Abstract
Study Objectives:
To compare NREM EEG power in primary insomnia (PI) and good sleeper controls (GSC), examining both sex and NREM period effects; to examine relationships between EEG power, clinical characteristics, and self-reports of sleep.
Design:
Overnight polysomnographic study.
Setting:
Sleep laboratory.
Participants:
PI (n = 48; 29 women) and GSC (n = 25; 15 women).
Interventions:
None.
Measurements:
EEG power from 1–50 Hz was computed for artifactfree sleep epochs across four NREM periods. Repeated measures mixed effect models contrasted differences between groups, EEG frequency bands, and NREM periods. EEG power-frequency curves were modeled using regressions with fixed knot splines.
Results:
Mixed models showed no significant group (PI vs. GSC) differences; marginal sex differences (delta and theta bands); significant differences across NREM periods; and group*sex and group*NREM period interactions, particularly in beta and gamma bands. Modeled power-frequency curves showed no group difference in whole-night NREM, but PI had higher power than GSC from 18–40 Hz in the first NREM period. Among women, PI had higher 16 to 44-Hz power than GSC in the first 3 NREM periods, and higher 3 to 5-Hz power across all NREM periods. PI and GSC men showed no consistent differences in EEG power. High-frequency EEG power was not related to clinical or subjective sleep ratings in PI.
Conclusions:
Women with PI, but not men, showed increased high-frequency and low-frequency EEG activity during NREM sleep compared to GSC, particularly in early NREM periods. Sex and NREM period may moderate quantitative EEG differences between PI and GSC.
Citation:
Buysse DJ; Germain A; Hall ML; Moul DE; Nofzinger EA; Begley A; Ehlers CL; Thompson W; Kupfer DJ. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. SLEEP 2008;31(12):1673–1682.
Keywords: Insomnia, non-rapid eye movement sleep, power spectral analysis, primary insomnia, sleep, electroencephalogram (EEG)
THE ETIOLOGY AND PATHOPHYSIOLOGY OF INSOMNIA ARE UNKNOWN, BUT IT IS OFTEN CONSIDERED TO BE A DISORDER OF INCREASED PHYSIOLOGICAL and cognitive arousal.1–3 Evidence for physiological arousal includes elevated resting metabolic rate,4,5 increased heart rate and sympathovagal tone as indicated by heart rate variability (HRV),6,7 increased cortisol secretion in the evening and early sleep hours,8,9 increased beta EEG activity during NREM sleep,10–14 and increased glucose metabolic rate during NREM sleep.15 Evidence for increased arousal defined in cognitive terms is found in the pre-sleep thoughts of insomnia patients compared to good sleepers,16–19 which are often described as “racing,” unstoppable, and sleep-focused. Contemporary models of the pathophysiology of insomnia suggest that, in individuals predisposed on the basis of genetic or affective factors, the acute experience of sleep difficulty leads to a positive feedback loop of selective attention, conditioned arousal, poor sleep, and impaired waking function.20–23
Of the various indicators of hyperarousal in insomnia, quantitative EEG characteristics have been studied most carefully, usually through the use of power spectral analysis of the sleep EEG. Particular attention has been focused on high frequency activity in the range of 16–32 Hz, or “beta activity,” which is thought to reflect a form of cortical activation. This interpretation is based on waking EEG studies in healthy adults, where even higher frequency EEG activity (in the “gamma” range of 30–100 Hz) is thought to represent an analog of sensory processing, focused attention, learning, or memory.13,24–26 Most insomnia studies have focused on quantitative EEG obtained during NREM sleep, as opposed to REM sleep, in part because phasic eye movements and muscle activity can lead to EEG artifacts during REM, and in part because there is little specific evidence to implicate disturbances of REM sleep in chronic insomnia. Although increased fast frequency EEG activity during NREM sleep has been demonstrated in several samples of insomnia subjects relative to controls, methodologies have varied widely among these studies. For instance, increased beta activity in one study was observed in subjects with “sleep state misperception” (paradoxical insomnia), but not psychophysiological insomnia, relative to controls.14 Another study showed increased beta in primary insomnia relative to insomnia with comorbid depression and controls.12 In addition to examining high-frequency EEG activity as an indicator of hyperarousal, some studies have also used quantitative EEG to measure delta EEG activity (0.5–4 Hz) as an indicator of homeostatic sleep drive in insomnia. These studies have also yielded variable results, with some showing reduced delta activity,11 but others showing no difference between insomnia and control samples,12,27 or a reduction only in “subjective” insomnia, but not “objective” insomnia.14 Although it may appear logical that EEG correlates of hyperarousal and homeostatic drive would inversely correlate in PI, previous published reports have presented only qualitative evidence to support this hypothesis.11,27
Most studies showing insomnia-control differences have focused on all-night NREM EEG activity, but others have focused on the sleep-onset interval.10,28 Some studies have also examined the time course of EEG power in different bands across successive NREM periods,11,27 generally finding stable differences across the night. However, other types of physiological data, such as plasma cortisol levels, suggest that individuals with PI may have particular evidence of hyperarousal in the first part of the night.8,9
Sex differences in quantitative EEG during sleep have also been identified in healthy individuals from adolescence to older adulthood,29–32 and among individuals with conditions such as major depressive disorder.33–35 However, sex differences have not been extensively examined in chronic insomnia. One study found higher relative beta EEG power in men than women among an older adult sample of insomnia and control subjects,14 but several other studies did not specifically examine sex differences.
Likewise, the relationship between quantitative EEG characteristics and subjective sleep characteristics has received little attention. If high-frequency EEG activity were indeed a correlate of hyperarousal, one might expect that this variable would correlate with insomnia severity or sleep self-reports. Krystal and colleagues14 found that delta and beta power differed between subjective insomnia subjects and controls regardless of the degree of subjective underestimation of sleep, but that subjective ratings of sleep quality, restedness, and sleep efficiency were related only to delta power, and only among subjective insomnia subjects. Perlis and colleagues12 found that subjective underestimation of sleep time was related to higher beta activity in a sample that included PI, GSC, and depressed subjects. Thus, whether quantitative EEG characteristics are related to overall insomnia severity or to specific features remains an unresolved issue.
In summary, previous findings regarding quantitative EEG characteristics of insomnia have provided inconsistent results, particularly regarding differences across NREM periods, sex effects, and clinical correlations. Therefore, the goals of the present analysis were: (1) to compare EEG power across successive NREM periods in well-characterized samples of primary insomnia (PI) and good sleeper controls (GSC); (2) to compare these findings for men and women separately; and (3) to examine relationships between quantitative EEG characteristics, baseline characteristics of insomnia, and self-reports of sleep.
METHODS
Data for the current analyses come from a study designed to examine mood, arousal, and response to pharmacologic treatment probes in individuals with PI and GSC (MH24652), and studies of sleep functional neuroanatomy in PI and GSC (MH061566). These studies were approved by the University of Pittsburgh Institutional Review Board, and all subjects provided written informed consent. After initial eligibility screening, participants complete a set of self-report retrospective symptom ratings, a one-week in-home evaluation including sleep diary and daily symptom ratings, and 3 nights of polysomnography (PSG).
Participants
Study participants included men and women with PI and GSC, recruited for research studies on the neurobiology of insomnia, and aged 20–50 years. PI and GSC groups did not differ in age (PI = 30.8 [7.2] years, GSC = 30.6 [7.4] years) or sex distributions (both groups 60% women). Furthermore, there was no significant difference in age between men and women (F = 1.13, P = 0.29), between PI and GSC (F = 0.03, P = 0.86), nor for the interaction of sex and group (F = 0.05, P = 0.83). Participants were recruited through media advertisements, word of mouth, and clinical referrals. All participants were evaluated with a medical history, medication/ substance history, physical examination, routine blood work, and urine drug screen; psychiatric history using the Structured Clinical Interview for DSM-IV (SCID)36,37; and sleep history using locally developed questionnaires and interviews to yield DSM-IV sleep disorder diagnoses.38 Inclusion criteria for PI and GSC included provision of informed consent and ability to speak and understand English. For PI, additional inclusion criteria included a current diagnosis of PI according to DSM-IV criteria and a score > 5 on the Pittsburgh Sleep Quality Index (PSQI).39 No specific quantitative criteria for sleep disturbance were used. Exclusion criteria for PI and GSC included significant or unstable medical conditions; current major syndromal mood, anxiety, psychotic, or substance use disorder; current sleep disorder (other than PI); apnea-hypopnea index > 15 or periodic limb movement arousal index > 20 on one night of screening polysomnography; use of medications or substances known to affect sleep; coffee consumption (or equivalent) of > 4 cups per 24 hours; and alcohol consumption > 14 drinks per week. Our thresholds for apnea and periodic limb movements (PLM) were chosen to reflect levels of apnea and PLMs that would typically elicit treatment in clinical settings. Despite our liberal inclusion criteria, the mean and median AHI and PLM arousal indices were quite low (Table 1), and not consistent with clinical apnea or PLM disorders. Additional specific exclusion criteria for PI included a history of any major psychiatric disorder within the past 6 months. Specific exclusion criteria for GSC involved current or past history of PI or any major psychiatric disorder.
Table 1.
Clinical Rating, Sleep Diary, PSG, And Post-Sleep Self-Report Measures1
| Variable | Good Sleeper Control N = 25 | Primary Insomnia N = 48 | Group Contrast (ANOVA) |
|---|---|---|---|
| Clinical Ratings | |||
| MANOVA: Group F = 24.91, P < 0.0001; Sex F = 0.49, P = 0.83; Group*Sex Interaction F = 1.74, P = 0.12; df = 7,47 | |||
| Inventory of Depressive Symptomatology (minus sleep item)2 | 4.0 (6.4) | 11.1 (5.4) | P < 0.0001 |
| Beck Anxiety Inventory4 | 1.3 (1.7) | 4.8 (4.3) | P = 0.0009 |
| Penn State Worry Questionnaire2,4 | 24.8 (15.0) | 32.4 (15.5) | P = 0.09 |
| Hyperarousal Scale2 | 24.4 (7.9) | 34.4 (6.7) | P < 0.0001 |
| Multidimensional Fatigue Inventory (General Fatigue Subscale)2 | 6.9 (6.9) | 13.4 (3.2) | P < 0.0001 |
| Pittsburgh Sleep Quality Index | 2.0 (1.2) | 11.2 (3.4) | P < 0.0001 |
| Epworth Sleepiness Scale2,4 | 4.1 (4.1) | 6.9 (3.6) | P = 0.007 |
| Sleep Diary3 | |||
| MANOVA: Group F = 8.28, P < 0.0001; Sex F = 0.98, P = 0.44; Group*Sex Interaction F = 0.21, P = 0.96; df = 5,62 | |||
| Time in Bed, min | 455.8 (54.7) | 460.4 (58.1) | P = 0.8 |
| Sleep latency, min | 8.1 (7.0) | 42.3 (38.8) | P = 0.0001 |
| Wake after sleep onset, min | 3.7 (4.9) | 29.6 (24.6) | P < 0.0001 |
| Total sleep time, min | 443.9 (53.5) | 389.0 (62.4) | P = 0.0007 |
| Sleep efficiency % | 97.4 (2.6) | 84.3 (10.7) | P < 0.0001 |
| Polysomnography (PSG) | |||
| MANOVA: Group F = 1.89, P = 0.08; Sex F = 0.56, P = 0.81; Group*Sex Interaction F = 1.62, P = 0.14; df = 8,62 | |||
| Apnea-hypopnea index, events per hour | 3.2 (3.6) | 2.7 (2.2) | — |
| Periodic Limb Movement Arousal Index, events per hour | 2.7 (2.9) | 3.2 (4.3) | — |
| Time in Bed, min | 458.1 (57.8) | 478.4 (47.7) | — |
| Sleep latency, min | 16.3 (13.5) | 26.4 (20.9) | — |
| Wake after sleep onset, min | 25.9 (24.1) | 28.6 (33.3) | — |
| Total sleep time, min | 416.0 (44.1) | 423.4 (51.8) | — |
| Sleep efficiency % | 91.2 (6.0) | 88.7 (8.6) | — |
| Stage 1% | 5.5 (2.9) | 4.6 (2.6) | — |
| Stage 2% | 57.2 (6.6) | 60.9 (7.6) | — |
| Stage 3+4% | 10.2 (7.6) | 9.9 (6.3) | — |
| Post-Sleep Self-Report | |||
| MANOVA: Group F = 7.31, P < 0.0001; Sex F = 0.29, P = 0.92; Group*Sex Interaction F = 1.07, P = 0.39; df = 5,59 | |||
| Sleep latency, min | 15.6 (12.0) | 37.2 (30.5) | P = 0.0002 |
| Wake after sleep onset, min | 10.1 (10.8) | 41.3 (42.7) | P = 0.0004 |
| Total sleep time, hours | 7.3 (0.7) | 6.6 (1.4) | P = 0.05 |
| Soundness of sleep, 0–100 | 72.7 (17.3) | 48.4 (24.1) | P < 0.0001 |
| Well-Rested, 0–100 | 72.3 (17.1) | 40.9 (28.3) | P < 0.0001 |
Data are shown as mean, (S.D.). P values are for ANOVA contrasting group (Primary Insomnia vs. Good Sleeper Control), and are shown only for domains in which MANOVA group contrast was significant.
Good sleeper controls n = 17
Good sleeper controls n = 22
Primary insomnia n = 42
Self-Report Measures
Baseline retrospective questionnaires were used to evaluate mood and arousal, and are reported here for descriptive purposes. For all retrospective psychological and sleep measures, higher scores indicate greater severity of symptoms. Because subjects in this report were drawn from 2 protocols that had identical inclusion criteria and PSG methods but slightly different clinical measures, the number of subjects available was reduced for some measures. Specific measures used in this study have been described in detail elsewhere.40 Global sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI).39 The Inventory of Depressive Symptomatology, Self-Report Version (IDS-SR)41 was used to measure symptoms of depression consistent with major depression criteria in DSM-III and DSM-IV. For analyses in this paper, IDS-SR score represented the total after excluding sleep-specific items. The Beck Anxiety Inventory (BAI)42 was used to assess self-report anxiety symptoms, with a focus on somatic symptoms rather than worry. By contrast, the Penn State Worry Questionnaire (PSWQ)43 was used to evaluate dispositional tendency to worry. The Hyperarousal Scale (HAS)44 was empirically designed to measure daytime alertness among individuals with insomnia with a “high arousal pattern,” and has been found to discriminate primary insomnia versus control subjects.
Daytime symptoms related to insomnia were measured using the Multidimensional Fatigue Inventory (MFI)45 and the Epworth Sleepiness Scale (ESS).46,47 For these analyses, we report the MFI “General Fatigue” subscale only, as recommended by the scale authors when only one scale is to be used.
Sleep patterns at home were assessed with the Pittsburgh Sleep Diary (PghSD).48 The PghSD is a diary of sleep-wake behaviors with bedtime and waketime portions. For this study, the PghSD was presented in a version adapted for hand-held computer. Specific outcome variables are indicated in Table 1.
Finally, the subjective experience of sleep in the laboratory was evaluated with the Post-Sleep Evaluation (PSE), a locally-developed questionnaire consisting of 23 quantitative and qualitative items. For the current analyses, we used self-reports of quantitative sleep parameters to contrast with PSG measures, and subjective ratings of restedness and soundness of sleep.
Polysomnography and Power Spectral Analysis
All subjects underwent one night of PSG to screen for sleep disorders followed by 2 nights of baseline PSG for sleep staging and quantitative analysis. Data for the current report come from Night 1 (sleep disordered breathing, periodic limb movements) or Night 2 (sleep stages, spectral analysis) unless otherwise specified. PSG was conducted using Grass Telefactor M15 bipolar Neurodata amplifiers and locally developed collection software.49 The recording montage consisted of bilateral central EEG leads referenced to A1+A2; right and left electrooculogram referenced to A1+A2; and bipolar submentalis electromyogram. On the screening night, additional channels were used to monitor sleep related breathing (nasal-oral thermistors, inductance plethysmography, fingertip oximetry, V2 EKG) and periodic limb movements (bilateral anterior tibialis EMG). EEG recordings used a high-frequency filter of 100 Hz, a low-frequency filter of 0.3 Hz, and a 60-Hz notch filter. Sleep stages were scored in 20-second epochs according to standard criteria.50
Methods for power spectral analysis have been previously published.51 Briefly, EEG signals were digitized at a rate of 256 Hz. The raw digitized data were band-limited to 64 Hz using a low pass finite impulse response (FIR) filter, then decimated to 128 Hz for quantitative analyses. Low frequency artifacts were excluded by eliminating epochs scored as wakefulness or movement time. High-frequency EEG artifacts were identified and excluded in 4-sec bins with a previously validated and published algorithm that uses a moving window threshold.52 Basically, this algorithm excludes 4-sec bins whose power in the frequency range of 26.25–32 Hz exceeds the power in adjacent bins by a factor of 4 or greater. A mean of < 1.5 min was excluded within each NREM period for both PI and GSC. PI tended to have more artifact-excluded minutes than GSC, but these differences approached statistical significance only in NREM 3 (1.11 ± 0.98 minutes excluded for PI, 0.76 ± 0.62 minutes excluded for GSC, t = 1.99, P = 0.051). Power spectral analysis was used to quantify the frequency content of the sleep EEG from 0.25–50 Hz.51 Non-overlapping 4-sec epochs were weighted with a Hamming window, and periodograms were then computed for these epochs using the Fast Fourier transform (FFT). EEG spectra for each artifact-free 4-sec epoch were then aligned with 20-sec visually scored sleep stage data to exclude epochs scored as awake or REM sleep. EEG power values from artifact-free 4-sec epochs at 0.25 Hz resolution were averaged into 1-Hz bins prior to modeling and analysis, to provide adequate resolution of frequencies while limiting the number of statistical comparisons.
Data Modeling and Statistical Analysis
For descriptive purposes, the 4 domains of clinical ratings, sleep diary data, PSG data, and post-sleep evaluation data were first compared using multivariate analyses of variance (MANOVA) with terms for diagnostic group (PI vs. GSC), sex, and their interaction. The α level for MANOVAs was conservatively set at 0.01. Significant results were then followed up with ANOVAs. Appropriate transformations were used prior to analysis for variables that were not normally distributed. Given the absence of age differences between diagnostic groups or sexes, we did not include age as a covariate in any analyses.
The major test of Aims 1 and 2 consisted of 6 repeated measures mixed effect models, one for each of the commonly used frequency bands: Delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), beta (16–32 Hz), and gamma (32–50 Hz). Based on our aims, we included factors for group (PI vs. GSC), sex, NREM period number (1–4), and interactions of group*sex and group*cycle. Given the restricted number of comparisons for the main test of study aims, we used an uncorrected α level of 0.05. Data analyses were conducted on modeled EEG power data, as described in the next paragraph.
Analyses of power in traditional frequency bins assumes that they are independent, and does not account for the possibility that power differences may not occur neatly within these bins. Therefore, we conducted additional exploratory analyses to test Aims 1 and 2. Power-frequency curves were generated in 1-Hz increments for each subject for whole-night NREM data, and for up to 4 individual NREM periods. The number of subjects is reduced for later NREM periods because subjects may not have had 4 NREM periods, and because we excluded NREM periods less than 20 min to ensure stable estimates of EEG power. Natural log transformation was used for power values in order to normalize distributions. Power-frequency curves were then modeled for each PI and GSC subject in each NREM period, using regression models with fixed-knot cubic splines.53 Group mean curves were then generated, allowing for a direct comparison of modeled power-frequency curves in the 2 groups. Fitted power values for each 1-Hz bin were compared across groups using F ratios for all-night NREM and for each NREM period separately. Specific comparisons involved the entire PI and GSC samples; women only; and men only. Since PI and GSC samples were well-matched for age, we did not use age as a covariate in these analyses. Given the exploratory nature of these analyses, we display horizontal lines in Figures 1–3 that correspond to α levels of 0.05, 0.01, and 0.001.
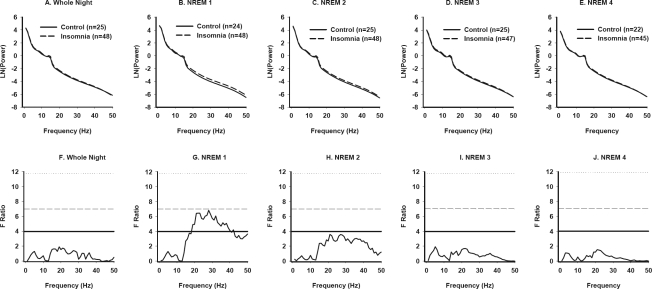
Figure 1.
Power spectral analysis of NREM sleep in Primary Insomnia and Good Sleeper Control subjects (All subjects). NREM EEG power across frequencies from 0.5–50 Hz for the whole night (A), and for individual NREM periods (B-E) in the entire set of PI and GSC subjects. Lines represent modeled data based on ln-transformed mean data in successive 1-Hz bins for each subject group. The number of subjects available for each NREM period is indicated in each panel. F tests conducted at each 1-Hz frequency bin are displayed for all-night NREM data (F) and for each successive NREM period (G-J). The solid horizontal line in each panel represents the critical F value to yield a P value of < 0.05 uncorrected; the dashed line corresponds to a P value < 0.01; and the dotted line corresponds to a P value of 0.001.
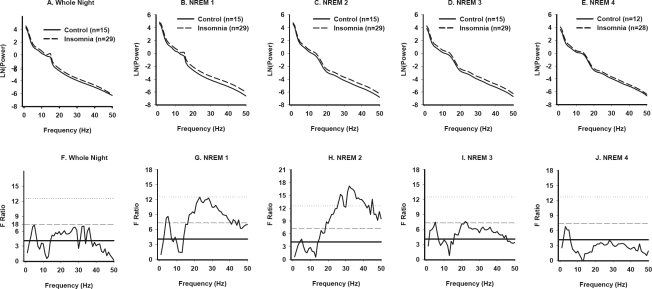
Figure 2.
Power spectral analysis of NREM sleep in Primary Insomnia and Good Sleeper Control subjects (Women). NREM EEG power across frequencies from 0.5–50 Hz for the whole night (A), and for individual NREM periods (B-E) in PI and GSC women. F tests conducted at each 1-Hz frequency bin are displayed for all-night NREM data (F) and for each successive NREM period (G-J). See Figure 1 legend for details.
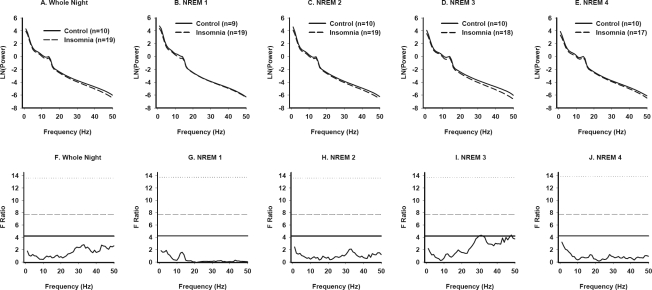
Figure 3.
Power spectral analysis of NREM sleep in Primary Insomnia and Good Sleeper Control subjects (Men). NREM EEG power across frequencies from 0.5–50 Hz for the whole night (A), and for individual NREM periods (B-E) in PI and GSC men. F tests conducted at each 1-Hz frequency bin are displayed for all-night NREM data (F) and for each successive NREM period (G-J). See Figure 1 legend for details.
Previous papers have reported either absolute EEG power, or relative EEG power in specific bins, normalized for total power across a range of EEG frequencies. In most cases, analyses using absolute and relative power yield similar findings.14 Because we were interested in the broadest possible understanding of differences between groups, we analyzed absolute power (natural log-transformed) as our primary analysis. We also conducted analyses using relative EEG data, and summarize these results briefly.
Finally, we examined exploratory correlations between NREM EEG beta and gamma power and selected clinical and self-report measures among PI subjects using Pearson correlations. These correlations were conducted for the entire PI group, and for male and female PI subjects separately. Given the large potential number of comparisons possible, we limited ourselves to seven dependent variables that we selected on conceptual grounds as potentially related to beta or gamma power. These included PSQI global score, Hyperarousal Scale score, Inventory of Depressive Symptomatology Self-Report score (minus sleep items), and diary-based sleep efficiency as baseline clinical ratings; Post-Sleep Evaluation ratings of soundness of sleep and well-restedness as self-report measures on the actual night of PSG; and the discrepancy between PSG and self-report WASO, since this variable showed a large group difference for self-report, but not PSG. We correlated each of these measures with beta and gamma EEG power in the first NREM period, which showed the greatest differences between PI and GSC. We again report findings using an uncorrected α level of 0.05, given the exploratory nature of these analyses.
RESULTS
Clinical ratings, sleep diary, PSG, and post-sleep evaluation measures for PI and GSC groups are shown in Table 1. MANOVAs showed differences among PI and GSC in domains of clinical ratings, sleep diary data, and post-sleep reports, but not in the domain of PSG sleep. Univariate ANOVAs confirmed significant PI-GSC differences on all measures except the Penn State Worry Questionnaire and sleep diary time in bed. MANOVAs did not indicate significant sex effects or group*sex interaction effects.
Analysis of 6 traditional EEG frequency bands in whole-night NREM sleep showed no significant group differences (Table 2). Significant sex differences were found in the theta band (higher power in women), and significant group*sex interactions for delta, theta, sigma, beta, and gamma (highest power in PI women). Significant effects of NREM period were found for each of the EEG frequency bands, and significant group*NREM period interactions for the beta and gamma bands (higher power in earlier NREM periods among PI). Post hoc ANOVAs for whole-night NREM EEG power values in women confirmed higher power among PI than GSC in delta (P = 0.04), theta (P = 0.03), sigma (P = 0.04), beta (P = 0.004) and gamma (P = 0.006) bands. Parallel analyses showed no significant PI-GSC differences among men.
Table 2.
Quantitative EEG Power1
| Good Sleeper Control N = 25 |
Primary Insomnia N = 48 |
Group | Sex | Group* Sex | NREM Period | Group* NREM Period | |||
|---|---|---|---|---|---|---|---|---|---|
| Men n = 10 | Women n = 15 | Men n = 19 | Women n = 29 | ||||||
| Delta (0.5–4 Hz) | 30.81 (11.59) | 33.15 (14.86) | 25.59 (11.87) | 45.82 (22.54) | P = 0.92 | P = 0.08 | P = 0.01 | P<0.0001 | P = 0.48 |
| Theta (4–8 Hz) | 2.93 (1.09) | 3.39 (1.66) | 2.67 (1.68) | 4.22 (1.60) | P = 0.71 | P = 0.04 | P = 0.03 | P<0.0001 | P = 0.38 |
| Alpha (8–12 Hz) | 1.61 (1.20) | 1.55 (0.93) | 1.37 (1.27) | 2.05 (1.51) | P = 0.84 | P = 0.17 | P = 0.07 | P<0.0001 | P = 0.64 |
| Sigma (12–16 Hz) | 0.81 (0.49) | 0.83 (0.43) | 0.67 (0.38) | 1.17 (0.66) | P = 0.50 | P = 0.11 | P = 0.04 | P<0.0001 | P = 0.94 |
| Beta (16–32 Hz) | 0.065 (0.022) | 0.060 (0.018) | 0.056 (0.024) | 0.085 (0.039) | P = 0.19 | P = 0.25 | P = 0.01 | P<0.0001 | P = 0.02 |
| Gamma (32–50 Hz) | 0.010 (0.007) | 0.007 (0.003) | 0.007 (0.004) | 0.010 (0.008) | P = 0.38 | P = 0.84 | P = 0.009 | P = 0.01 | P = 0.04 |
Values represent the group mean and standard deviation of average whole-night absolute power (μ V2/Hz). Data were derived from each subject's modeled and smoothed data within each bandwidth. Statistical analyses were also conducted on the modeled data. P values are from factorial ANOVA with terms for group (PI vs. GSC), sex, and their interaction.
Analyses for relative EEG power indicated no significant main effects of diagnostic group; a significant sex effect for gamma power (higher in men; F = 4.03, P = 0.05); and significant NREM period effects for all frequencies (P values ≤ 0.01). A significant group*sex interaction was found for delta (highest relative power in GSC men, lowest in GSC women; F = 4.04, P = 0.05), and a significant group*NREM period interaction for beta (higher relative power in early NREM periods among PI; F = 3.19, P = 0.03). Thus, relative EEG power findings differed from absolute EEG power findings mainly with regard to sex and group*sex interactions.
To further explore the significant interactions identified above, modeled EEG power-frequency curves and F ratio values for the entire sample are displayed in Figure 1. No significant group differences were observed at any frequency for whole-night NREM sleep. However, PI had higher power than GSC in frequencies of 18–40 Hz during the first NREM period (Figure 1g).
Modeled EEG power-frequency curves and F ratio values for women are displayed in Figure 2. PI women had higher power than GSC women for frequencies in the high delta-low theta range (3–6 Hz) across all NREM periods. In addition, PI women had higher power in frequencies >15 Hz for the first 3 NREM periods (Figure 2g–2i).
Modeled EEG power-frequency curves and F ratio values for men are displayed in Figure 3. The only difference observed between groups was in the 30-Hz range in NREM 3, but the magnitude of this difference was small.
Given the finding of higher power values for PI women than GSC women in both low- and high-frequency EEG bands, we examined correlations between whole-night NREM delta, beta, and gamma power among PI subjects. Delta power was positively correlated with both beta power (r = 0.65, P < 0.0001) and gamma power, (r = 0.33, P = 0.02). Beta and gamma power were also positively correlated (r = 0.70, P < 0.0001). The delta-beta and beta-gamma correlations, but not the delta-gamma correlation, were significant at P < 0.01 for PI men and women separately. Among GSC, delta power was significantly correlated with beta power (r = 0.52, P = 0.009) but not gamma power (r = 0.29, P = 0.17), and beta and gamma power were positively correlated (r = 0.69, P = 0.0002).
Finally, we examined correlations between quantitative EEG power and selected clinical measures related to self-reported insomnia severity, mood, and arousal (see Methods). For the entire sample, NREM 1 beta EEG power correlated only with subjective-PSG WASO discrepancy (r = −0.34, P = 0.02, n = 44). The correlation was significant in men (r = −0.52, P = 0.03, n = 17), but not in women. However, the direction of this correlation is opposite to that expected, i.e., greater subjective-PSG discrepancy was related to lower beta power. NREM EEG gamma activity was not related to any of the self-report measures.
DISCUSSION
We compared clinical, self-report, PSG, and NREM EEG power characteristics in a sample of individuals meeting clinical criteria for PI and an age-and sex-matched group of GSC. The groups showed robust differences on clinical and self-report sleep measures, but few differences on traditional PSG measures. The PI group had increased high-frequency EEG power during NREM sleep in the first NREM period. However, PI-GSC group differences in EEG power were seen more prominently among women than men. Finally, we did not find robust correlations between high-frequency EEG activity and clinical ratings or self-report sleep characteristics in PI. Taken together, our findings provide only partial support for the hypothesis of high-frequency EEG activity during NREM as a correlate of chronic insomnia, and suggest that sex differences may play a more important role than previously recognized.
Increased high-frequency EEG power during NREM sleep is often considered to be an indicator of “hyperarousal” in insomnia.2,13 However, the total number of published studies addressing this issue is small (fewer than 10), and all of these have included relatively small sample sizes of 20 subjects or fewer per group. Moreover, the diagnostic characteristics and subgroups, specific methods for EEG data collection and quantification of EEG power, statistical approaches, and the specific nature of group differences have varied considerably in these studies. Our results again differ from previously published studies in several of these particulars. For these reasons, the finding of increased high frequency EEG power during NREM sleep in chronic insomnia should still be considered a preliminary observation. However, we believe that the current report adds important information to the field in several ways.
First, we selected participants on the basis of clinical insomnia criteria, and without reference to specific sleep parameters measured by either self-report or PSG. Contemporary diagnostic criteria for insomnia focus on sleep disturbance and associated daytime impairment, but do not include any specific quantitative sleep criteria.38,54 Our sample of PI patients was not further characterized according to ICSD-2 diagnoses, e.g., psychophysiological insomnia, paradoxical insomnia. It is possible that such classification would identify more specific EEG correlates, as has been reported in previous studies.12,14 On the other hand, features such as subjective-objective discrepancy likely exist on a continuum rather than as a discrete phenomenon, and cluster analyses of clinical and polysomnographic features in insomnia patients have not provided strong support for ICSD insomnia subtypes.55 Our inclusion/exclusion criteria were designed to yield a sample of PI that would be more similar to that typically seen in treatment settings. Likewise, defining insomnia subtypes according to predominant symptom type (sleep onset, sleep maintenance, nonrestorative sleep) may appear to be a logical way to limit variance, but does not account for the fact that multiple symptom presentations are much more common than isolated symptom types.56,57 The lack of traditional PSG criteria for study entry (e.g., sleep latency, WASO) may also have diminished the likelihood of finding group differences in quantitative EEG. Other studies have also reported that PSG differences are smaller in magnitude, or even absent, when comparing unselected insomnia patients to GSC.58
Second, our primary analyses examined both whole-night NREM EEG power values, as well as individual NREM period values. Our findings differ from those of some previous studies,11,27 which found stable increases in high frequency power across NREM periods. However, other studies have reported time-dependent differences in quantitative EEG characteristics of PI compared to other groups, most often within the examination of the sleep onset period.10,28,59 Some other types of physiological studies have also identified differences between insomnia and non-insomnia subjects that differ by time of night or across the course of the day,5–9 suggesting that any underlying hyperarousal may be a time-dependent phenomenon. This type of hyperarousal could be related to context-dependent or learned arousal,2 and may suggest that treatments for insomnia should focus on improving sleep and cognition in the first part of the night.
Third, we examined EEG power across a broad range of frequencies both with and without traditional EEG bands. Contrasting PI and GSC groups at each individual frequency imposes fewer assumptions about the nature of the sleep EEG, and better demonstrates the evolution of group changes across frequencies and across NREM periods. Although our statistical technique should be viewed as exploratory, the major findings were nevertheless confirmed with analyses of traditional EEG bands.
Fourth, we analyzed men and women separately and in doing so, found a clear distinction in PI-GSC differences. Previous studies of PI have not always identified sex differences in PI using standard visually-scored PSG measures.60 In our study of quantitative EEG, differences between PI and GSC men were few and inconsistent, being limited to the 30-Hz range in NREM 3. This is also the frequency range with most pronounced PI-GSC differences in women. Women with PI had greater high-frequency EEG activity than GSC women, as well as higher delta and theta EEG power. Men and women have different quantitative EEG sleep characteristics across the adult lifespan.29–32 Previous studies in disorders such as major depression demonstrate interactions between diagnosis and sex.33–35 These studies have, for the most part, demonstrated deficits in delta EEG activity among depressed men, with fewer differences observed between depressed and control women. Thus, the current findings and previous reports suggest that PI and major depression have distinct quantitative EEG profiles, and distinct patterns of sex differences.
The finding of increased low frequency (delta/theta) and high frequency (beta/gamma) power in PI women may reflect an overall increase in EEG power in the PI group, but it also suggests that hyperarousal and “deep” sleep may coexist in PI. Indirectly, this finding also argues against the conceptualization of PI as a disorder characterized by deficient homeostatic sleep drive. Indeed, previous evidence regarding reduced homeostatic sleep drive in chronic insomnia is inconclusive,61 with varying reports of reduced delta activity, no difference, or reduction only in a subgroup of insomnia patients compared to controls.11,14,27 Two of these publications show delta activity that is numerically, but not statistically significantly, greater in PI than GSC.12,27 Individuals with PI have increased SWS following TSD,62,63 demonstrating a capacity to respond to a homeostatic challenge. This response was comparable to GSC in some studies,63,64 but reduced in magnitude or duration in others.63,65,66 PI show increased sleepiness measured on the MSLT following TSD, similar to that seen in GSC.65,67 Finally, as noted in the Introduction, there is no published evidence that high-frequency and delta EEG activity are inversely correlated in insomnia, although such data do exist for GSC.68 Indeed, delta and beta power were positively correlated among PI and GSC in this sample. Thus, what may at first appear to be a contradictory finding, that women with PI may have EEG markers of both increased arousal and increased homeostatic drive, is not necessarily inconsistent with principles of sleep regulation or with previously published studies. The simultaneous occurrence of hyperarousal and increased sleep drive may also relate to common but seemingly contradictory clinical presentations of insomnia, which include disturbed nighttime sleep, daytime sleepiness and fatigue, but difficulty napping. Behavioral treatments such as sleep restriction may be seen as correcting homeostatic sleep deficiency, but could just as plausibly be interpreted as taking advantage of a relatively intact homeostatic mechanism to overcome hyperarousal. Finally, it is also plausible that the increased delta activity seen in women with PI represents something very different, e.g., a manifestation of cyclic alternating pattern (CAP), an arousal pattern in the EEG that has previously been observed in PI.69
The neurobiological significance of changes in EEG power within any specific range remains unknown. Although previous studies have interpreted NREM EEG activity above 16 Hz as being consistent with “hyperarousal” and increased cognitive activity, this interpretation is based mainly on analogies to waking EEG activation patterns. However, the gamma oscillations associated with attention and cognitive activity during wakefulness occur at higher frequencies (typically 40–100 Hz),13,24–26 and are distinct from power in individual EEG waveforms as measured in this and most other sleep studies. Furthermore, the use of 60-Hz notch filters, traditionally used in PSG studies (including the present one), may attenuate power near the 50-Hz frequency. For these reasons, the high-frequency EEG power during NREM sleep cannot be taken as a direct correlate of findings during wakefulness. Additional techniques such as functional brain imaging, brain electrical activity mapping, or magneto-encephalography may help to clarify the significance of high-frequency EEG activity during NREM sleep. For instance, Nofzinger et al.70 found a significant positive association between NREM beta EEG power and orbitofrontal glucose metabolism in both healthy control subjects and depressed individuals.
We found little relationship between high frequency EEG power and specific clinical characteristics of insomnia, and the one significant correlation was in a direction opposite to what would have been predicted. Previous examinations of this question have identified various relationships, but with little consistency across reports. Therefore, it does not yet appear that we can identify consistent subjective correlates of quantitative EEG findings in insomnia.
We acknowledge two other limitations of the current data set. First, our exploratory statistical approach relied upon F tests at successive EEG frequencies, resulting in a large number of statistical comparisons that may not adequately account for correlations among adjacent EEG frequencies. Second, our sample size, while large by the standards of previous quantitative EEG studies in insomnia, it was not large for examining separate effects of sex, NREM periods, and multiple EEG frequencies. In light of these last two points, these findings should be viewed as preliminary, and in need of replication.
Our results highlights again a more general set of weaknesses in insomnia studies: Their limited theoretical or neurobiological basis, variability in selection criteria, limited sample size, variability in specific data methods, and variability in analytic methods. Developing consensus around these issues, as has begun to occur in diagnosis and assessment,71 may help to address in a more definitive way the neurophysiological correlates of insomnia disorder, and of specific symptoms.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of the staff of the Neuroscience Clinical and Translational Research Center and individual research studies for their assistance. Spectral analysis programs were written by Ray Vasko, Ph.D., and Rob Seres, with additional technical assistance from David Cashmere and Jim Havstad.
Supported by NIH grants MH24652, AG00972, AG20677, RR00056, RR024153, MH30915
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Buysse consults for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Somnus Therapeutics, Stress Eraser, Takeda, and Transcept. Dr. Moul has received research support from Takeda. Dr. Nofzinger has received research support from Sepracor, Sanofi-Aventis, and Respironics and honoraria from Takeda, Sanofi-Aventis, and Sepracor. Dr. Kupfer has consulted for Servier and is on the advisory board of Eli Lilly, Forest, Pfizer, and Solvay-Wyeth. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.NIH State of the Science Conference Statement Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 2.Perlis ML, Smith MT, Pigeon WR. Etiology and pathophysiology of insomnia. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 714–25. [Google Scholar]
- 3.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH, Arand DL. Physiological activation in patients with sleep state misperception. Psychosom Med. 1997;59:533–40. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. 2005 doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 9.Rodenbeck A, Hajak G. Neuroendocrine dysregulation in primary insomnia. Rev Neurol (Paris) 2001;157:S57–S61. [PubMed] [Google Scholar]
- 10.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63:408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 11.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 12.Perlis ML, Smith MT, Andrews PJ, et al. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Perlis ML, Merica H, Smith MT, et al. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- 14.Krystal AD, Edinger JD, Wohlgemuth WK, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 15.Nofzinger EA, Buysse DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–31. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AG. Pre-sleep cognitive activity: a comparison of sleep-onset insomniacs and good sleepers. Br J Clin Psychol. 2000;39(Pt 3):275–86. doi: 10.1348/014466500163284. [DOI] [PubMed] [Google Scholar]
- 17.Nelson J, Harvey AG. An exploration of pre-sleep cognitive activity in insomnia: imagery and verbal thought. Br J Clin Psychol. 2003;42:271–88. doi: 10.1348/01446650360703384. [DOI] [PubMed] [Google Scholar]
- 18.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 19.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Semler CN, Harvey AG. An investigation of monitoring for sleep-related threat in primary insomnia. Behav Res Ther. 2004;42:1403–20. doi: 10.1016/j.brat.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Semler CN, Harvey AG. Monitoring for sleep-related threat: a pilot study of the Sleep Associated Monitoring Index (SAMI) Psychosom Med. 2004;66:242–50. doi: 10.1097/01.psy.0000114870.50968.90. [DOI] [PubMed] [Google Scholar]
- 22.Perlis ML, Giles DE, Mendelson WB, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. [Review] [115 refs] J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 23.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. Neuroreport. 2005;16:207–11. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–42. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- 26.Lee KH, Williams LM, Breakspear M, et al. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 27.Perlis ML, Kehr EL, Smith MT, et al. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10:93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 28.Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: A discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- 29.Feinberg I, Higgins LM, Khaw WY, et al. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongrain V, Carrier J, Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005;28:819–27. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- 31.Latta F, Leproult R, Tasali E, et al. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep. 2005;28:1525–34. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 32.Carrier J, Land S, Buysse DJ, et al. The effects of age and gender on sleep EEG power spectral density in the middle years of life (aged 20–60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 33.Armitage R, Hudson A, Trivedi M, et al. Sex differences in the distribution of EEG frequencies during sleep: unipolar depressed outpatients. J Affect Disord. 1995;34:121–9. doi: 10.1016/0165-0327(95)00009-c. [DOI] [PubMed] [Google Scholar]
- 34.Armitage R, Hoffmann R, Trivedi M, et al. Sleep macro- and microarchitecture in depression: age and gender effects. Sleep Res. 1997;26:283. [Google Scholar]
- 35.Armitage R, Hoffmann R, Fitch T, et al. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;23:607–17. [PubMed] [Google Scholar]
- 36.First M, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P). Version 2.0 ed. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 37.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis I Disorders SCID I: Clinician version, administration booklet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 38.American Psychiatric Association. Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [Google Scholar]
- 39.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 44.Regestein QR, Dambrosia J, Hallett M, et al. Daytime alertness in patients with primary insomnia. Am J Psychiatry. 1993;150:1529–34. doi: 10.1176/ajp.150.10.1529. [DOI] [PubMed] [Google Scholar]
- 45.Smets EMA, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI); Psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 46.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 47.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 48.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 49.Doman J, Detka C, Hoffman T, et al. Automating the sleep laboratory: Implementation and validation of digital recording and analysis. Int J Biomed Comput. 1995;38:277–90. doi: 10.1016/s0020-7101(05)80010-8. [DOI] [PubMed] [Google Scholar]
- 50.Rechtschaffen A, Kales A. NIH Publication 204. Washington, DC: US Government Printing Office, Department of Health Education and Welfare; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 51.Vasko RC, Brunner DP, Monahan JP, et al. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. Int J Med Informatics. 1997;46:175–84. doi: 10.1016/s1386-5056(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 52.Brunner DP, Vasko RC, Detka CS, et al. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 53.Buysse DJ, Hall M, Begley A, et al. Sleep and treatment response in depression: New findings using power spectral analysis. Psychiatry Res. 2001;103:51–67. doi: 10.1016/s0165-1781(01)00270-0. [DOI] [PubMed] [Google Scholar]
- 54.American Academy of Sleep Medicine. (ICSD-2): diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. [Google Scholar]
- 55.Edinger JD, Fins AI, Goeke JM, et al. The empirical identification of insomnia subtypes: A cluster analytic approach. Sleep. 1996;19:398–411. [PubMed] [Google Scholar]
- 56.Hohagen F, Kappler C, Schramm E, et al. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–4. [PubMed] [Google Scholar]
- 57.Roth T, Jaeger S, Jin R, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–71. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62:474–82. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Staner L, Cornette F, Maurice D, et al. Sleep microstructure around onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12:319–30. doi: 10.1046/j.0962-1105.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 60.Voderholzer U, Al-Shajlawi A, Weske G, et al. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17:162–72. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- 61.Pigeon WR, Perlis ML. Sleep homeostasis in primary insomnia. Sleep Med Rev. 2006;10:247–54. doi: 10.1016/j.smrv.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Bonnet MH, Arand DL. Activity, arousal, and the MSLT in patients with insomnia. Sleep. 2000;23:205–12. [PubMed] [Google Scholar]
- 63.Besset A, Villemin E, Tafti M, et al. Homeostatic process and sleep spindles in patients with sleep-maintenance insomnia: effect of partial (21 h) sleep deprivation. Electroencephalogr Clin Neurophysiol. 1998;107:122–32. doi: 10.1016/s0013-4694(98)00048-0. [DOI] [PubMed] [Google Scholar]
- 64.Benoit O, Aguirre A. Homeostatic and circadian aspects of sleep regulation in young poor sleepers. Neurophysiol Clin. 1996;26:40–50. doi: 10.1016/0987-7053(96)81533-4. [DOI] [PubMed] [Google Scholar]
- 65.Stepanski E, Zorick F, Roehrs T, et al. Effects of sleep deprivation on daytime sleepiness in primary insomnia. Sleep. 2000;23:215–19. [PubMed] [Google Scholar]
- 66.Bonnet MH. Effect of 64 hours of sleep deprivation upon sleep in geriatric normals and insomniacs. Neurobiol Aging. 1986;7:89–96. doi: 10.1016/0197-4580(86)90145-4. [DOI] [PubMed] [Google Scholar]
- 67.Salin-Pascual RJ, Valencia-Flores M, Campos RM, et al. Caffeine challenge in insomniac patients after total sleep deprivation. Sleep Med. 2006;7:141–5. doi: 10.1016/j.sleep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Merica H, Blois R. Relationship between the time courses of power in the frequency bands of human sleep EEG. Neurophysiol Clin. 1997;27:116–28. doi: 10.1016/S0987-7053(97)85664-X. [DOI] [PubMed] [Google Scholar]
- 69.Parrino L, Ferrillo F, Smerieri A, et al. Is insomnia a neurophysiological disorder? The role of sleep EEG microstructure. Brain Res Bull. 2004;63:377–83. doi: 10.1016/j.brainresbull.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 70.Nofzinger EA, Price JC, Meltzer CC, et al. Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Research: Neuroimaging. 2000;98:71–91. doi: 10.1016/s0925-4927(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 71.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]