Abstract
Study Objective:
To produce a compromise circadian phase position for permanent night shift work in which the sleepiest circadian time is delayed out of the night work period and into the first half of the day sleep period. This is predicted to improve night shift alertness and performance while permitting adequate late night sleep on days off.
Design:
Between-subjects.
Setting:
Home and laboratory.
Participants:
24 healthy subjects.
Interventions:
Subjects underwent 3 simulated night shifts, 2 days off, and 4 more night shifts. Experimental subjects received five, 15 minute bright light pulses from light boxes during night shifts, wore dark sunglasses when outside, slept in dark bedrooms at scheduled times after night shifts and on days off, and received outdoor afternoon light exposure (the “light brake”). Control subjects remained in normal room light during night shifts, wore lighter sunglasses, and had unrestricted sleep and outdoor light exposure.
Measurements and Results:
The final dim light melatonin onset (DLMO) of the experimental group was ∼04:30, close to our target compromise phase position, and significantly later than the control group at ∼00:30. Experimental subjects performed better than controls, and slept for nearly all of the allotted time in bed. By the last night shift, they performed almost as well during the night as during daytime baseline. Controls demonstrated pronounced performance impairments late in the night shifts, and exhibited large individual differences in sleep duration.
Conclusions:
Relatively inexpensive and feasible interventions can produce adaptation to night shift work while still allowing adequate nighttime sleep on days off.
Citation:
Smith MR; Eastman CI. Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off. SLEEP 2008;31(12):1639–1645.
Keywords: Human, shift work, circadian rhythms, bright light, melatonin, sleep, performance
SCHEDULED EXPOSURE TO LIGHT AND DARKNESS ARE ESTABLISHED METHODS OF PHASE SHIFTING THE CIRCADIAN CLOCK, AND HAVE BEEN USED IN laboratory and field studies to facilitate entrainment to a night work and day sleep schedule.1–8 Shifting the circadian clock to reduce the misalignment with daytime sleep and night work is an effective countermeasure for the alertness and performance decrements that are prevalent during night shifts.9 Although appropriately timed bright light and sleep/dark has been shown to completely shift the circadian clock to reestablish a normal phase relationship with a daytime sleep period,10 these interventions have not been widely applied in the real world. One problem may be the long duration of bright light used in most experimental studies (e.g. 7.5 h per night shift2). In the current study we used brief 15-min periods of light exposure totaling only 1.25 h per night shift. Another problem limiting the feasibility of translating laboratory protocols to the field with real shift workers is that complete circadian adjustment to night work leaves the night shift worker completely out of phase with the daytime-working world on days off and makes it difficult for a night worker to interact with friends and family.11
We therefore tested the feasibility of a compromise phase position for permanent night shift work in which the circadian clock is delayed to only partially align with the day sleep period. This partial entrainment should attenuate the performance and alertness decrements during night shifts and permit sufficient daytime sleep after night shifts.9 Importantly, because entrainment to the delayed sleep schedule is partial, a compromise phase position is also conducive to late nighttime sleep on days off.
We scheduled night shifts from 23:00 – 07:00, with sleep starting at 08:30 after night shifts and at 03:00 on days off. In this schedule, we define a compromise phase position as a dim light melatonin onset (DLMO) at ∼03:00. At this phase position, the body temperature minimum (Tmin), which occurs about 7 h after the DLMO,12–20 would fall at ∼10:00. The Tmin is an estimate for the sleepiest circadian time. A Tmin of ∼ 10:00 means that the sleepiest circadian time is early in the sleep period after night shifts and late in the sleep period on days off. Our goal was thus to delay the sleepiest circadian time out of the night work period so that it fell within sleep episodes after night shifts and on days off, but to keep it from delaying too far, beyond the scheduled sleep episodes on days off.
In the current study circadian phase was assessed before and after 7 night shifts interspersed with a weekend off. Final circadian phase for the experimental group was hypothesized to be later than that of a control group, and close to the compromise circadian phase position. The experimental group was also predicted to perform better than the control group during night shifts and sleep longer during the daytime.
METHODS
The present study is study # 3 in a series of studies (each a between-subjects design with an experimental group and a control group and each involving different groups of subjects). These studies assessed circadian phase, sleep, and performance at different time points during a sequence consisting of blocks of night shifts alternating with days off. The methodology for the other studies in this series has been described elsewhere: for study 0,21 for study 1,22 and for study 2.23 Study 1 describes the common methods in the most detail. All the studies were run between May and October.
Subjects
Twenty-four healthy subjects completed the present study. The experimental and control groups (n = 12 in each group, 5 male in each group) had similar morningness-eveningness24 scores (56.3 ± 9.3 [SD] and 53.6 ± 6.3, respectively). All subjects were young, but the experimental group (28.9 ± 5.8 y) was significantly older than the control group (23.7 ± 3.6), P = 0.04. Subjects were nonsmokers, had a BMI < 30 kg/m2, habitually drank < 300mg caffeine/day, and did not take prescription medication, except for 5 female subjects who used hormonal contraceptives. A urine toxicology screen at the start of the study confirmed that subjects were free from recreational drug use. Subjects did not work night shifts in the 3 months preceding the study and did not travel across more than 3 time zones in the month preceding the study. The Rush University Medical Center Institutional Review Board approved this study. All subjects provided written informed consent.
Sunglasses
All subjects wore sunglasses at all times when outside during daylight hours. Subjects in the control group wore lightly tinted sunglasses (average visible light transmission [VLT] of 36%, varying from 0% at 400 nm to about 55% at 650 nm). Sunglasses for experimental subjects had darker lenses (15% average VLT, varying from 0% at 400 nm to about 25% at 650 nm). Sunglasses for experimental subjects more strongly attenuated short wavelength light. The spectral transmission graphs of both of these lenses have been published.22
Baseline Sleep and Morning Light Schedule
Subjects maintained a structured sleep/wake schedule with outdoor morning light exposure for 15 baseline days before the baseline phase assessment (Figure 1). On weeknights during the baseline, subjects remained in bed in the dark from 23:00–07:00. On weekends, subjects were permitted to go to sleep between 23:00–00:00, wake up between 07:00–08:00, and nap between 13:30–16:30. After the baseline phase assessment on days 15–16 (described below) subjects resumed the structured sleep/wake schedule with morning light exposure for an additional 6 days before beginning simulated night shift work. Night shifts began one week after the baseline phase assessment. Study 0 of this series21 showed that one week after the baseline phase assessment, circadian phase was similar to what it was during the baseline phase assessment. For this reason, the position of the DLMO and DLMOff assessed during the baseline phase assessment is a good approximation for their position before the first night shift.
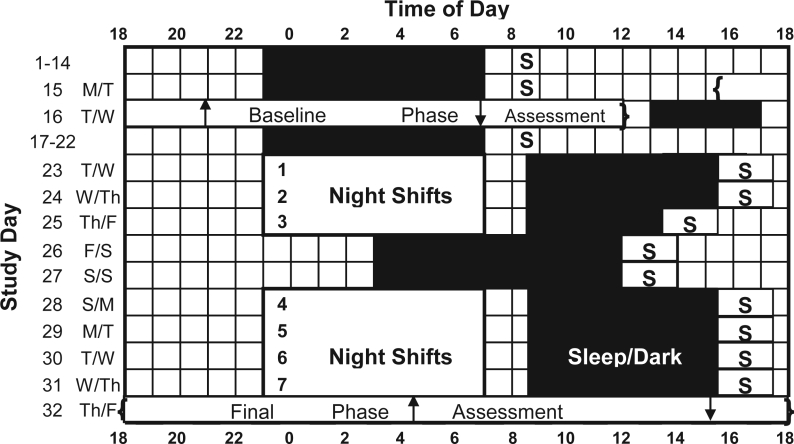
Figure 1.
Diagram illustrating the study protocol. The sleep schedule shown during the section with night shifts (starting with day 23) is for the experimental group only. Control subjects followed the same baseline sleep schedule and sequence of night shifts and days off but chose when to sleep starting with day 23. On days 1–22 the “S” indicates that all subjects were required to go outside for ≥15 minutes of sunlight between 8:00 and 9:00. Experimental subjects received brief bright light pulses during night shifts, designed to delay circadian rhythms, while control subjects remained in room light. Upward arrows show the dim light melatonin onset (DLMO), and downward arrows show the offset (DLMOff) for the experimental group during the baseline phase assessment (day 16) and the final phase assessment (day 32). After day 23, the “S” symbols depict the “light brake” for experimental subjects, designed to keep circadian rhythms from delaying too far. In the text, study day numbers correspond to the rows shown in the figure, the 24 h from 18:00 to 18:00.
Night Shift Light
Subjects underwent 7 simulated night shifts (23:00 to 07:00, study days 23–25 and 28–31). Subjects in the experimental groups were exposed to five 15-min intermittent bright light pulses each night shift. The first pulse began at 00:45 and the last pulse ended at 05:00. Pulses were interspersed by 45 min of normal room light (< 50 lux). Four light boxes (Sun Ray, Sun Box Company, Inc) containing fluorescent lamps (5095K) were positioned around a table at which subjects were seated. At a typical distance and angle of gaze, the average illuminance was ∼ 4100 lux, the average irradiance was ∼ 1200 μW/cm2, and the average photon density was ∼ 3.1 × 1015 photons/cm2/second. Subjects in the control group remained in normal room light during the night shifts.
Daytime Sleep and Afternoon Light Schedule
After night shifts began on day 23, experimental subjects were required to remain in bed during scheduled times in bedrooms that we made dark by putting black plastic over their windows. Sleep was scheduled from 08:30 to 15:30 (7 h) after the first 2 night shifts, from 08:30 to 13:30 (5 h) after the third night shifts, from 03:00 to 12:00 (9 h) on the 2 weekend days off, and again from 08:30 to 15:30 after the final 4 night shifts (Fig 1). Experimental subjects were required to go outside for ≥ 15 minutes of light exposure within 2 h after the end of these scheduled sleep periods (the “light brake”). Sleep and light exposure for control subjects was unrestricted.
Circadian Phase Assessments
The baseline phase assessment occurred from 15:30 on day 15 until 12:00 on day 16. The final 24-h phase assessment began at 18:00 on day 32. Light intensity was < 5 lux during phase assessments. Saliva was sampled every 30 min using a salivette (Sarstedt, Newton, NC). Samples were frozen until shipped on dry ice to Pharmasan Labs (Osceola, WI), where they were radioimmunoassayed for melatonin. The sensitivity of the assay was 0.7 pg/mL. The intra-assay variability was 12.1%, and the inter-assay variability was 13.2%. Additional details of the phase assessment procedures have been published elsewhere.22
Performance Testing
Measurements of performance were assessed during both day and night shifts. During 3 day shifts on study days 17, 18, and 21, subjects completed the Automated Neurophysiological Assessment Metrics (ANAM) test battery25,26 on desktop computers beginning at 10:05, 12:05, 14:05, and 16:05. During night shifts, the ANAM battery was administered beginning at 00:05, 02:05, 04:05, and 06:05 (see Figure 2 in reference23). The ANAM battery included simple reaction time (SRT), procedural memory, code substitution, mathematical processing and matching sample tasks.
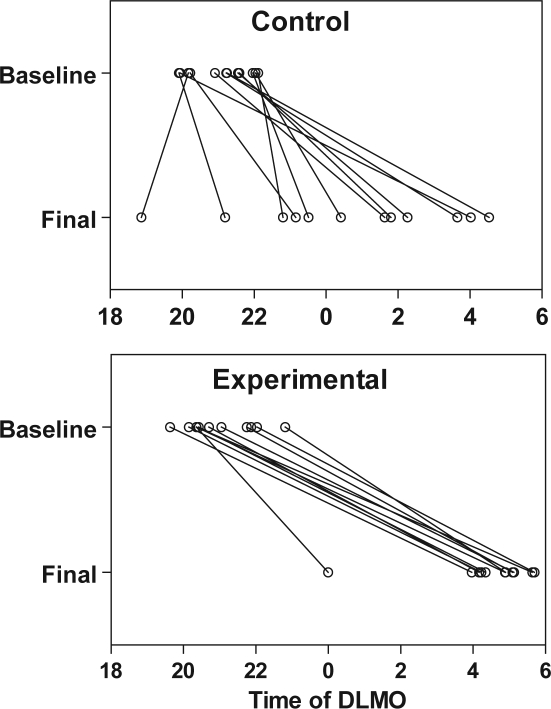
Figure 2.
Position of the DLMO for individual subjects during the baseline and final phase assessments. Lines are included to connect the DLMOs of each subject, and may not reflect the time course of the phase shift, which may not have been linear.
Additional Procedures
Subjects completed daily sleep logs recording bed, sleep, and wake times, as well as nighttime awakenings > 5 min. Subjects wore an Actiwatch-L (AWL) device (Mini Mitter, Bend, OR) around the dominant wrist to verify compliance with the sleep schedule and to measure sleep quantity. To estimate exposure to ambient light, a second AWL was worn on a cord around the neck like a medallion. Outside light exposure and use of sunglasses were recorded on daily light logs, and compliance with required outdoor light was verified with light recordings from the neck-worn AWL.
All alcohol, caffeine, and over-the-counter medication intake were recorded on daily event logs. During the baseline portion of the study, ≤ 2 alcoholic drinks per day were allowed. Alcohol was prohibited the day before a night shift and in the 24 h prior to and during each phase assessment. Caffeine (≤ 300mg) was permitted before 17:00 on baseline days 1–15 and 18–22, but was prohibited during night shifts and in the 6 h before and during both phase assessments. For the experimental group, caffeine was also prohibited during the travel home period in between the night shifts and daytime sleep, and in the 6 h preceding sleep on the 2 weekend days off.
Data Analysis
Circadian Phase
The threshold to determine the DLMO for each melatonin profile was the average of the 5 lowest consecutive raw data points plus 15% of the average of the 5 highest consecutive raw data points.21–23 A locally weighted least squares (LOWESS) curve was fit to each melatonin profile (GraphPad Prism). The DLMO was defined as the time the fitted curve exceeded and remained above the threshold, while the DLMOff was the time the fitted curve dropped and remained below the threshold.
Circadian phase was analyzed with a repeated measure multivariate analysis of variance (MANOVA), with DLMO and DLMOff as dependent variables. The between-subjects factor of group had 2 levels: experimental and control. The within-subjects factor of time had 2 levels: baseline and final. A significant MANOVA was followed by a repeated measure ANOVA on each phase marker. Simple main effects27 were used to elucidate a significant interaction effect.
Sleep
Measurements of total sleep time (TST) were calculated from sleep logs and actigraphy. Sleep log TST was defined as the difference between wake time and sleep onset, minus any awakenings > 5 min during the sleep period. Actigraphic TST (Actiware version 5.0) was calculated from wrist actigraphy using the medium setting for wake threshold.
Experimental subjects had a single prescribed sleep period on each day, whereas after night shifts began control subjects could have multiple sleep bouts per day. For control subjects, multiple sleep bouts occurring on the same day were summed to derive a daily measurement of TST. TST for control subjects between consecutive night shifts (days 23, 24, and 28–30) was the sum of all sleep after one night shift and before the next. Sleep on the weekend off (study days 25–27) was divided into 3 “days.” Day 25 was from the end of the third night shift at 07:00 until 22:30 on day 26. Day 26 was from 22:30 on day 26 until 22:30 on day 27. Day 27 was from 22:30 on day 27 until the start of the night shift at 22:30 on Day 28. TST on day 31 was all sleep from the end of the last night shift at 07:00 until the start of the final phase assessment at 18:00 on day 32.
Sleep log and actigraphic TST after night shifts were each analyzed with a repeated measure ANOVA. The between subjects factor of group had 2 levels (experimental and control group), while the within-subjects factor of time had 10 levels (baseline sleep [the average of days 1–15], and study days 23–31). Greenhouse-Geisser corrections were used to correct for violations of sphericity, but the original degrees of freedom are reported. A significant group by time interaction was explored with simple main effects.27
For the control group, total sleep time was also correlated with the final DLMO, with the hypothesis that those subjects with later DLMOs during the final phase assessment would have greater TST after night shifts. Average wake time for control subjects on the weekend off was correlated with final DLMO, with the hypothesis that later wake times would be associated with later final DLMOs.
Ambient Light Exposure
Light (lux) recorded from the AWL around the neck was analyzed using the Actiware version 5.0 software. For the experimental group, light exposure was determined for the travel home period after night shifts (07:00–08:30) and for the light brake, which was when subjects were required to receive ≥15 min of outdoor light within the first 2 hours after awakening from daytime sleep (15:30–17:30 on days 23–24 and 28–31, 13:30–15:30 on day 25, and 12:00–14:00 on days 26–27). For the control group, light exposure was summarized for the time between 07:00–12:00 for study days 23–31.
Three light measures were calculated: number of minutes to > 10 lux, exposed to > 1000 lux (primarily when outside), and average lux/minute. Values from the medallion photosensor were corrected for when subjects were outside wearing sunglasses (15% or 36% VLT) according to their light logs. Thus, for experimental subjects minutes > 1000 lux at the AWL corresponded to minutes > 150 lux at the eye; for control subjects minutes > 1000 lux corresponded to minutes > 360 lux. Light exposure was correlated with the final DLMO, with the hypothesis that increased intensity and duration of light exposure during these intervals would be associated with an earlier final DLMO.
Performance Testing
Reaction time (RT), standard deviation (SD) of RT, and number of lapses (RT > 500 msec) were analyzed for the SRT task. For each of these dependent variables, difference-from-baseline scores were calculated. Performance on the second and third day shifts was averaged to form a baseline value. This baseline value was subtracted from scores on each test bout during the night shifts to obtain a difference-from-baseline score.
Reaction time, SD of RT, and lapses on the SRT task were each compared with repeated measure ANOVA. The between-subjects factor had 2 levels (experimental and control), the first within-subjects factor of shift had 7 levels (7 night shifts), and the second within-subjects factor of time-of-night had 4 levels (4 test bouts).
Summary statistics for all data are means and standard deviations unless otherwise indicated. A 2-tailed significance level of 0.05 was used.
RESULTS
Circadian Phase
Individual DLMOs of the control and experimental subjects are shown in Figure 2. The average position of the DLMO and DLMOff at the baseline and final phase assessments are presented in Table 1, and the average baseline and final DLMO and DLMOff for the experimental subjects are illustrated in Figure 1. The average final DLMO of the experimental group was about 04:30, which is slightly later than the target compromise phase position of 03:00. The average final DLMO of the control group was about 00:30, but as shown in Figure 2, there were large individual differences. Three of the 12 control subjects had final DLMOs as late as the experimental subjects.
Table 1.
Circadian Phase Markers Determined from Analysis of Salivary Melatonin. Values are Mean Clock Time (SD in Hours).
| Experimental | Control | |
|---|---|---|
| DLMO | ||
| Baseline | 20:58 (1.0) | 21:04 (0.8) |
| Final | 4:34 (1.6)* | 0:39 (2.9) |
| DLMOff | ||
| Baseline | 6:55 (0.9) | 7:11 (0.7) |
| Final | 15:15 (1.5)* | 11:24 (3.1) |
signifi cantly later than the fi nal phase position of control group, P < 0.001.
There was a significant group × time interaction for both the DLMO (F1,22 = 18.92, P < 0.001) and the DLMOff (F1,22 = 17.69, P < 0.001). The position of the DLMO and DLMOff for the 2 groups did not differ during the baseline phase assessment (P > 0.05), but the experimental group had a significantly later DLMO and DLMOff than the control group during the final phase assessment (P < 0.001 for both phase markers).
Ambient Light Exposure
For the experimental group, exposure to bright light (> 1000 lux at the medallion or > 150 lux at the eye) during the travel home period after night shifts ranged from 0 to 55 min, with an average duration of 13.3 ± 12 min. The experimental group exceeded the “light brake” requirement for ≥ 15 min afternoon outdoor light exposure within 2 h after awakening. Average exposure to bright light during those 2 h was 28.0 ± 15 min. There were a few significant correlations between the final DLMO and light exposure from the light brake, but these analyses were limited by the low variability in final phase position for the experimental subjects.
The control group averaged 23 ± 28 min of morning bright light (> 1000 lux at the light medallion or > 360 at the eye) between 07:00 and 12:00 per day. Greater morning bright light exposure was associated with an earlier final DLMO. When averaged over days 23–31 for each subject, the correlation between minutes of bright light exposure and the final DLMO was r = −0.57 (P < 0.05).
Sleep
Sleep log TST for experimental subjects closely paralleled the permitted time in bed. The duration of sleep log TST for control subjects, who self-selected sleep times, was similar, with a few small but statistically significant differences. The main effect of group was not significant, nor were there significant group differences when examining weekday (daytime) and weekend (late night) sleep episodes separately. There was a significant group x study day interaction (F9,198 = 3.00, P = 0.02). The experimental group had about 45 min less TST on day 25 when their time in bed was restricted to 5 h (P < 0.05), and had about 1 h more TST on study days 26 and 31 (P < 0.01). The pattern of actigraphic measurements of TST across study days was similar to sleep logs.
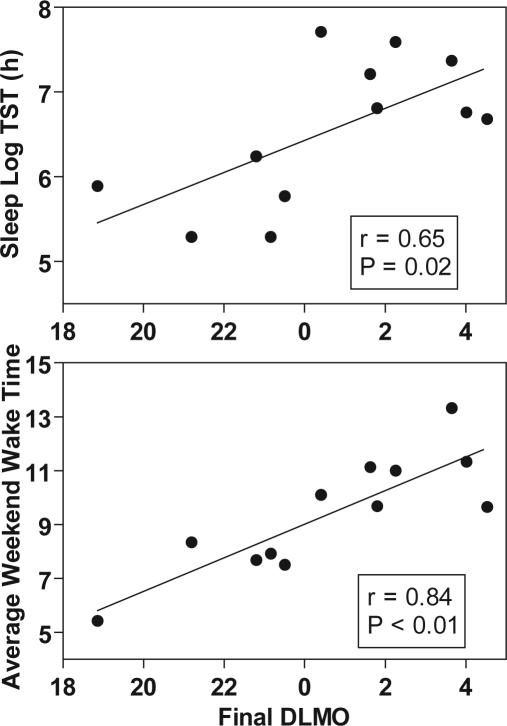
Although the average sleep duration for the control group was nearly as long as the experimental group on many days, control subjects showed large individual differences in the duration and pattern of sleep episodes. Some control subjects selected a pattern of sleep and light exposure similar to that of the experimental group, initiating daytime sleep soon after night shifts, and adopting late bed and wake times on the 2 weekend days off. Other control subjects had a more erratic sleep pattern, with shorter and more disrupted sleep after night shifts, and earlier bed and wake times on the weekend days off. Average TST for control subjects was positively correlated with the time of the final DLMO (top panel of Figure 3; r = 0.65, P = 0.02). Those control subjects who delayed so far that their final DLMO was close to a compromise phase position (02:00–04:00) averaged ≥ 7 h TST across days 23–31, while control subjects with final DLMOs before 00:00 averaged 5–6 h TST (Figure 3, top). The timing of weekend sleep for control subjects was also associated with final circadian phase position. Later wake times and bed times on the weekend were strongly correlated with a later final DLMO (bottom panel of Figure 3; r = 0.84, P < 0.01 for wake time; r = 0.63, P = 0.03 for bed time). Subjects whose average weekend wake time was close to the prescribed weekend wake time of the experimental group (which was 12:00) had later final DLMOs that were close to a compromise circadian phase position (Figure 3, bottom)
Figure 3.
Scatterplots of average total sleep time (days 23–31, top) and weekend wake time (days 26–27, bottom) versus final DLMO for control subjects.
Performance
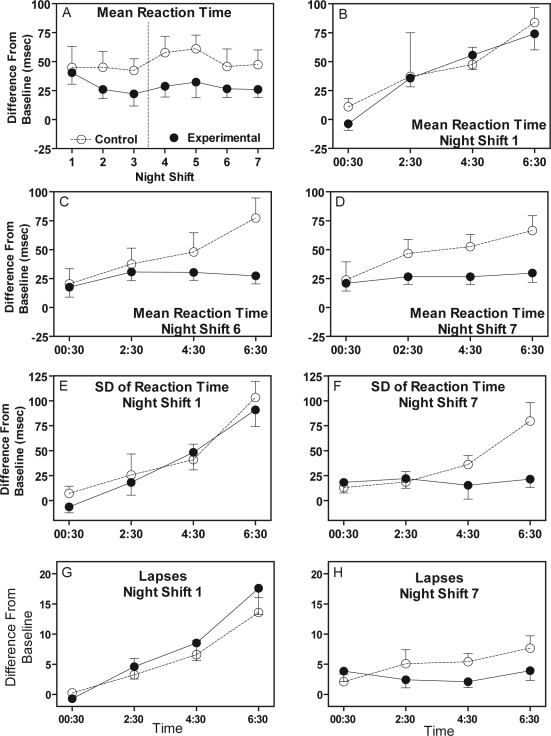
The control group had longer reaction times on the SRT task than the experimental group (Figure 4, panel A). There was a significant main effect of group for mean reaction time (F1,22 = 5.26, P = 0.03). For the first few night shifts there was gradual increase in RT across the night shift for both experimental and control subjects, with the longest RT during the last test bout (06:30) (panel B in Figure 4). By night shifts 6 and 7 the increase in RT across the night persisted for the control group, but RT for the experimental group remained the same across the night, and was only slightly longer than baseline (Figure 4, panels C and D).
Figure 4.
Performance on the simple reaction time task. All scores are difference-from-baseline. Error bars show SEM. Dotted vertical line in panel A indicates the weekend off occurred between night shifts 3 and 4.
Standard deviation of RT showed a pattern similar to mean RT. The control group had more variable reaction times, and the increase in variability later in night shifts persisted across night shifts, while for the experimental group variability in reaction time leveled off close to baseline by night shift 6 (Figure 4, panels E and F). There was a significant main effect of group for SD of reaction time (F1,22 = 8.00, P = 0.01). The number of lapses on the SRT task followed the same trend as RT and SD of RT, but there were no statistically significant differences between the groups (Figure 4, panel G and H). The results from the other ANAM tests (data not shown) showed a pattern that was similar to the results for SRT.
DISCUSSION
Complete circadian adaptation to night shifts and day sleep has been achieved in laboratory studies with scheduled exposure to bright light and dark/sleep. However, this approach leaves the worker completely out of phase with family and friends on days off, which is a sacrifice few night workers are likely willing to make. We tested a schedule including brief intermittent bright light pulses and a sleep/dark pattern designed to produce a compromise circadian phase position compatible with both night shifts and with days off that could be maintained throughout alternations of night shifts with days off. The final circadian phase for the experimental group after 3 consecutive night shifts, a weekend off, and 4 more consecutive night shifts was slightly later (DLMO ∼ 04:30) than the targeted final phase position (03:00). However, these subjects were able to sleep for almost all of the scheduled time both after night shifts and on days off and performed better than control subjects during night shifts. To our knowledge, apart from the earlier studies in this series, this is one of the first attempts to balance phase delaying and phase advancing light and dark exposure to achieve a specific circadian phase position.
Although we aimed for a target compromise position of the DLMO at 03:00, we expect that circadian phase will vary somewhat across a week of night work. It would likely be earlier on the first night shift in a work week immediately following a weekend off, due to the relatively advanced sleep/dark periods and earlier advancing light brake on that weekend, and would become progressively later across the work week due to the delaying bright light pulses during night shifts and the relatively delayed sleep schedule and later afternoon light brake. Thus, the final phase position in this study with the DLMO at ∼04:30 is consistent with achieving our overall goal because it occurred towards the end of a series of night shifts, when circadian phase might be expected to be slightly later than the target compromise position.
The position of the final DLMO for the control group was earlier and more variable than for the experimental group. A number of control subjects self-selected a pattern of sleep and light exposure similar to that of the experimental group, initiating daytime sleep soon after night shifts, and adopting late bed and wake times on the 2 weekend days off. Some of these subjects had a final DLMO as late as those of experimental subjects, but others did not. This observation is consistent with a previous study in our lab10 showing that having 5 consecutive night shifts in only room light followed by regular daytime sleep/dark periods and using dark sunglasses was not sufficient to substantially delay the circadian clock of all subjects (see top panels of Figure 2 in that publication). Our data suggest that just adopting the recommended sleep pattern and wearing light sunglasses was sufficient to delay the circadian clocks of some but not all the control subjects, and thus the use of additional countermeasures, such as bright light during night shifts and/or darker sunglasses, is also necessary.
The position of the final DLMO was modestly correlated with outside light exposure. For the control group, greater morning light exposure during the night shift section of the study (days 23–31) was associated with smaller phase delays, which can be attributed to the advancing effect of morning light.8 Correlations between afternoon light exposure (the advancing light brake) and final DLMO for the experimental group were largely unremarkable, but limited by the small variability in final phase position for experimental subjects. The one experimental subject with a substantially earlier final DLMO than the remainder of the group (Figure 2) also had much greater afternoon light exposure. This subject averaged 951 lux during the first 2 h of awakening from the last 3 sleep episodes of the study (days 29–31), whereas the remainder of the group averaged 337 ± 221 lux.
There were few differences in TST between the experimental and control group, and the differences were small. This reflects the fact that as a group both the experimental and control subjects slept fairly well. The experimental subjects had little trouble sleeping at the prescribed times, and slept for nearly all of the allotted time in bed. Although the average TST for the control group was similar to the experimental group on most days, the individual differences in TST for the control subjects were much larger than for experimental subjects. There was a significant positive correlation between TST and final circadian phase for the control subjects, in that a later final DLMO was associated with longer sleep episodes after night shifts and on days off (Figure 3). Sleep duration is dependent on circadian phase,28,29 and it is thus likely that shorter duration sleep for some control subjects was due to the relatively small delays of their circadian clocks.
Performance on the SRT task was better for the experimental subjects than the controls. When beginning the first series of night shifts, both experimental and control subjects demonstrated prolonged and variable reaction times with occasional lapses in the later hours of the night shift, like real night shift workers. We speculate that for experimental subjects the target compromise phase position was likely reached by the 6th night shift. This is based upon the final circadian phase in the previous study in this series,23 in which the final DLMO for experimental subjects was about 01:00 on day 28, and assumed a gradual delay from day 28 to day 32 in the current study. Thus, by the 6th and 7th night shifts, when the phase delays of the experimental group had likely reached the compromise circadian position, performance for this group was close to the level during day shifts, demonstrating fast reaction times with low variability and few or no lapses. In contrast, the control group continued to show longer and more variable reaction times on all night shifts. Their responses became progressively slower later in each shift, with some lapses at the end of the night shifts.
We have demonstrated that the target compromise circadian phase position can be reached during the second series of night shifts, with concomitant improvements in night shift alertness and sleep duration. The next and final study in this series assesses circadian phase after 1 more night shift (on day 32) followed by a second weekend off. In this final study we hope to demonstrate that the target compromise circadian phase position can be maintained after the second weekend off. These will be important steps towards convincing employers and shift workers that these interventions are feasible and worthwhile.
ACKNOWLEDGMENTS
We thank Stephanie J. Crowley for her contribution to writing the grant that supported this research; Dr. Helen Burgess, Dr. Victoria Revell, Meredith Durkin, Valerie Ellios, Thomas Molina, Vanessa Meyer, Daniel Alderson, and Erin Cullnan for assistance with data collection; Dr. Louis F. Fogg for statistical consultation, our medical director Dr. Keith Callahan, and Erin Cullnan for comments on the manuscript. We also thank Uvex Safety and the Sun Box Company. This work was supported by R01 OH003954 from NIOSH and the Centers for Disease Control and Prevention (CDC) to C.I.E. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIOSH or the CDC.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Eastman CI. Bright light in work-sleep schedules for shift workers: Application of circadian rhythm principles. In: Rensing L, an der Heiden U, Mackey MC, editors. Temporal Disorder in Human Oscillatory Systems. Berlin-Heidelberg-New York: Springer-Verlag; 1987. pp. 176–85. [Google Scholar]
- 2.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N. Engl. J. Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 3.Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–6. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- 4.Eastman CI, Stewart KT, Mahoney MP, Liu L, Fogg LF. Dark goggles and bright light improve circadian rhythm adaptation to night-shift work. Sleep. 1994;17:535–43. doi: 10.1093/sleep/17.6.535. [DOI] [PubMed] [Google Scholar]
- 5.Stewart KT, Hayes BC, Eastman CI. Light treatment for NASA shiftworkers. Chronobiol. Int. 1995;12:141–51. doi: 10.3109/07420529509064509. [DOI] [PubMed] [Google Scholar]
- 6.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann. Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 7.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J. Biol. Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 8.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J. Biol. Rhythms. 2005;20:353–65. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night shift work. Sleep. 2004;27:1077–87. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- 10.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J. Biol. Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 11.Budnick LD, Lerman SE, Nicolich MJ. An evaluation of scheduled bright light and darkness on rotating shiftworkers: trial and limitations. Am. J. Ind. Med. 1995;27:771–82. doi: 10.1002/ajim.4700270602. [DOI] [PubMed] [Google Scholar]
- 12.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behavioral Brain Research. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 13.Goel N. An arousing, musically enhanced bird song stimulus mediates circadian rhythm phase advances in dim light. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R822–7. doi: 10.1152/ajpregu.00550.2005. [DOI] [PubMed] [Google Scholar]
- 14.Goel N. Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R209–R16. doi: 10.1152/ajpregu.00754.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J. Appl. Physiol. 1996;80:25–9. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol. Int. 2000;17:807–26. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- 17.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J. Biol. Rhythms. 2004;19:248–57. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- 18.Griefahn B. The validity of the temporal parameters of the daily rhythm of melatonin levels as an indicator of morningness. Chronobiol. Int. 2002;19:561–77. doi: 10.1081/cbi-120004226. [DOI] [PubMed] [Google Scholar]
- 19.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 20.Griefahn B, Kunemund C, Golka K, Thier R, Degen G. Melatonin synthesis: A possible indicator of intolerance to shiftwork. Am. J. Ind. Med. 2002;42:427–36. doi: 10.1002/ajim.10122. [DOI] [PubMed] [Google Scholar]
- 21.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J. Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Smith M, Eastman C. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol. Int. 2006;23(4):859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Cullnan E.E, Eastman C.I. Shaping the light/dark pattern for circadian adaptation to night shift work: Study 2. Physiol. Behav. 2008;95:449–56. doi: 10.1016/j.physbeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 25.Reeves D KR, Elsmore T, Winter K, Bleiberg J. San Diego, CA: National Cognitive Recovery Foundation Publication NCRF-SR2002-1; 2002. ANAM 2001 User's Manual: Clinical and Research Modules. [Google Scholar]
- 26.Cernich A, Reeves D, Sun W, Bleiberg J. Automated Neuropsychological Assessment Metrics sports medicine battery. Arch Clin Neuropsychol. 2007;22(Suppl 1):S101–14. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. pp. 529–532. [Google Scholar]
- 28.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflugers Archives. 1981;391:314–8. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]
- 29.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]