/BF00235282. [DOI] [PubMed] [Google Scholar]
- 36.Olzewski J. On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol. 1950;92:401–13. doi: 10.1002/cne.900920305. [DOI] [PubMed] [Google Scholar]
- 37.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–81. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 39.Baghdoyan HA, Lydic R. M2 muscarinic receptor subtype in the feline medial pontine reticular formation modulates the amount of rapid eye movement sleep. Sleep. 1999;22:835–47. doi: 10.1093/sleep/22.7.835. [DOI] [PubMed] [Google Scholar]
- 40.Sugita S, Uchimura N, Jiang Z, North RA. Distinct muscarinic receptors inhibit release of gamma aminobutyric acid and excitatory amino acids in mammalian brain. Proc Nat Acad Sci. 1991;88:2608–11. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee LH, Friedman DB, Lydic R. Respiratory nuclei share synaptic connectivity with pontine reticular regions regulating REM sleep. Am J Physiol. 1995;268:L251–62. doi: 10.1152/ajplung.1995.268.2.L251. [DOI] [PubMed] [Google Scholar]
- 42.Cairns BE, Fragoso MC, Soja PJ. Active-sleep related suppression of feline trigeminal sensory neurons: evidence implicating presynaptic inhibition via a process of primary afferent depolarization. J Neurophysiol. 1996a;75:1152–62. doi: 10.1152/jn.1996.75.3.1152. [DOI] [PubMed] [Google Scholar]
- 43.Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: a synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. The Neurochemistry of Sleep and Wakefulness. Cambridge, UK: Cambridge University Press; 2008. pp. 109–43. [Google Scholar]
- 44.Yaksh TL. The spinal action of opioids. In: Herz A, editor. Handbook of Experimental Pharmacology. New York, NY: Springer-Verlag; 1993. pp. 53–90. [Google Scholar]
- 45.Gutstein HB, Akil H. Opioid analgesics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2006. pp. 547–90. [Google Scholar]
- 46.Kushida K, Ishida K, Kikuta J, Kato M, Uchiyama T, Taguchi K. Alpha 2-adrenoceptor modulates the release of acetylcholine from the rostral ventrolateral medulla in response to morphine. Biol Pharm Bull. 2003;26:1548–51. doi: 10.1248/bpb.26.1548. [DOI] [PubMed] [Google Scholar]
- 47.Kalyuzhny AE, Dooyema J, Wessendorf MW. Opioid- and GABAA-receptors are co-expressed by neurons in rat brain. Neuroreport. 2000;11:2625–8. doi: 10.1097/00001756-200008210-00004. [DOI] [PubMed] [Google Scholar]
- 48.McDavid S, Lund JP, Auclair F, Kolta A. Morphological and immunohistochemical characterization of interneurons within the rat trigeminal motor nucleus. Neuroscience. 2006;139:1049–59. doi: 10.1016/j.neuroscience.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Aldes LD, Boone TB. Organization of projections from the principal sensory trigeminal nucleus to the hypoglossal nucleus in the rat: an experimental light and electron microscopic study with axonal tracer techniques. Exp Brain Res. 1985;59:16–29. doi: 10.1007/BF00237661. [DOI] [PubMed] [Google Scholar]
- 50.Bonica JJ. Vol. I. Philadelphia, PA: Lea – Febiger; 1990. The Management of Pain. [Google Scholar]
- 51.Aakerlund LP, Rosenberg J. Postoperative delirium: treatment with supplementary oxygen. Br J Anaesth. 1994;72:286–90. doi: 10.1093/bja/72.3.286. [DOI] [PubMed] [Google Scholar]
- 52.Bonafide CP, Aucutt-Walter N, DiVittore N, King T, Bixler EO, Cronin AJ. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology. 2008;108:627–33. doi: 10.1097/ALN.0b013e3181684bc3. [DOI] [PubMed] [Google Scholar]
- 53.Kay DC, Eisenstein RB, Jasinski DR. Morphine effects on human REM state, waking state, and NREM sleep. Psychopharmacologica (Berlin) 1969;14:404–16. doi: 10.1007/BF00403581. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev. 2001;5:129–37. doi: 10.1053/smrv.2000.0121. [DOI] [PubMed] [Google Scholar]
- 55.Shaw IR, Lavigne G, Mayer P, Choinière M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28:677–82. doi: 10.1093/sleep/28.6.677. [DOI] [PubMed] [Google Scholar]
- 56.Walder B, Tramèr MR, Blois R. The effects of two single doses of tramadol on sleep: a randomized, cross-over trial in healthy volunteers. Eur J Anaesthesiol. 2001;18:36–42. doi: 10.1046/j.1365-2346.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 57.Arankowsky-Sandoval G, Gold PE. Morphine-induced deficits in sleep patterns: attenuation by glucose. Neurobiol Learn Mem. 1995;64:133–8. doi: 10.1006/nlme.1995.1052. [DOI] [PubMed] [Google Scholar]
- 58.Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–21. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- 59.Mayo-Michelson L, Young GA. Genetic profiles of morphine-induced EEG, EEG power spectra, and behavior in two inbred rat strains. Brain Res Bull. 1993;30:79–84. doi: 10.1016/0361-9230(93)90041-9. [DOI] [PubMed] [Google Scholar]
- 60.Menefee LA, Cohen MJA, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1:156–72. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 61.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Onen SH, Alloui D, Jourdan D, Eschalier A, Dubray C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001;900:261–7. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 64.Chiu YH, Silman AJ, Macfarlane GJ, et al. Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain. 2005;115:316–21. doi: 10.1016/j.pain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 66.Baghdoyan HA. Hyperalgesia induced by REM sleep loss: a phenomenon in search of a mechanism. Sleep. 2006;29:137–9. [PubMed] [Google Scholar]
- 67.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 68.Phillips DM. JCAHO pain management standards are unveiled. JAMA. 2000;284:428–9. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- 69.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 70.Katayama Y, Tsubokawa T, Hirayama T, Yamamoto T. Pain relief following stimulation of the pontomesencephalic parabrachial region in humans: brain sites for nonopiate-mediated pain control. Appl Neurophysiol. 1985;48:195–200. doi: 10.1159/000101127. [DOI] [PubMed] [Google Scholar]
- 71.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Hood DD, Mallak KA, James RL, Tuttle R, Eisenach JC. Enhancement of analgesia from systemic opioid in humans by spinal cholinesterase inhibition. J Pharmacol Exp Ther. 1997;282:86–92. [PubMed] [Google Scholar]
- 73.Eisenach JC. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–54. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- 74.Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;101:1417–21. doi: 10.1097/00000542-200412000-00023. [DOI] [PubMed] [Google Scholar]
- 75.Harvig P, Gillberg PG, Gordh T, Post C. Cholinergic mechanisms in pain and analgesia. Trends Pharmacol Sci. 1989;10(Suppl):75–9. [PubMed] [Google Scholar]
- 76.Klamt JG, Prado WA. Antinociception and behavioral changes induced by carbachol microinjected into identified sites of the rat brain. Brain Res. 1991;549:9–18. doi: 10.1016/0006-8993(91)90593-k. [DOI] [PubMed] [Google Scholar]
- 77.Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid eye movement sleep. Sleep. 1998;21:677–85. doi: 10.1093/sleep/21.7.677. [DOI] [PubMed] [Google Scholar]
- 78.Ma H-C, Dohi S, Wang Y-F, Ishizawa Y, Yanagidate F. The antinociceptive and sedative effects of carbachol and oxycodone administered into brainstem pontine reticular formation and spinal subarachnoid space in rats. Anesth Analg. 2001;92:1307–15. doi: 10.1097/00000539-200105000-00043. [DOI] [PubMed] [Google Scholar]
- 79.Ortega-Legaspi JM, Lopez-Avila A, Coffeen U, del Angel R, Pellicer F. Scopolamine into the anterior cingulate cortex diminishes nociception in a neuropathic pain model in the rat: an interruption of ‘nociception-related memory acquisition’? Eur J Pain. 2003;7:425–9. doi: 10.1016/s1090-3801(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 80.Dussor GO, Helesic G, Hargreaves KM, Flores CM. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain. 2004;107:22–32. doi: 10.1016/j.pain.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 81.Prado WA, Segalla DK. Antinociceptive effects of bethanechol or dimethylphenylpiperazinium in models of phasic or incisional pain in rats. Brain Res. 2004;1018:272–82. doi: 10.1016/j.brainres.2004.05.085. [DOI] [PubMed] [Google Scholar]
- 82.Lynch JJ, Wade CL, Mikusa JP, Decker MW, Honore P. ABT-594 (a nicotinic acetylcholine agonist): anti-allodynia in a rat chemotherapy-induced pain model. Eur J Pharm. 2005;509:43–8. doi: 10.1016/j.ejphar.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 83.Wamsley JK, Lewis MS, Young WS, 3rd, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. 1981;1:176–91. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baghdoyan HA, Mallios VJ, Duckrow RB, Mash DC. Localization of muscarinic receptor subtypes in brain stem areas regulating sleep. Neuroreport. 1994;5:1631–4. doi: 10.1097/00001756-199408150-00022. [DOI] [PubMed] [Google Scholar]
- 85.Capece ML, Baghdoyan HA, Lydic R. Carbachol stimulates [35S]guanylyl 5′-(gamma-thio)-triphosphate binding in rapid eye movement sleep-related brainstem nuclei of rat. J Neurosci. 1998;18:3779–85. doi: 10.1523/JNEUROSCI.18-10-03779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeMarco GJ, Baghdoyan HA, Lydic R. Differential cholinergic activation of G proteins in rat and mouse brainstem: relevance for sleep and nociception. J Comp Neurol. 2003;457:175–84. doi: 10.1002/cne.10548. [DOI] [PubMed] [Google Scholar]