Abstract
Objective:
Previous data have suggested that a prolonged QTc interval during the first days of life can be associated with some cases of sudden infant death syndrome (SIDS). Analysis of heart rate variability during sleep in future SIDS victims has shown findings compatible with an imbalance in autonomic tone. We hypothesized that some future SIDS infants could have longer QTc intervals during sleep, compared with healthy control infants, and that this difference would correlate with the autonomic imbalance already found in these infants.
Methods:
QTc intervals and a heart rate autoregressive power spectral analysis were calculated during the same periods in the polysomnographic sleep recordings of 18 infants who eventually died of SIDS and of 18 control infants. The control infants were matched for sex, gestational age, postnatal age, birth weight, and sleep position. The median postnatal age was 8 weeks.
Results:
Compared with control infants, future SIDS victims were characterized by having longer QTc intervals during total sleep (P = 0.019), rapid eye movement sleep (P = 0.045) and non-rapid eye movement sleep (P = 0.029). When the night was divided into 3 equal parts, this difference was always present but was most marked during the last part of the night. There was, respectively, a negative and a positive correlation between parasympathetic activity and sympathovagal balance and median and maximum QTc interval values.
Conclusion:
Compared with QTc intervals in matched control infants, QTc intervals were increased in future SIDS victims. Such a prolongation could be related to the autonomic dysfunction already reported in these patients.
Citation:
Franco P; Groswasser J; Scaillet S; Lanquart JP; Benatar A; Sastre JP; Chevalier P; Kugener B; Kahn A; Lin JS. QT interval prolongation in future SIDS victims: a polysomnographic study. SLEEP 2008;31(12):1691–1699.
Keywords: Autonomic nervous system, infant, QTc, sleep, Sudden Infant Death Syndrome
SUDDEN INFANT DEATH SYNDROME (SIDS) IS DEFINED AS THE SUDDEN DEATH OF AN INFANT UNDER THE AGE OF 1 YEAR THAT REMAINS UNEXPLAINED after a complete postmortem examination, including an investigation of the death scene and a review of the case history. Such deaths occur during sleep, which may be a daytime nap or a night sleep.1 Despite extensive research, the etiology of SIDS is still unknown. Cardiac mechanisms, including life-threatening arrhythmias, have been suspected of causing a proportion of SIDS cases.2 A large prospective cohort study provided evidence of an association between neonatal QT prolongation recorded on electrocardiogram (ECG) during the first days of life and the subsequent occurrence of SIDS.3 Various mechanisms could be implicated in the association between SIDS and QT prolongation. In the late 1970s, Schwartz et al suggested that abnormal development of cardiac sympathetic innervation could occur with a difference in the timing of the maturation between left and right cardiac sympathetic innervation during the first months of life.2 Such QT prolongation could also be an early manifestation of congenital long QT syndrome.2,4 Long QT syndrome is a primary cardiac channelopathy with 7 cardiac ion-channel genes currently implicated: KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, and CAV3.5 Potentially lethal cardiac ion-channel gene mutations have been found in 9.5% of SIDS infants, suggesting that de novo mutations in cardiac ion channels could provide a lethal arrhythmogenic substrate in some infants at risk for SIDS.5 Another possibility is that prolongation of the QTc intervals could be related to the autonomic dysfunction already reported in some of these patients.6–8 In a previous study, following analysis of night polygraphic recordings of 18 infants who died some weeks later of SIDS, compared with those of matched control infants, we reported a higher sympathetic activity in SIDS infants, especially at the end of the night when most cases of SIDS occur.9 In Schwartz' prospective study of QT values,3 an ECG was obtained in the first week of life—when transitional QT prolongation is a relatively common finding. The purpose of the present study was to determine if certain future SIDS victims recorded at 2 to 3 months of age could also present with prolongations of QTc intervals, compared with matched control infants, and if this QTc prolongation was related to sleep stages, time of night, and autonomic nervous system activity.
METHODS
Patients
Diverse sleep research programs on sleep maturation in infants were initiated in Belgium during the 1980s. Parents could have a polysomnographic night recording of their infant during the first year of life to relieve anxiety about SIDS. The indications were very broad and were based on personal and family history (e.g., multiple births, prematurity, maternal smoking, inappropriate weight for gestational age, sibling of a SIDS victim) and also on clinical signs (e.g., apparent life-threatening events, breath-holding spells, episodes of fatigue during feeding, profuse sweating, snoring or noisy breathing during sleep).10 More than 45,000 sleep studies have been recorded in the last 25 years. The prevalence of SIDS during this period was 0.8 per 1000 live births. Forty infants eventually died some days or weeks after their recordings.11 The recordings of the 18 controls were consecutively selected when matching and technical criteria were found. For each SIDS recording, 1 recording of a control infant was selected. Control and SIDS infants were matched for sex, gestational age, postnatal age, birth weight, and sleep position. All control infants were healthy, had no family history of SIDS, and survived the first year of life. Only polysomnographic recordings with digitized ECG signals sampled at 300 Hz were used for the autonomic nervous system studies (Morpheus system, Medatec, Belgium). This system has been available since 1992. All of the recordings of these SIDS and control infants were performed during the same period from 1992 to 1995. Following the SIDS prevention campaign in 1994, the number of SIDS victims decreased abruptly.12 The 18 recordings of future SIDS victims and matched control infants were analyzed, and the results on autonomic controls have been published.9,13 In this population of 18 SIDS victims, 13 were boys, and 6 were preterm. Two of the preterm infants had inappropriate weights for their gestational ages. One infant was a sibling of a SIDS victim. At the time of recording, no infant had signs of infection. No infant was being monitored at the time of death. The 18 deaths were unexpected and remained unexplained despite complete postmortem studies. Data on the children's histories and usual behavior were collected using a standard questionnaire before sleep monitoring was undertaken. The questionnaires were coded and analyzed together with the sleep recordings. Treatment by cisapride was noted, since this prokinetic agent, which facilitates gastrointestinal motility, was widely used for the treatment of gastroesophageal reflux disease in the 1990s.14 Cisapride was subsequently reported to prolong QT intervals and to induce ventricular arrhythmia.15,16 The aims and methodology of the present study were approved by the university ethics committee.
Polygraphic Recordings
The infants were admitted for a night monitoring session that lasted 8 to 9 hours. The data were collected on a computerized polygraph recording system (Morpheus system). The following variables were recorded simultaneously: 8 channels of electroencephalography, 2 channels of electrooculography, digastric electromyography, ECG (DII), thoracic and abdominal respiratory movements by inductive plethysmography, and airflow by means of thermistors taped under each nostril and on the side of the mouth. Oxygen saturation was continuously recorded using a transcutaneous sensor (Ohmeda Box, Madison, WI).
Data Analysis
Sleep Stages
Each recording was allocated a random code number. The code was disclosed after completion of the analysis. Two independent scorers analyzed the sleep recordings to ensure reliability. The 2 scorers, who had not taken part in the collection of the data, analyzed the coded recordings without knowing the patient's identity. Conflicting data were discussed and, after consensus, subsequently agreed-upon codes were used for analysis of the data. Each 30-second period of the recordings was analyzed and categorized as non-rapid eye movement sleep (NREM), rapid eye movement sleep (REM), or movement or wakefulness according to the guidelines in the literature.17 NREM refers to NREM 2 and 3 stages. Sleep efficiency was defined as the ratio of the total sleep time divided by the total recording time, expressed as a percentage. Movement time represents gross body movements detected by movement sensors or seen as movement artifact in the somatic channels (ECG, electroencephalogram, respiratory parameters) for less than 15 seconds and reported as a percentage of total recording time.
Cardiorespiratory Parameters
Sleep apneas were scored only if they lasted 3 seconds or more. A central apnea was scored when flat tracings were obtained simultaneously from thoracic/abdominal movements and thermistors. An obstructive apnea was scored when continuous deflections were obtained from thoracic and abdominal movements while a flat tracing was recorded from thermistors. To avoid artificial scoring due to thermistor displacement, obstructive apneas preceded by body movements, crying, or sighs were rejected. Mixed apneas were defined as central apneas followed directly by obstructive episodes and were scored with obstructive apneas.
QT Intervals
QT and preceding RR intervals were manually measured on the screen using electronic calipers in 3 consecutive heart cycles in each successive REM and NREM sleep stage throughout the night. To ensure signal stability, QT intervals were always measured 5 minutes after the onset of REM and NREM sleep. Care was taken to exclude episodes of desaturation, obstructive apnea, and central apnea. QT was measured from the onset of the Q wave to the end of the T wave, at its point of return to the isoelectric line. The computer “zoom-in” feature made it possible to enlarge the P-QRS-T complex, which provided accurate measurement of the end of the T wave.18 The QT interval was corrected for heart rate with the Bazett formula.19 The median, minimal, and maximal values of QTc intervals were calculated for total sleep, NREM sleep, and REM sleep. Recordings were divided into 3 periods (21:00 to 00:00, 00:01 to 03:00, and 03:01 to 06:00) to evaluate QTc values throughout the night.
Heart Rate Spectral Analysis
Heart rate spectral analysis (HRSA) was obtained as follows: digitized ECG signals were sampled at 300 Hz. Premature ventricular contractions or artifactual RR intervals due to gross body movements or arousals were eliminated by visual analysis of the heart rate data before HRSA was performed. HRSA was computed for periods of 256 successive RR intervals. QT intervals and HRSA were selected at the same time during the night. An HRSA of the trendgram was calculated for each period according to the method proposed in references 20 and 21 and the Task Force recommendations.22 The trendgram was defined by the RR time according to the index of the cardiac beat. The autoregressive analysis was directly applied to this time series. The frequencies were expressed in Hzeq to point out the approximation involved in this procedure. The validity of this approach has been extensively demonstrated.8,13,,20,21,22
Two major peaks were recognizable: a low-frequency (LF) component, defined by a center frequency of 0.1 Hzeq (0.04–0.15 Hzeq) related to sympathetic and parasympathetic activities, and a high-frequency (HF) component, defined by a center frequency of 0.4 Hzeq (> 0.15–2 Hzeq), reflecting parasympathetic tone.20 Respiratory frequency during the selected period was measured manually after being printed. For each 256 RR-interval period, the major component in the LF band of the heart rate spectrum was related to the major component in the HF band, corresponding to the mean respiratory frequency as determined by analysis of breath-to-breath intervals. The LF/HF power ratio for each episode was calculated as an index of the sympathovagal interaction.23 Spectral components were represented as the RR intervals (in ms), power (in msec2), bandwidth (in Hzeq),20,21 and normalized power obtained by dividing the power of the period by the total power component (in %), after subtraction of the direct current component.24
Statistical Analysis
Wilcoxon matched-paired signed rank test was used to compare the SIDS cases with the matched control subjects and to compare QTc values during the 3 periods of the night and between REM and NREM sleep. χ2 test was used for 2 × 2 tables. Spearman correlation coefficient was used to describe the relationship between QT intervals and the HRSA data and the frequency of obstructive apnea. The results were considered as being significant at P < 0.05.
RESULTS
The general characteristics of the infants studied are reported in Table 1. Due to the study design, no differences were noted between the future SIDS victims and the control subjects, except that mothers of future SIDS victims were younger (P = 0.038). Only 1 infant died after 6 months of life, at the age of 36 weeks. Two SIDS infants and 3 control infants were receiving cisapride at the recommended pediatric dosage. At the time of recording, no infant was receiving other medication.
Table 1.
Major Characteristics of the Infants Studied
| SIDS infants | Control infants | |
|---|---|---|
| No. | 18 | 18 |
| Sex, M/F | 13/5 | 13/5 |
| Gestational age, wks | 38 (27–41) | 38 (27–41) |
| Birth weight, gm | 2.925 (0.96–3.98) | 2.790 (1.090–3.840) |
| Age at sleep study, wks | 8 (5–19) | 8 (5–19) |
| Weight at sleep study, gm | 4.380 (2.220–5.900) | 4.845 (2.000–6.400) |
| Sleep position, prone/supine | 9/9 | 9/9 |
| Age at death, wks | 13.5 (10–36) | — |
| Maternal age, ya | 24 (20–29) | 27 (21–36) |
| History | ||
| Sibling of SIDS cases | 1 | 0 |
| Preterm infants | 6 | 6 |
| Inappropriate weight for gestational age | 2 | 2 |
| Maternal smoking | 5 | 1 |
| Multiple births | 3 | 3 |
| Cyanosis episode during the first day of life | 1 | 0 |
| Noisy breathing or snoring during sleep | 3 | 4 |
| Breath-holding spells | 1 | 2 |
| Feeding difficulties (fatigue, choking) | 2 | 5 |
| Regurgitations | 5 | 5 |
| Cisapride therapy (0.80 mg/kg per d) | 2 | 3 |
Data are presented as absolute, median, and range values or number.
Wilcoxon matched-paired test and χ2 test between sudden infant death syndrome (SIDS) cases and controls, P < 0.05.
Sleep Characteristics
No significant differences were noted between the two groups of infants for the main sleep variables (Table 2). As previously reported,13 future SIDS victims had more obstructive and mixed apnea episodes, as compared with their matched control subjects (P = 0.004).
Table 2.
Sleep and Cardiorespiratory Characteristics of SIDS Victims and Control Infants
| Future SIDS infants | Control infants | P Value | |
|---|---|---|---|
| Total recording time, min | 480 (330–510) | 480 (290–510) | NS |
| TST, min | 347 (228–480) | 370 (198–440) | NS |
| Sleep effi ciency, % | 80.2 (48–100) | 77.3 (56.3–91.7) | NS |
| Sleep stage, % TST | |||
| NREM | 29.9 (16.6–43.1) | 32.6 (10.4–40.7) | NS |
| REM | 46.1 (24.4–69.8) | 42.6 (25.2–61.6) | NS |
| Movement time | 4.65 (0–15.5) | 4 (0.1–10.7) | NS |
| Central apnea events | |||
| Frequency, /h sleep | 4.8 (0.7–19.4) | 6.4 (3.2–15.4) | NS |
| Duration, sec | 7.8 (5.1–10.2) | 7.4 (4.3–10.1) | NS |
| Obstructive apnea events | |||
| Frequency, /h sleep | 0.88 (0–4.85) | 0.22 (0–1.91) | 0.004 |
| Duration, sec | 8.1 (4.1–18.9) | 7 (4–9.1) | NS |
| Basal heart rate, bpm | |||
| NREM | 133 (105–147) | 125.5 (109–150) | NS |
| REM | 136 (109–156) | 131 (111–155) | NS |
| Basal breathing rate, bpm | |||
| NREM | 39 (21–45) | 31 (19–39) | NS |
| REM | 43 (24–48) | 36 (20–40.5) | NS |
| Oxygen saturation, % | 93.7 (86.5–98.6) | 95.8 (88.5–97.3) | NS |
Data are presented as median and ranges. Statistical analyses were performed with Wilcoxon matched-paired test between sudden infant death syndrome (SIDS) cases and control subjects, P < 0.05.
QTc Values
There were no significant differences between the two groups for the frequency of studied periods for QT analyses in total sleep (224 in future SIDS victims vs 219 in control infants), in NREM sleep (103 in future SIDS victims vs 102 in control infants), and in REM sleep (121 in future SIDS victims vs 117 in control infants).
As shown in Table 3, compared with the control infants, future SIDS victims were characterized by longer median QTc intervals during total sleep (P = 0.019), NREM (P = 0.029) and REM sleep (P = 0.045). Max QTc intervals were longer during total sleep (P = 0.022) and NREM sleep (P = 0.023) in future SIDS victims. There were no significant differences between REM and NREM sleep for QTc intervals in both SIDS and control infants (Table 3). Only 1 SIDS infant, but no control infants, had median QTc intervals values longer than 440 ms (Table 4). The sibling of the SIDS infant had normal QTc values. There was no significant correlation between the frequency of obstructive apnea and QTc intervals.
Table 3.
QTc Values of SIDS and Matched Control Infants During Total Sleep Time, REM Sleep, and NREM Sleep
| QTc values, ms | Future SIDS Infants | Control infants | P Value |
|---|---|---|---|
| Total Sleep | |||
| Median | 396.2 (342.5–452) | 373 (327.5–409) | 0.019 |
| Max | 405 (358–480) | 392.5 (335–428) | 0.022 |
| NREM sleep | |||
| Median | 393.5 (340–451) | 375.25 (413) | 0.029 |
| Max | 401 (349–459) | 387 (334–428) | 0.023 |
| REM sleep | |||
| Median | 387.2 (345–466) | 371 (328.5–408) | 0.045 |
| Max | 398 (354–480) | 386.5 (333–428) | NS |
| NREM sleep/REM sleep | |||
| Median | NS | NS | |
| Max | NS | NS |
Data are presented as median, and ranges. Statistical analyses were performed with Wilcoxon matched-paired test between sudden infant death syndrome (SIDS) cases and controls, P < 0.05.
NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep.
Table 4.
Individual Median Heart Rate and Median and Max QTc Values for SIDS and Control Infants
| SIDS Infants |
Control infants |
|||||
|---|---|---|---|---|---|---|
| Median HR | Mean QTc | Max QTc | Median HR | Mean QTc | Max QTc | |
| 1 | 104 | 342.5 | 358 | 105 | 327.5 | 335 |
| 2 | 100.5 | 353 | 367 | 112.5 | 350.5 | 365 |
| 3 | 89 | 400 | 420 | 103 | 406 | 409 |
| 4 | 106 | 374 | 392 | 105.5 | 372 | 381 |
| 5 | 103 | 452 | 480 | 123 | 333 | 407 |
| 6 | 114 | 420 | 444 | 109 | 388 | 401 |
| 7 | 111 | 400 | 412 | 89 | 409 | 428 |
| 8 | 92.5 | 357 | 372 | 93 | 371 | 392 |
| 9 | 109.5 | 397 | 425 | 87.5 | 377.5 | 398 |
| 10 | 105 | 390 | 398 | 94 | 384 | 392 |
| 11 | 82.5 | 409.5 | 417 | 94 | 364 | 386 |
| 12 | 99 | 384 | 401 | 92 | 379 | 400 |
| 13 | 84 | 378 | 391 | 84 | 371.5 | 394 |
| 14 | 109 | 352 | 362 | 94 | 364 | 387 |
| 15 | 103 | 396 | 407 | 94 | 387.5 | 401 |
| 16 | 101 | 402 | 415 | 96 | 380 | 393 |
| 17 | 96.5 | 396.5 | 408 | 95 | 374 | 386 |
| 18 | 108 | 399 | 403 | 93 | 360 | 375 |
The data represent median heart rate (HR) and median and max QTc values in total sleep time.
SIDS refers to sudden infant death syndrome.
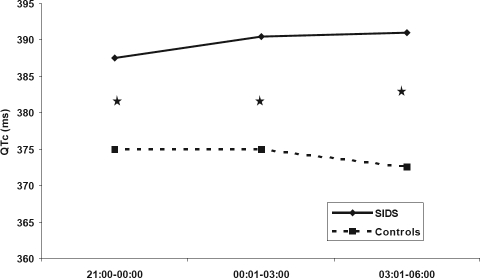
Nycthemeral Variations of QTc Values
Differences in median QTc intervals between the two groups of infants were found throughout the night (Table 5, Figure 1). Max QTc intervals tended to differ between future SIDS victims and control infants in the last part of the night (P = 0.05). These differences were noted when QTc interval values during REM and NREM sleep were considered together. There were no significant differences when the values in REM and NREM were considered separately.
Table 5.
QTc Values of SIDS and Matched Control Infants at 3 Time Intervals Throughout the Night
| QTc values (ms) | Future SIDS Infants | Control infants | P Value |
|---|---|---|---|
| Median values | |||
| 21:00–00:00 | 387.5 (344–413) | 375 (350–399) | 0.019 |
| 00:01–03:00 | 390.5 (354–466) | 375 (349.5–422) | 0.016 |
| 03:01–06:00 | 391 (343.5–457.5) | 372.5 (346–423) | 0.010 |
| Max values | |||
| 21:00–00:00 | 394 (344–425) | 380 (354–400) | NS |
| 00:01–03:00 | 396 (354–480) | 384 (350–428) | NS |
| 03:01–06:00 | 394 (343.5–467) | 378 (351–428) | 0.050 |
The data represent median and range values. Statistical analyses were performed with Wilcoxon matched-paired test between sudden infant death syndrome (SIDS) cases and controls, P < 0.05.
Figure 1.
Nycthemeral variations of median QTc values in sudden infant death syndrome (SIDS) and matched control infants. ★Statistically significant. The figures represent median values.
Short-term Heart Rate Spectral Analysis
Table 6 provides a summary of the short-term spectral analysis in REM and NREM sleep. Compared with control subjects, SIDS infants were characterized by lower HF powers and HF normalized powers and higher LF/HF power ratios in total sleep and NREM sleep. The results were the same in both groups for REM sleep concerning HF powers and LF/HF ratios. There were no significant differences in RR intervals, total power of the spectrum, or LF values in REM and NREM sleep between the two populations.
Table 6.
Short-Term Spectral Analysis in SIDS and Control Infants
| Future SIDS infants | Control infants | P Value | |
|---|---|---|---|
| Total Sleep | |||
| HF power, ms2 | 13.07 (5.06–98) | 31.64 (3.56–174.61) | <0.001 |
| HF normalized power, % | 6.25 (2.5–23.60) | 13.10 (2.45–54.50) | 0.002 |
| LF/HF power ratio, % | 8.99 (1.38–24.24) | 5.04 (0.71–18.82) | 0.005 |
| NREM sleep | |||
| HF power, ms2 | 10.63 (5.06–46.94) | 36.59 (3.56–167.68) | 0.006 |
| HF normalized power, % | 10.20 (5–17.3) | 21.75 (4.5–54.5) | 0.008 |
| LF/HF power ratio, % | 6.65 (2.06–13.98) | 1.96 (0.71–18.82) | 0.050 |
| REM sleep | |||
| HF power, ms2 | 14.09 (5.6–98) | 29.99 (11.91–174.61) | 0.027 |
| HF normalized power, % | 5.4 (2.5–23.6) | 7.68 (2.45–14.9) | NS |
| LF/HF power ratio, % | 9.53 (1.38–24.24) | 5.33 (2.52–17.44) | 0.046 |
The data are presented as median and range values. Statistical analyses were performed with Wilcoxon matched-paired test between sudden infant death syndrome (SIDS) cases and controls, P < 0.05.
NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep; HF, high-frequency; LF, low-frequency.
Correlation Between Short-term HRSA and QTc Values
For the control infants, there was a negative correlation between parasympathetic tone and the median and maximum duration of QTc intervals (respectively r = −0.48, P = 0.048 and r = −0.55, P = 0.023) in NREM sleep. In REM sleep, there tended to be a correlation between median QTc intervals and parasympathetic tone (r = −0.45). For SIDS victims, there was a positive correlation between sympathovagal balance and the minimum of QTc intervals (r = 0.36, P = 0.048) in total sleep. When the data of control and SIDS victims were considered together, there was a negative correlation between parasympathetic tone and the median and maximum QTc intervals (respectively r = −0.25, P = 0.043 and r = −0.25, P = 0.045). There was also a positive correlation with sympathovagal tone and median, minimum, and maximum QTc intervals (respectively r = 0.26, P = 0.033; r = 0.28, P = 0.024; r = 0.301, P = 0.016).
No significant differences were found between infants receiving cisapride and those without treatment in both SIDS and control infants. Prone position was not associated with significant differences in QTc interval duration in either group of infants.
DISCUSSION
Schwartz and colleagues have already suggested, in a prospective study of more than 33,000 infants, that prolongation of the QT interval on the ECG recorded in the first week of life is strongly associated with incidence of SIDS.3 Our study demonstrated that, a few weeks before death, SIDS victims had longer QT intervals than control infants throughout the night in REM and NREM sleep. This was especially observed in the late hours of the night when most SIDS occur.25, 26 QT intervals were significantly correlated with other aspects of autonomic regulation, showing, respectively, a negative and a positive correlation between parasympathetic tone and sympathovagal balance.
The QT interval represents repolarization of the ventricular myocardial cells.27 If ventricular repolarization is prolonged, there is a risk of torsades de pointes when a ventricular extra beat occurs, leading to ventricular fibrillation with sudden death as a terminal event.28 QT prolongation can occur from a genetic abnormality or acquired factors. The inherited forms of long QT syndrome are mostly secondary to a dysfunction in the transport of potassium and sodium ions through channels across the myocardial cell membranes.29 Seven genotypes of long QT syndrome have been identified in families with long QT syndrome.30 The 3 most common types are KCNQ1, KCNH2, and SCN5A. Mutations in cardiac ion channels, responsible for the long QT syndrome, were identified in cases initially diagnosed as SIDS.30,31 In the first genetic analysis of the LQT genes in SIDS victims, SCN5A defects were reported in 5% of a prospective SIDS population-based cohort.4 Recently, Arnestad et al found 9.4% of the mutations as likely contributors to sudden death in more than 200 cases of SIDS.5 We found that most of the QTc values of SIDS victims were longer than those of the control infants but were usually within normal ranges. In our study, only 1 SIDS infant, but no control infants, had a markedly long QTc interval, with a QTc max at 480 ms. It is probable that this infant had long QT syndrome.
The QT interval is influenced by many factors, including medications (such as cisapride), myocardial disease, biochemical factors (particularly hypokalemia), and physiologic factors (e.g., autonomic nervous system, sleep).14,32–39 The autonomic nervous system can modify the QT interval by its parasympathetic and sympathetic influences on the sinoatrial node by modulating cardiac rhythm or directly through its sympathetic ventricular innervation.34–37 A circadian modulation of the QT-RR relationship by the sympathetic-vagal balance has shown prolongation of the QT interval during sleep independent of heart rate in normal subjects.38,39 QT and QTc intervals reach their peaks during the early waking hours, which could reflect the increased autonomic instability at this time of increased vulnerability to ventricular tachycardia and sudden cardiac death.39
Cerebral injury at all ages can cause QT prolongation.40 Pathologic and immunohistochemical studies in SIDS infants have demonstrated diffuse lesions within different nuclei of the central nervous system, especially at the brainstem level41–43 but also in the cerebellum.44–45 Serotonergic neuron abnormalities have been reported in the ventral medulla of SIDS victims, in brainstem structures associated with respiratory, cardiovascular, and arousal control.46,47 Dysregulation in the autonomic nervous system has recently been recorded in a future SIDS victim whose postmortem investigation later demonstrated brainstem serotoninergic abnormalities.48 Otherwise, the cerebellum may play a critical role in compensatory responses, particularly to autonomic challenges, and mediate failure mechanisms in SIDS.49
Evidence of changes in cardiac autonomic controls—such as higher heart rate,6 decreased heart rate variability, profuse night sweating, lower parasympathetic tone, or higher sympathovagal balance7,8,11—has been observed in infants who eventually died of SIDS. The obstructive sleep apneas found in future SIDS victims could also be associated with abnormal autonomic control of the upper airways.9,12,50
The correlation between QT interval values and autonomic control found in this study confirmed the relation between QT prolongation and autonomic imbalance. The most marked difference in QT intervals between SIDS and control infants was found during the last part of the night when a high desynchronized sympathetic peak appears in SIDS victims.9 QT prolongation could act as an arrhythmogenic substrate that requires a trigger, such as a stress condition for the development of life-threatening arrhythmias. Infants could be exposed to several conditions that increase cardiac electrical instability: REM sleep with bursts of vagal and sympathetic activation; minor upper respiratory tract infections that, in infants, easily induce hypoxemia and trigger chemoreceptive reflexes; and environmental risk conditions that increase sympathetic activity, such as the prone position,51 maternal smoking,52 high ambient room temperature, and sleep deprivation. The factors reported to be protective for SIDS, such as the use of a pacifier53 and sleeping supine in a swaddled condition, have an inverse effect on the autonomic system, increasing parasympathetic tonus, decreasing sympathetic activation, or both. The increase in sympathetic activity could reduce the electrical stability of the heart and precipitate ventricular fibrillation and sudden cardiac death.54
Moreover, most deaths from SIDS occur in the first 6 months of life, with a specific peak between 2 and 4 months of age, which coincides with the period when the QTc interval tends to be longest after the first week of life.55 It has been postulated that this QT prolongation represents a transient development imbalance between the innervation by the right and left sympathetic nerves.2,55 In agreement with other authors, we did not find statistical differences in QT intervals between the prone and supine positions in 2- to 3-month-old infants.56,57 Ariagno et al found longer QTc in the prone position only in preterm infants at 1 month but not at 3 months corrected age.57
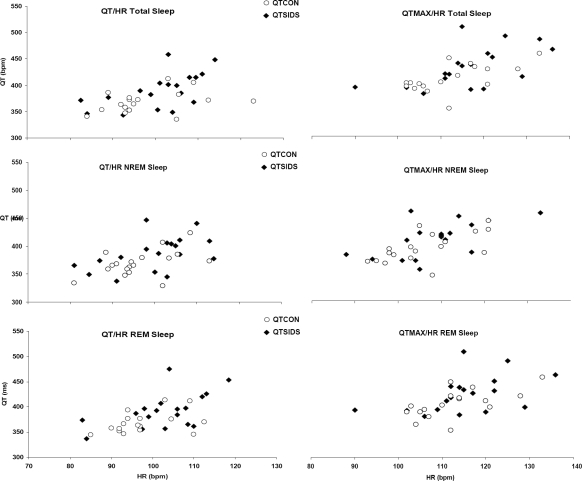
We must admit that there were several limitations on our study. Firstly, because of the limited number of SIDS subjects available for analysis, this report was restricted to the description of QTc and heart rate spectral analysis. No multiple analyses were performed on the various infant characteristics that could have led to the identification of determinant factors in QTc interval prolongation. It can also not be excluded that some biases exist in the selection of these infants in the general population. However, there were no significant differences between the two analyzed groups (Table 1). All control infants were healthy and survived the first year of life. Secondly, it is of interest to note that there are no official guidelines on QT-interval measurement in childhood. There is no consensus on the number of successive RR intervals required for an accurate evaluation of the QTc. With the advent of modern technology, clinicians have at their disposal various measurement procedures, including digital automatic devices, manual measurement with calipers or rulers, application of a digitizing board with or without magnification, on-screen measurement with electronic calipers, and others.58 The digital manual method in our study could be more accurate than manual measurement. QT interval is often corrected for heart rate. In pediatric practice, the Bazett formula is a widely used method for QT heart rate correction.18 Some investigators have questioned the appropriateness of this formula for correction of the QT interval. More specifically, the Bazett formula overadjusts the QT interval at high heart rates.17 In our study, there were no statistical differences in heart rate values between SIDS and control infants. We cannot exclude the possibility that application of the Bazett formula influenced QT values. Such a bias would, however, affect the data from infants in both groups. In Figure 2, we show heart rate and noncorrected QT values on scatter plots. More SIDS infants had higher median and maximum noncorrected QT values, as compared with control infants, in total sleep, NREM sleep, and REM sleep. Thirdly, as for heart rate spectral analysis, crossspectral analysis of respiration and heart rate changes were not evaluated.7 Our previous studies comparing future SIDS victims with control infants have provided results11 that are similar to those reported by authors using cross-spectral analysis of respiration and heart rate changes.7 Moreover, the LF/HF ratio must be interpreted with care.22,59 Although it is generally accepted that, in the HF band, the respiratory peak is principally vagally mediated and can be used as a measure of parasympathetic activity22,60; within the LF range, heart rate fluctuations depend on both sympathetic and parasympathetic controls.22 Vasomotor or thermal influences can be observed at less than 0.09 Hz,61 and baroreceptor controls contribute to changes in the 0.1- to 0.15-Hz frequency band.62 With these restrictions in mind, the ratio of LF/HF powers is usually considered as an index of sympathovagal interaction.22,23,59 Finally, genetic analysis of the implicated cardiac ion-channel genes could not be performed in this retrospective study.
Figure 2.
Individual noncorrected median and max QT values presented according to their heart rate values in total sleep time, non-rapid eye movement (NREM) sleep and rapid eye movement 2
To conclude, SIDS victims, as a group, had longer QTc intervals than control infants. This prolongation could be related to the autonomic dysfunction already reported in these patients. In our study, 1 infant probably had long QT syndrome. The concept of systematic ECG screening in newborns to prevent SIDS is still being debated.28 SIDS is a multifactorial disease. The huge body of epidemiologic and physiologic data would appear to suggest that a deficit in cardiorespiratory control of arousal could be implicated as a determinant key cause of SIDS.63 Schwartz and al propose ECG screening as a cost-effective program to prevent all long QT syndrome-related deaths and, in particular, early long QT syndrome SIDS-labeled deaths.64,65 We have asked ourselves if the death of the infant with the QTc of 480 milliseconds and who was probably affected by long QT syndrome could have been prevented by using a β-adrenergic receptor-blocking treatment. This study advances our comprehension of the mechanisms favoring the unexpected death of an infant during sleep and provides additional data for subsequent discussion of the benefit of newborn ECG screening programs.66
ACKNOWLEDGMENTS
We thank Etienne Denayer, Elie Troch, and Gabriel Debilly for their technical assistance; Catherine Limoge for her dedicated administrative work; Susan Higgins and Peter Tucker for the English revision of the manuscript; and Rene Ecochard for his advice on statistical analysis.
ABBREVIATIONS
- HF
high frequency
- HRSA
heart rate power spectral analysis
- Hzeq
Herz equivalent
- LF
low frequency
- LF/HF
low frequency to high frequency power ratio
- QTc
Corrected QT interval
- NREM
Non-rapid eye movement sleep
- REM
Rapid eye movement sleep
- SIDS
sudden infant death syndrome
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–8. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ. Cardiac sympathetic innervation and the sudden infant death syndrome. A possible pathogenetic link. Am J Med. 1976;60:167–72. doi: 10.1016/0002-9343(76)90425-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Stramba-Badiale M, Segantini A, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–14. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman MJ, Siu BL, Sturner WQ, et al. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–9. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 5.Arnestad M, Crotti L, Rognum TO, et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–7. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of sudden infant death syndrome. J Pediatr. 1986;109:249–54. doi: 10.1016/s0022-3476(86)80380-8. [DOI] [PubMed] [Google Scholar]
- 7.Kluge KA, Harper RM, Schechtman VL, Wilson AJ, Hoffman HJ, Southall DP. Spectral analysis assessment of respiratory sinus arrhythmia in normal infants and infants who subsequently died of sudden infant death syndrome. Pediatr Res. 1988;24:677–82. doi: 10.1203/00006450-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kitney RI. New findings in the analysis of heart rate variability in infants. Automedica. 1984;5:289–310. [Google Scholar]
- 9.Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin Auton Res. 1998;8:243–9. doi: 10.1007/BF02277969. [DOI] [PubMed] [Google Scholar]
- 10.Kahn A, Groswasser J, Sottiaux M, et al. Clinical symptoms associated with brief obstructive sleep apnea in normal infants. Sleep. 1993;16:409–13. doi: 10.1093/sleep/16.5.409. [DOI] [PubMed] [Google Scholar]
- 11.Kato I, Groswasser J, Franco P, Scaillet S, Kelmanson I, Togari H, Kahn A. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am J Respir Crit Care Med. 2001;64:1464–9. doi: 10.1164/ajrccm.164.8.2009001. [DOI] [PubMed] [Google Scholar]
- 12.Ministère de la Communauté française de Belgique. Statistiques de décès en Communauté Française 1990–1997. Institut Scientifique de la Santé Publique. 2003 ( http://www.iph.fgov.be/epidemio/spma)
- 13.Franco P, Szliwowski H, Dramaix M, Kahn A. Decreased autonomic responses to obstructive sleep events in future victims of sudden infant death syndrome. Pediatr Res. 1999;46:33–9. doi: 10.1203/00006450-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Barone JA, Jessen LM, Colaizzi JL, Bierman RH. Cisapride: a gastrointestinal prokinetic drug. Ann Pharmacother. 1994;28:488–500. doi: 10.1177/106002809402800413. [DOI] [PubMed] [Google Scholar]
- 15.Lewin MB, Bryant RM, Fenrich AL, Grifka RG. Cisapride-induced long QT interval. J Pediatr. 1996;128:279–81. doi: 10.1016/s0022-3476(96)70409-2. [DOI] [PubMed] [Google Scholar]
- 16.Hill SL, Evangelista JK, Pizzi AM, Mobassaleh M, Fulton DR, Berul CI. Proarrhythmia associated with cisapride in children. Pediatrics. 1998;101:1053–6. doi: 10.1542/peds.101.6.1053. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Souquet M. Sleep states and related pathology. In: Korobkin R, Guilleminault C, editors. Advances in Perinatal Neurology. New York, NY: Spectrum Publications; 1979. pp. 225–47. [Google Scholar]
- 18.Benatar A, Ramet J, Decraene T, Vandenplas Y. QT interval in normal infants during sleep with concurrent evaluation of QT correction formulae. Med Sci Monit. 2002;8:CR351–6. [PubMed] [Google Scholar]
- 19.Bazett JC. An analysis of time relation of electrocardiograms. Heart. 1920;7:353–67. [Google Scholar]
- 20.Baselli G, Cerutti S, Civardi S, et al. Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Comput Biomed Res. 1986;19:520–34. doi: 10.1016/0010-4809(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 21.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 22.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 23.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 24.Akaike H. Statistical predictor identification. Ann Inst Stat Math. 1970;22:203–17. [Google Scholar]
- 25.Nelson EA, Taylor BJ, Mackay SC. Child care practices and the sudden infant death syndrome. Aust Paediatr J. 1989;25:202–4. doi: 10.1111/j.1440-1754.1989.tb01455.x. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 26.Norvenius SG. Sudden infant death syndrome in Sweden in 1973–1977 and 1979. Acta Paediatr Scand Suppl. 1987;333:1–138. doi: 10.1111/j.1651-2227.1987.tb17289.x. [DOI] [PubMed] [Google Scholar]
- 27.Skinner J. Is there a relation between SIDS and long QT syndrome. Arch Dis Child. 2005;90:445–9. doi: 10.1136/adc.2004.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman M. The long QT syndrome. Pediatr Rev. 1998;19:232–8. doi: 10.1542/pir.19-7-232. [DOI] [PubMed] [Google Scholar]
- 29.Moss A. Long QT syndrome. JAMA. 2003;289:2041–4. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz PJ, Priori SG, Dumaine R, et al. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–7. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PJ, Priori SG, Bloise R, et al. Molecular diagnosis in a child with sudden infant death syndrome. Lancet. 2001;20(358):1342–3. doi: 10.1016/S0140-6736(01)06450-9. [DOI] [PubMed] [Google Scholar]
- 32.Yelamanchi VP, Molnar J, Ranade V, Somberg JC. Influence of electrolyte abnormalities on interlead variability of ventricular repolarization times in 12-lead electrocardiography. Am J Ther. 2001;8:117–22. doi: 10.1097/00045391-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Taran LM, Szilagyi N. The duration of the electrical systole (Q-T) in acute rheumatic carditis in children. Am Heart J. 1947;33:14–26. doi: 10.1016/0002-8703(47)90421-3. [DOI] [PubMed] [Google Scholar]
- 34.Cappato R, Alboni P, Pedroni P, Gilli G, Antonioli GE. Sympathetic and vagal influences on rate-dependent changes of QT interval in healthy subjects. Am J Cardiol. 1991;68:1188–93. doi: 10.1016/0002-9149(91)90192-n. [DOI] [PubMed] [Google Scholar]
- 35.Magnano AR, Holleran S, Ramakrishnan R, Reiffel JA, Bloomfield DM. Autonomic nervous system influences on QT interval in normal subjects. J Am Coll Cardiol. 2002;39:1820–6. doi: 10.1016/s0735-1097(02)01852-1. [DOI] [PubMed] [Google Scholar]
- 36.Browne KF, Zipes DP, Heger JJ, Prystowsky EN. Influence of the autonomic nervous system on the Q-T interval in man. Am J Cardiol. 1982;50:1099–103. doi: 10.1016/0002-9149(82)90425-8. [DOI] [PubMed] [Google Scholar]
- 37.Zaza A, Malfatto G, Schwartz PJ. Sympathetic modulation of the relation between ventricular repolarization and cycle length. Circ Res. 1991;68:1191–203. doi: 10.1161/01.res.68.5.1191. [DOI] [PubMed] [Google Scholar]
- 38.Molnar J, Zhang F, Weiss J, Ehlert FA, Rosenthal JE. Diurnal pattern of QTc interval: how long is prolonged? Possible relation to circadian triggers of cardiovascular events. J Am Coll Cardiol. 1996;27:76–83. doi: 10.1016/0735-1097(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 39.Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol. 1983;52:55–9. doi: 10.1016/0002-9149(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 40.Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 41.Takashima S, Becker LE. Delayed dendritic development of catecholaminergic neurons in the ventrolateral medulla of children who died of sudden infant death syndrome. Neuropediatrics. 1991;22:97–9. doi: 10.1055/s-2008-1071424. [DOI] [PubMed] [Google Scholar]
- 42.Filiano JJ, Kinney HC. Arcuate nucleus hypoplasia in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1992;51:394–403. doi: 10.1097/00005072-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Waters KA, Meehan B, Huang JQ, Gravel RA, Michaud J, Cote A. Neuronal apoptosis in sudden infant death syndrome. Pediatr Res. 1999;45:166–72. doi: 10.1203/00006450-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Kinney HC, McHugh T, Miller K, Belliveau RA, Assmann SF. Subtle developmental abnormalities in the inferior olive: an indicator of prenatal brainstem injury in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2002;61:427–41. doi: 10.1093/jnen/61.5.427. [DOI] [PubMed] [Google Scholar]
- 45.Lavezzi AM, Ottaviani G, Mauri M, Matturri L. Alterations of biological features of the cerebellum in sudden perinatal and infant death. Curr Mol Med. 2006;6:429–35. doi: 10.2174/156652406777435381. [DOI] [PubMed] [Google Scholar]
- 46.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–47. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- 47.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 48.Kinney HC, Myers MM, Belliveau RA, et al. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol. 2005;64:689–94. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- 49.Harper RM, Woo MA, Alger JR. Visualization of sleep influences on cerebellar and brainstem cardiac and respiratory control mechanisms. Brain Res Bull. 2000;53:125–31. doi: 10.1016/s0361-9230(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan CE, Grunstein RR, Marrone O. Sleep apnea-pathophysiology: upper airway and control of breathing. In: Guilleminault C, Partinnen M, editors. Obstructive sleep Apnea Syndrome: Clinical Research and Treatment. New York, NY: Raven Press Ltd; 1990. pp. 49–69. [Google Scholar]
- 51.Franco P, Groswasser J, Sottiaux M, Broadfield E, Kahn A. Decreased cardiac responses to auditory stimulation during prone sleep. Pediatrics. 1996;97:174–8. [PubMed] [Google Scholar]
- 52.Franco P, Chabanski S, Szliwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatr Res. 2000;47:215–20. doi: 10.1203/00006450-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Franco P, Chabanski S, Scaillet S, Groswasser J, Kahn A. Pacifier use modifies infant's cardiac autonomic controls during sleep. Early Hum Dev. 2004;77:99–108. doi: 10.1016/j.earlhumdev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–70. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz PJ, Montemerlo M, Facchini M, Salice P, Rosti D, Poggio G, et al. The QT interval throughout the first 6 months of life: a prospective study. Circulation. 1982;66:496–501. doi: 10.1161/01.cir.66.3.496. [DOI] [PubMed] [Google Scholar]
- 56.Baker SS, Milazzo AS, Jr, Valente AM, et al. Measures of cardiac repolarization and body position in infants. Clin Pediatr (Phila) 2003;42:67–70. doi: 10.1177/000992280304200110. [DOI] [PubMed] [Google Scholar]
- 57.Ariagno RL, Mirmiran M, Adams MM, Saporito AG, Dubin AM, Baldwin RB. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months' corrected age. Pediatrics. 2003;111:622–5. doi: 10.1542/peds.111.3.622. [DOI] [PubMed] [Google Scholar]
- 58.Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749–66. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 59.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 60.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigated by spectral analysis. Am J Physiol. 1985;249:H867–75. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 61.Burton AC. The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. Am J Physiol. 1939;127:437–453. [Google Scholar]
- 62.Schweitzer A. Rhythmical fluctuations of the arterial blood pressure. J Physiol. 1945;104:25P. [Google Scholar]
- 63.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–87. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz P. Newborn ECG screening to prevent sudden cardiac death. Heart Rhythm. 2006;3:1353–5. doi: 10.1016/j.hrthm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 65.Quaglini S, Rognoni C, Spazzolini C, et al. Cost-effectiveness of neonatal ECG screening for the long QT syndrome. Eur Heart J. 2006;15:1824–32. doi: 10.1093/eurheartj/ehl115. [DOI] [PubMed] [Google Scholar]
- 66.Berul C, Perry J. Contribution of long-QT syndrome genes to sudden infant death syndrome. Circulation. 2007;115:294–6. doi: 10.1161/CIRCULATIONAHA.106.675470. [DOI] [PubMed] [Google Scholar]