Abstract
Study Objectives:
This paper aims to determine whether experimental arousals from sleep delay the sleep related fall in cardiovascular activity in healthy adults.
Design:
We report the results of 2 studies. The first experiment manipulated arousals from sleep in young adults. The second compared the effect of frequent arousals on young and middle-aged adults. The influence of arousals were assessed in 2 ways; (1) the fall in cardiovascular activity over sleep onset and the early sleep period, and (2) the underlying sleep levels during the sleep periods in between arousals.
Setting:
Both experiments were conducted in the sleep laboratory of the Department of Psychology, The University of Melbourne, Australia.
Participants:
There were 5 male and 5 female healthy individuals in each experiment between the ages of 18–25 years (Experiment 1) and 38–55 years (Experiment 2).
Interventions:
Participants in Experiment 1 were aroused by auditory stimuli every (i) 2 min, (ii) 1 min, and (iii) 30 sec of sleep for 90 min after the first indication of sleep. In a control condition, participants slept undisturbed for one NREM sleep cycle. Experiment 2 compared the control with the 30-sec condition in the young adults and in an additional group of middle-aged adults.
Measurements and Results:
The dependent variables were blood pressure (BP) and heart rate (HR). In Experiment 1, sleep fragmentation at higher frequencies retarded the fall in BP over sleep onset but did not affect the underlying sleep levels. Experiment 2 showed that there were no age differences on the effect of arousals on changes in BP and HR during sleep.
Conclusions:
This paper supports the hypothesis that repetitive arousals from sleep independently contribute to elevations in BP at night.
Citation:
Carrington MJ; Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. SLEEP 2008;31(12):1701–1712.
Keywords: Sleep Fragmentation, heart rate, blood pressure, sleep onset
AN EXTENSIVE LITERATURE DEMONSTRATES THAT SLEEP INDUCES CHANGES IN BLOOD PRESSURE (BP) AND HEART RATE (HR) ACTIVITY. THESE CHANGES occur rapidly at sleep onset (SO)1–3 with a decrease in both BP and HR. The sleep related drop in BP is termed dipping, defined as a proportional day/night BP difference of >10%, whereas a diminished fall in BP is referred to as non-dipping.4 A non-dipping BP profile is often observed in individuals with obstructive sleep apnea (OSA).5–7
Non-dipping in OSA may be a consequence of repetitive arousals from sleep. Arousals dramatically increase BP and HR and are associated with sympathetically mediated peripheral vasoconstriction.5,8,9 The cardiovascular response may be exacerbated in individuals with OSA because of the added consequences of episodes of hypoxemia and excessive negative intrathoracic pressure that characterize the disorder. However the hypothesis that repetitive arousals from sleep cause a non-dipping BP profile is difficult to test in OSA patients because of the difficulty isolating arousals from the other physiologic changes involved in the disorder. Therefore we induced experimental arousals from sleep in normal individuals.
We have previously shown in healthy young adults that spontaneous arousals delay the fall in BP over SO.10 This suggests that sleep stability is an important factor for the development of the BP dip that is typical of NREM sleep in normal sleepers. It further suggests that experimental arousals extending into NREM sleep may continue to delay the fall in BP. The first experiment of this paper evaluated the frequency of experimental arousals necessary to inhibit the fall in BP and produce a non-dipping effect. The second study investigated whether age influences the ability to create a non-dipping BP profile. Additionally in these studies, we evaluated the effect of repetitive arousals from sleep on the underlying sleep levels of BP and HR during periods of sleep between arousals.
EXPERIMENT ONE
METHODS
Participants
There were 5 male and 5 female participants with an average age of 20 ± 2 years (range 18–25 years) and a median body mass index (BMI) of 23 kg/m2 with an interquartile range (IQR) of 22–25 kg/m2. Participants were screened via questionnaire at an intake interview and were excluded from participating for a personal or familial history of a sleep, cardiovascular, or respiratory disorder; smoking; intense regular physical exercise (> 10 h/wk); excessive alcohol (> 5 standard drinks/wk) or caffeine (> 350 mg/day, ∼ 2 medium-sized coffees) intake; regular daytime napping; and abnormal sleep/wake schedules (including shiftwork) or transmeridian travel in the previous 3 months. During the study, thoracic and abdominal effort measures were recorded, and participants' sleep records were subsequently scrutinized for evidence of a sleep disorder or sleep disordered breathing. Participants were not taking any medication other than birth control pills and did not have any physical illness or hearing limitations, as established by verbal reports. As the intensity of auditory stimulation was adjusted to achieve arousal from sleep, undetected hearing loss would not have been an issue. Menstrual phase was not controlled for. Informed consent to participate was given, and participants were reimbursed for their time commitment to the study. The project was approved by the Human Research Ethics Committee of The University of Melbourne.
Design
The study consisted of a repeated measures design with 3 experimental and one control condition. In all conditions 2 nights of data were collected, with the order of nights counterbalanced across participants. Two consecutive nights were conducted, provided that the first was a control night. Participants were aroused by auditory stimuli following each; a) 2 min, b) 1 min or c) 30 sec of sleep. This protocol of sleep fragmentation approximated levels of sleep disturbance seen in patients with OSA. The duration of the intervention lasted 90 min following the first indication of sleep (10 sec of continuous theta activity) or until REM sleep occurred. In the control condition, participants engaged in their usual sleep routines and slept undisturbed until the first NREM sleep cycle was completed, although only the first 20 min of stable sleep was subsequently analyzed. The dependent variables were systolic blood pressure (BPsys), diastolic blood pressure (BPdia), and HR.
The decision to study sleep onset (SO) and the first 20 min of stable sleep was based on 2 considerations. First, the current studies follow from an earlier experiment in which spontaneous arousals during SO were found to delay the sleep related fall in cardiovascular activity10; that is, the specific interest was the onset of sleep. Second, whole night effects would have confounded the specific effects of arousal from sleep with the consequences of extended sleep fragmentation, the latter being a question of interest, but not the aim of the current studies.
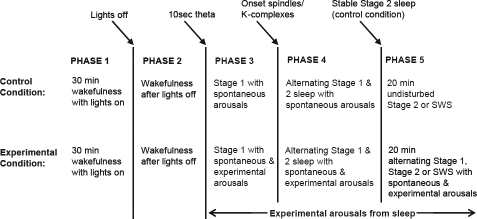
The transition from wakefulness to sleep follows a variable time course over nights within individuals and between individuals. In order to average data over nights and subjects and to compare between conditions, we have developed a method by which the SO transition is divided into 5 consecutive phases using procedural and electrophysiological factors identified from the sleep recordings (Figure 1).10
Figure 1.
Five phases of SO based on procedural events and electrophysiological factors for sleep staging in the control and experimental conditions.
Only phases 1 (30 min pre-sleep wakefulness) and 5 (20 min stable sleep for the control condition and 20 min experimental arousal from sleep for the experimental conditions) were of a constant length for all participants' nights. Within these 2 phases, the data were averaged into 15 (phase 1) or 10 (phase 5) consecutive 2-min epochs. In phase 5 for the control condition, periods where the subject aroused from sleep were discarded until the participant had returned to stable sleep for 1 min. With the exception of one night, all subjects entered SWS on each control night during the 20 min of phase 5. In phase 5 for the experimental conditions, data including extended periods of α activity were discarded until the participant went back to sleep and the arousal procedure resumed. This was to ensure that periods of stable sleep in the control condition were being compared to periods of fragmented sleep in the experimental conditions. Phases 2, 3, and 4 did not have constant lengths across nights or across participants. Therefore, these data were averaged within proportions of the total time spent in the phase, such that each phase was divided into 10 equal segments, with one epoch representing 10% of the total phase length.
Procedure
Participants were requested to maintain their regular sleep/ wake routine in the week prior to, and on nights in between the test nights, and were asked to eliminate alcohol and caffeine intake in the 24 h before each session. On each night, participants went to bed at least 45 min before their usual SO time in order to collect baseline wakefulness data. During this time they were permitted to read or watch television/video while their recordings were monitored to ensure they did not go to sleep. Both ambient light and room temperature were kept constant at approximately 50 lux and 22–24°C, respectively. The lights were then turned off at participants' normal time of SO, and they were instructed to go to sleep. Participants remained supine throughout the recording period.
On experimental nights, to avoid startle responses to the arousing stimuli, the intensity of the auditory stimuli was manipulated to be just sufficient to produce an arousal from sleep. The procedure for presenting auditory stimuli was adapted from Philip et al.11 Initially, a 70-dB tone was presented for 5 sec, and an immediate judgment by the experimenter was made on whether an ASDA arousal response occurred.12 The decision was based primarily on the electroencephalogram (EEG), but other channels, particularly the electromyogram (EMG), could be used for assistance. If no EEG arousal occurred, a 70-dB tone was presented for 10 sec. Thereafter if no response was observed, stimulus intensities were progressively increased by increments of 10 dB, lasting 10 sec, to a maximum of 100 dB, until an arousal from sleep was achieved. If no EEG arousal was produced, the time between subsequent arousal attempts was < 2 sec from the offset of the previous tone. Occasionally, presentation of the maximal intensity stimulus was unsuccessful in producing an arousal from sleep, and the investigator entered the bedroom to physically awaken the participant. After a short period of repetitive sleep fragmentation, an intensity that typically elicited an arousal from a particular individual was reached, so that arousals could be induced near to the specified time intervals, thus minimizing consecutive tone presentations. Following an arousal, participants returned to sleep for 2 min, 1 min, or 30 sec, depending on the experimental condition, before applying the next stimulus.
Equipment and Materials
Auditory stimuli were delivered by 2 Media Theater speakers (Boston Acoustics Inc, Peabody, MA, USA) positioned 70 cm apart and 70 cm above participants' heads. The tones were digitally generated by a 16-bit sound blaster (Vibra 16 CT4180) audio card (Creative Technology Ltd, Singapore) and Pentium IV PC with a continuous 1000-Hz frequency.
In accordance with standardized sleep recordings and procedures,13 participants' sleep/wake state was assessed by a central (C3-A2) and occipital (O1-A2) EEG, a submental EMG, and an electrooculogram (EOG) (left and right outer canthi offset from horizontal).
Continuous noninvasive measurement of BP was via a Portapres Model-2 device (TNO-TPD Biomedical Instrumentation, Amsterdam, The Netherlands) that utilized the arterial volume-clamp method of BP assessment.14 Inflatable cuffs that alternated every 15 min were firmly placed around the index and middle fingers of the right hand. By regularly alternating measurements between 2 fingers, ischemic discomfort associated with continuous cuff inflation was reduced, and the apparatus was deemed minimally intrusive. With the exception of a small number of participants who read prior to the lights being turned off, the cuffed fingers were maintained at heart level throughout the recording procedure; however, this was not considered critical because of the automated height adjustment feature of the equipment.
The BP data were inspected for inappropriate cuff application (that can cause baseline differences in BP values between the 2 cuffs) and for body movement artefact that may have shifted the cuffs, thus preventing BP from being measured accurately. BP values were not retained in these instances, but there were few events of this type, and a full-night recording was never lost. Additionally, because temperature affects peripheral arterial tone of the finger, the laboratory temperature was maintained at 22–24°C, although obviously body temperature per se could not be controlled.
Three Meditrace Ag/AgCl spot electrodes (Graphic Controls Corp., Buffalo, NY, USA) were used to assess HR with an electrocardiogram (ECG). Electrodes were placed on the lower left and lower right rib cage (ground) and another on the right clavicular notch. The left rib cage and clavicular notch electrodes served as recording sites.
Respiratory effort was assessed via thoracic and abdominal bands using a Respitrace Ambulatory Interface Model 10, 4200 (Research Instrumentation Associates, Inc. Beachwood, OH, USA) attached to elastic belts with piezoelectric sensors. During the initial pre-sleep wakefulness period (before the 30 min of baseline wakefulness), the thoracic and abdominal bands were calibrated against ventilation, as measured by a pneumotachograph. The airflow system was calibrated prior to each testing session using a Shorate, Model 1355 flow generator and flow meter (Brook Instruments, Hatfield, PA, USA). While respiratory measurements were not formally analyzed for this paper, these data have been published,15 and in this study, the respiratory signals were scrutinized in participants' sleep records for evidence of any sleep disordered breathing.
The data were collected using a 12-channel Grass Model 7D pen-chart recorder (Grass Instrument Co., West Warwick, RI, USA). The signals were amplified, filtered with a 50-Hz notch filter, and displayed on paper chart. The bandwidth of the respiratory bands was within 0.1 Hz–0.1 kHz. The ECG bandwidth was within 0.03 Hz–75 Hz. The high-frequency filter for BP was set at 75 Hz. The ECG, BP, and respiratory band measures were also recorded on a Pentium IV PC, via a 12-bit A/D converter using an acquisition program developed within the laboratory. The rate of digitization was 1000 Hz for ECG and 100 Hz for both BP and the respiratory bands.
Data Reduction and Statistical Analyses
Following the elimination of any suspect data in the BP or ECG waveforms, detection of the maximum and minimum BP points and the R-wave of each cardiac cycle was made via an automated computer algorithm; automated results were visually checked and corrected where necessary. BPsys and BPdia were identified by the program from the maximum and minimum points (respectively) for each cardiac cycle, and HR was calculated from the time interval between consecutive R-waves. A subset of these data have also been subjected to mathematical modeling and published separately.15
Two main aspects of the data were evaluated. The first was the effect of frequent arousal from sleep on BP and HR over the recording period. This investigation used all data, including the activation response to transient arousals as well as the intervening sleep data. In the second assessment, the underlying sleep levels of BP and HR between arousals from sleep during phase 5 were assessed and compared to the overall phase average. In this analysis, only the intervening periods of sleep data were used; activation responses to transient arousals were disregarded. The sleep values were generated from the mean of the pre-arousal beats within each condition (90 beats, 2-min condition; 45 beats, 1-min condition; 20 beats, 30-sec condition). This method of analyzing pre-arousal beats was deemed to be the best measure of the “sleep” level of activity.
For both forms of analysis, BP and HR were standardized within a night's recording by normalizing to the nightly mean and representing values as percentage change scores from the nightly mean. This was necessary because the internal calibration mechanism of the Portapres BP device causes differences in finger cuff attachment/placement and inter-individual differences in cardiovascular control mechanisms, which result in absolute values of BP being relatively unreliable, producing random variability between nights. In contrast, change values within a recording session are considered highly reliable.16
Graphically, the change in BP and HR over the development of sleep was represented by 55 data points (15 values for phase 1 and 10 each for phases 2 through 5). For statistical analyses, the data were averaged for each participant within phases and then over nights within conditions resulting in a 5 (phases) by 4 (conditions) analysis of variance (ANOVA) with repeated measures on each factor. The interaction between phase and condition gave an indication of the effect of sleep fragmentation on the change in cardiovascular activity over time between conditions. However, the critical phase in which the effects of experimental manipulation were likely to become apparent was phase 5, after participants in the control condition ceased to have spontaneous arousals and differences between the conditions were complete. Thus, the accumulated effect of experimentally induced arousal from sleep on BP and HR was further assessed during phase 5 in a 4 (conditions) by 10 (2-min epochs) ANOVA with repeated measures on each factor.
To determine the influence of arousals from sleep on the underlying sleep levels, pre-arousal values during phase 5 were compared with the phase 5 (overall) average data. In the control condition, in which spontaneous arousals did not occur and participants were continuously in stable NREM sleep, the mean phase value was also used to represent the underlying sleep value. Statistically, the pre-arousal levels in each of the 4 conditions were initially compared in a 1-way ANOVA. The effect of frequent arousals on the underlying sleep levels were then assessed in the experimental conditions via a 3-condition (2 min, 1 min, 30 sec) by 2-sleep level (phase average v prearousal) repeated measures ANOVA. The statistical outcomes of interest were the main effect of sleep level and the interaction effect between condition and sleep level.
RESULTS
Following lights out (LO), the time to the onset of phase 3 (stage 1, SO latency) was similar in all conditions (11.4, 10.4, 10.9, and 11.9 min for the control, 2 min, 1 min, and 30 sec, respectively). The intervention with tone-induced arousals essentially doubled the delay to the onset of phase 4 (the first occurrence of a spindle or K-complex) in the experimental conditions compared with the control condition (7.7, 14.0, 11.5, and 15.1 min for the control, 2 min, 1 min, and 30 sec, respectively). Despite these differences, the effect of the arousals on the development of sleep was highly variable, and a 2 (phases) by 4 (conditions) ANOVA indicated that there was no significant interaction effect (F3,27 = 2.59, P > 0.05).
Preliminary analyses were conducted to establish that auditory arousals elicited cardiovascular activation. There were 2 statistically significant findings. First, there was a main effect of condition, indicating that tone-induced arousals in the experimental conditions produced larger responses than spontaneous arousals in the control condition. Second, there was a general trend for larger responses for less frequently induced arousals during phases 3 and 4 for BPsys (22.37, 20.02, 17.17, 13.20 mm Hg for the 2 min, 1 min, 30 sec, and spontaneous arousals, respectively), for BPdia (24.57, 19.88, 15.16, 14.44 mm Hg, respectively), and for HR (19.30, 12.43, 10.88, 15.78 bpm, respectively). This likely reflected subjects progressing further into sleep in the less frequent arousal condition. The duration of arousals did not differ between spontaneous and experimentally induced arousals (19 v 16 sec respectively; F3,27 = 0.60, P > 0.05), or between the arousal durations of the 3 experimentally induced arousal frequencies (16, 17, 15 sec for 2 min, 1 min, and 30 sec, respectively; F2,18 = 0.32, P > 0.05), or as a function of phase (F4,36 = 0.07, P > 0.05).
The Influence of Arousals on the Fall in BP and HR in the Initial Sleep Period
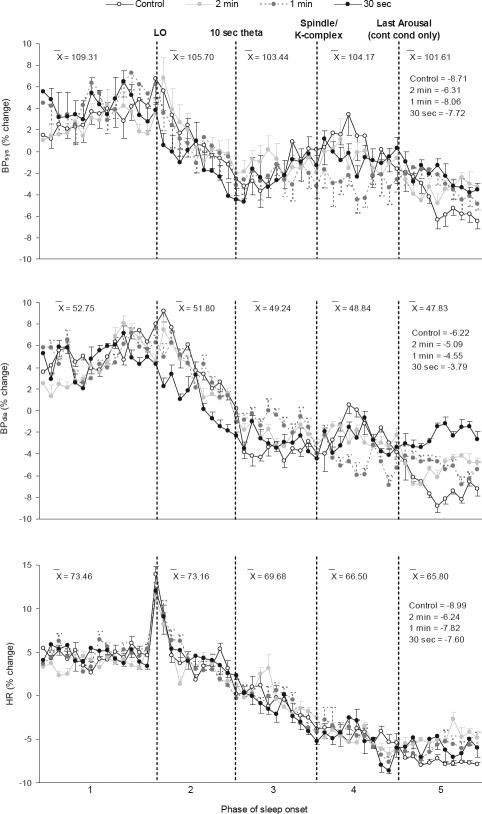
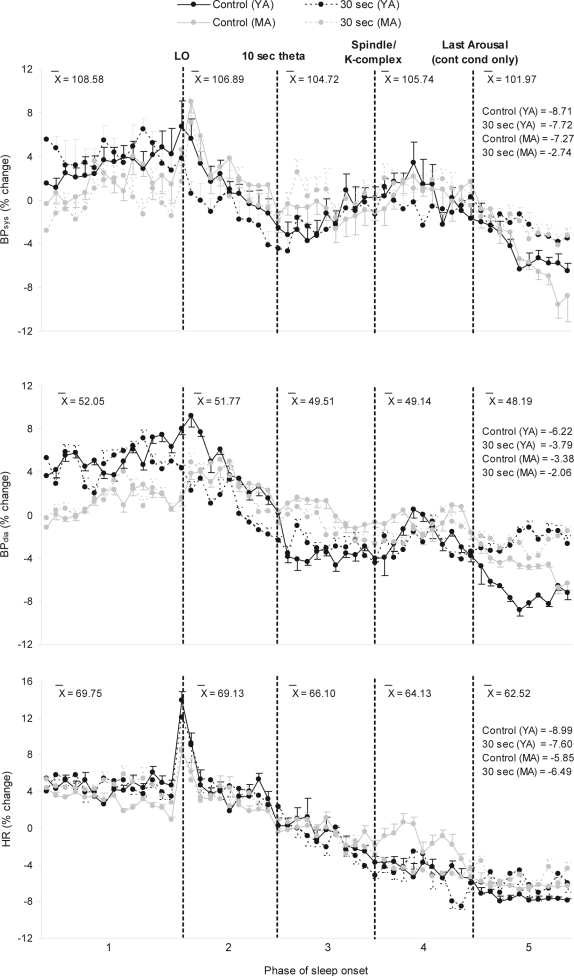
Figure 2 illustrates that from phase 1 to phase 5, there were marked reductions across all conditions in BPsys and BPdia of approximately 8 and 5 mm Hg, respectively. A repeated-measures ANOVA indicated that the interaction effect over the 5 phases was not significant, although it did approach significance for BPsys; the direction of the effect suggested, as predicted, that there was a larger fall for BPsys in the control condition than the experimental conditions (BPsys: F12,108 = 1.83, P = 0.052; BPdia: F12,108 = 1.47, P > 0.05). The fall in BP was significant across phases (BPsys: F4,36 = 5.95, P < 0.01; BPdia: F4,36 = 11.86, P < 0.001). As seen in Table 1, pairwise comparisons between phase averages confirmed the pattern apparent in Figure 2; the major fall in BP occurred during the period following LO but before the onset of sleep (and before the beginning of the experimental manipulation).
Figure 2.
% change from nightly mean values in BPsys, BPdia, and HR over Phase of sleep onset (SO) in each condition as a function of time (2-min epochs) in phases 1 (30 min) and 5 (20 min), and 10% epochs in phases 2, 3, and 4.
Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate; LO, lights out; X̄, overall raw score phase average values between conditions in mm Hg (BPsys and BPdia) and bpm (HR). Labeled values on the right of the graph in phase 5 are the % changes from phase 1 to phase 5 for each condition (N=10). Standard error bars indicate within-subject variability (variance in the change within subjects over time).
Table 1.
F-Ratios for All Pair-Wise Comparisons Between Phase Means
| Comparison | BPsys | BPdia | HR |
|---|---|---|---|
| Phase 1 v 2 | 3.97 | 0.97 | 0.49 |
| Phase 1 v 3 | 6.78* | 8.69* | 16.84** |
| Phase 1 v 4 | 3.56 | 10.85** | 62.33*** |
| Phase 1 v 5 | 9.17* | 13.63** | 64.97*** |
| Phase 2 v 3 | 11.93** | 65.75*** | 36.10*** |
| Phase 2 v 4 | 1.62 | 36.98*** | 72.72*** |
| Phase 2 v 5 | 12.75** | 42.14*** | 40.27*** |
| Phase 3 v 4 | 0.90 | 0.23 | 52.39*** |
| Phase 3 v 5 | 2.30 | 4.98 | 18.50** |
| Phase 4 v 5 | 14.10** | 4.38 | 1.24 |
BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate.
df (1,9) for all variables.
P < 0.05;
P < 0.01;
P < 0.001.
See Figure 1 for description of each Phase.
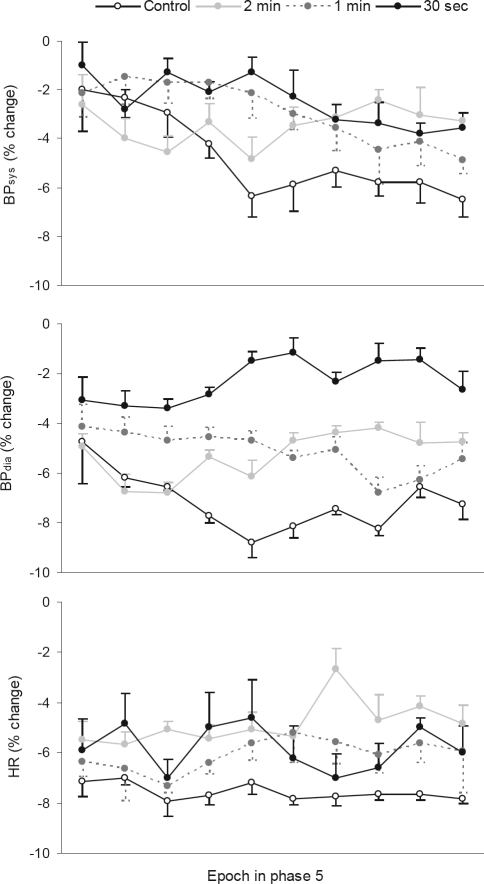
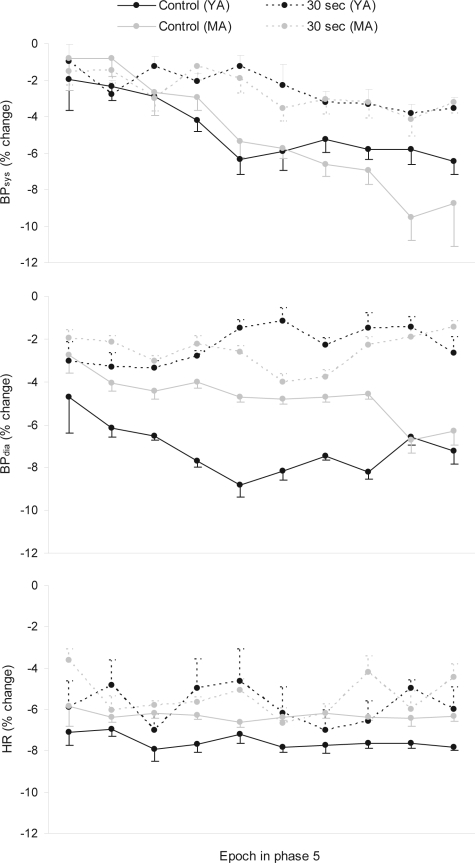
Figure 3 indicates that during phase 5, once spontaneous arousals had ceased in the control condition, the magnitude of reductions in BP were greater in this condition than following the accumulated effect of experimental arousal from sleep in the experimental conditions. For BPsys, the main effect was not significant (F3,27 = 1.47, P > 0.05) but the interaction effect was (F27,243 = 1.81, P < 0.05). Figure 3 shows that for BPsys, there was a larger fall across time in phase 5 in the control condition than the experimental conditions. Further, the overall fall in BPsys was significant across epochs in phase 5 (BPsys: F9,81 = 4.76, P < 0.001). For BPdia, the trend was reflected in a significant difference between conditions (BPdia: F3,27 = 3.04, P < 0.05) but not a significant interaction effect (BPdia: F27,243 = 0.90, P > 0.05). Pairwise comparisons of the phase 5 means confirmed that BPdia in the control condition was lower than in the 30-sec experimental condition, albeit the difference was relatively small (4.5%). Finally, there were no other significant pairwise differences in BPdia in phase 5 (Table 2), and the overall fall in BPdia was not significant across epochs in phase 5 (F9,81 = 0.38, P > 0.05).
Figure 3.
% change from nightly mean values in BPsys, BPdia, and HR in YA in each condition during phase 5
Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate. N=10. Standard error bars indicate within subject variability (variance in the change within subjects over time).
Table 2.
F-Ratios for All Pair-Wise Comparisons Between Condition Means During Phase 5
| Comparison | BPsys | BPdia | HR |
|---|---|---|---|
| Control v 2.0 min | 1.14 | 2.15 | 10.04* |
| Control v 1.0 min | 3.60 | 4.96 | 1.70 |
| Control v 30 sec | 2.26 | 8.32* | 3.44 |
| 2.0 min v 1.0 min | 1.46 | 0.18 | 6.16* |
| 2.0 min v 30 sec | 0.44 | 1.89 | 1.59 |
| 1.0 min v 30 sec | 0.01 | 2.30 | 1.63 |
BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate.
df (1,9) for all variables.
P < 0.05.
For HR, Figure 2 shows that in all conditions, HR significantly decreased by an average of 8 bpm from pre-sleep wakefulness to stable sleep, following an abrupt, transient increase at LO. The interaction between phase and condition was not significant for HR, indicating that there was not a differential fall in HR over the conditions (F12,108 = 1.41, P > 0.05). The fall in HR was significant across phases of SO (F4,36 = 36.21, P < 0.001). Unlike BP, HR continued to fall across phases 3 and 4, whereas the fall in BP was suspended during this time. HR did not significantly decrease from phase 1 to 2 and phase 4 to 5; however, all other pairwise comparisons between phase averages were significant (Table 1).
During phase 5, Figure 3 shows that the cessation of spontaneous arousals in the control condition was associated with a greater reduction in HR than the accumulated effect of experimental arousal from sleep in the experimental conditions. There was a significant difference in HR between conditions (HR: F3,27 = 4.35, P < 0.05), although the interaction effect was not significant (HR: F27,243 = 0.77, P > 0.05). HR in the control condition was lower than the 2-min experimental condition, while the latter condition was also different from the 1-min experimental condition (Table 2). As seen in Figure 3 and confirmed by ANOVA, the overall fall in HR was not significant across epochs in phase 5 (HR: F9,81 = 0.61, P > 0.05).
The Influence of Arousals on Underlying Sleep Levels of BP and HR
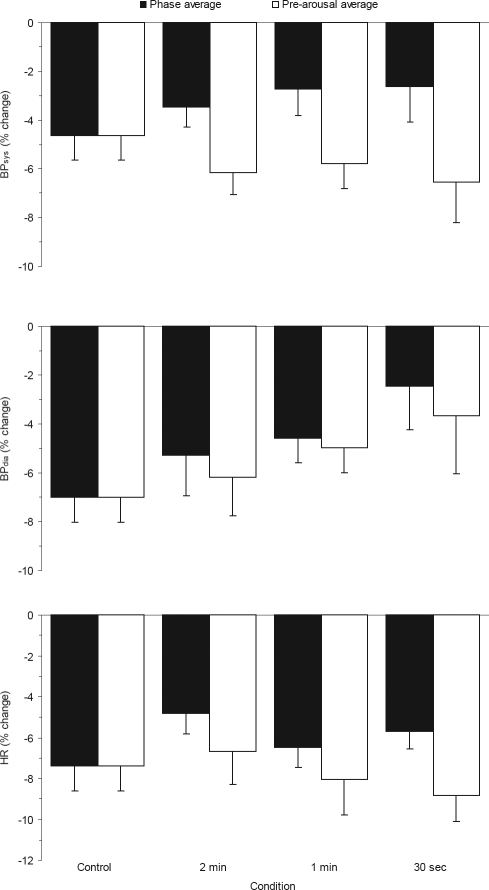
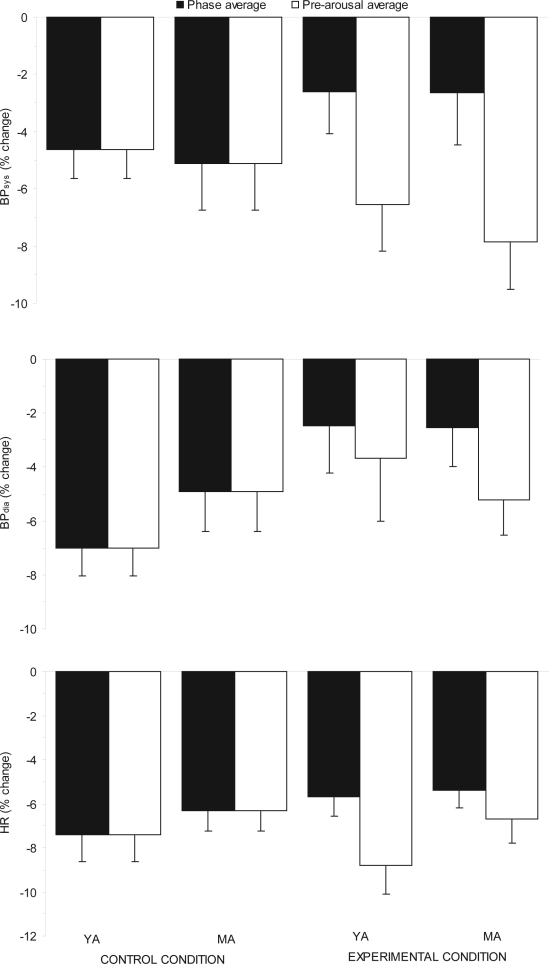
Figure 4 illustrates the phase 5 average values in contrast to the phase 5 pre-arousal values (i.e. the underlying sleep-specific levels,). The values represent the percentage change from nightly mean values with a more negative value indicating a greater fall. Comparisons between the pre-arousal levels for the control and experimental conditions did not show any significant differences (BPsys: F3,27 = 0.65, P > 0.05; BPdia: F3,27 = 1.52, P > 0.05; HR: F3,27 = 1.61, P > 0.05). However, the average levels in the experimental conditions for both BPsys and HR, but not BPdia were significantly higher than pre-arousal values, as identified from the main effect of sleep level in a 2-way repeated-measures ANOVA (BPsys: F1,9 = 278.91, P < 0.001; BPdia: F1,9 = 2.88, P > 0.05; HR: F1,9 = 7.29, P < 0.05). There were no significant main effects of condition for any variable (BPsys: F2,18 = 0.14, P > 0.05; BPdia: F2,18 = 1.42, P > 0.05; HR: F2,18 = 2.95, P > 0.05), nor were there any significant condition by sleep level interaction effects (BPsys: F2,18 = 1.93, P > 0.05; BPdia: F2,18 = 0.37, P > 0.05; HR: F2,18 = 1.93, P > 0.05). Therefore in this study, there was no convincing evidence that experimental arousal from sleep retarded the underlying sleep related fall in BP and HR.
Figure 4.
Comparison of phase average values with pre-arousal values in BPsys, BPdia, and HR in each condition during phase 5 of SO
Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate. N=10. Error bars indicate standard errors.
DISCUSSION
A small effect of sleep fragmentation on the fall in BP and HR over SO and the initial sleep period was tentatively identified. During phase 5, when sleep was stable in the control group, the data indicated significant differences between conditions for all 3 variables. BPdia and HR were lower in the control condition than in the experimental conditions, and there was a significant interaction effect for BPsys. Results from sleep periods between arousals indicated that phase 5 pre-arousal levels for BPsys and HR were lower than the phase averages, although there were not significant differences as a function of the frequency of arousals. This suggests that experimental arousal from sleep did not have a strong effect on the underlying sleep related fall in BP and HR, and that average values were higher because of the inclusion of transient activation at each arousal in the average.
While the effects were small in magnitude, they suggest that sleep fragmentation may retard the sleep-induced falls in cardiovascular activity. This result is consistent with a previous study showing that the fall in BP during SO is retarded by spontaneous arousals during SO.10
There was some suggestion that more frequent sleep disruption caused a greater effect on the degree of fall in BP, particularly BPsys, which was affected in approximately a dose-response manner. There were significantly greater differences between the 2 most divergent conditions, that is, between the control condition and arousals occurring every 30 sec, but not between intermediate conditions characterized by less frequent arousals (occurring every 1 min or 2 min). For reasons that were not anticipated, certain features of the design appear to have worked against each other. In the 30-sec condition, arousals were induced at a higher frequency but with a smaller cardiovascular activation response, whereas in the 2 min condition, arousals were produced less frequently but had a greater activation response. Thus, owing to the trade-off between frequency of arousals and the magnitude of the cardiovascular arousal activation response, there may not have been as big a difference between the 3 experimental conditions as one may have anticipated, giving rise to small condition effects.
EXPERIMENT TWO
There was preliminary evidence from Experiment 1 and Carrington et al.10 that in healthy young adults, repetitive arousals from sleep over the SO period retard the fall in BP and HR, although the magnitude of the effects was small. However, there was reason to suspect that the effects would be stronger in older individuals. First, there is a greater prevalence of OSA17 and hypertension,18 and an associated non-dipping BP profile in older individuals. Second, overall sleep structure and quality is diminished by the fragility of sleep with advancing age.19–22 Third, autonomic nervous system (ANS) function deteriorates in older individuals.23–28 However, in contrast to these arguments, the arousal response is weaker with advancing age.29–32 Nevertheless, considering all findings, it was expected from the first 3 points that average cardiac activity would be more susceptible to arousals from sleep in older adults, particularly because it was not necessarily the case that a small HR arousal response would be associated with small fluctuations in BP.
The aim of Experiment 2 was to experimentally manipulate arousals from sleep in order to retard the fall in BP and HR during the SO period in middle-aged adults (MA), and to compare these individuals to the sample of young adults from Experiment 1. It was hypothesized that frequent arousals from sleep would retard the sleep related fall in BP and HR more in MA than young adults (YA). It was also hypothesized that in contrast to YA, the retardation of the fall in BP and HR would be due to an effect on the underlying sleep levels of BP and HR.
METHODS
Participants
The study consisted of 2 groups, 10 young adults (YA) from Experiment 1 and 10 middle-aged (MA) adults. There were equal numbers of males and females in each group. The mean age of YA was 20 ± 2 years (range 18–25 years) and the average age of MA was 47 ± 6 years (range 38–55 years). YA had a median BMI of 23 kg/m2 with an IQR of 22–25 kg/m2, while the median BMI of MA was 24 kg/m2 with an IQR of 23–26 kg/m2. MA were screened in the same way as YA in Experiment 1. Additionally, female MA did not report being on hormone replacement therapy. The laboratory procedures were approved by The University of Melbourne Human Research Ethics Committee. All participants gave informed consent to undertake the project and were reimbursed for their time commitment to the study.
Design
Experiment 2 assessed differences in cardiovascular activity among 2 age groups in a repeated-measures design with 2 conditions. Using the YA data from Experiment 1, the MA were compared to YA for 2 nights each in the control and 30-sec experimental conditions, since this was the frequency at which the greatest effect was obtained in YA. Thus, MA adults were not administered the 2-min and 1-min experimental conditions. The dependent variables were identical to Experiment 1, and the equivalent phase structure and manipulation of the data within the phases were employed (refer Figure 1).
Procedure
The same laboratory conditions and general testing procedures as in Experiment 1 were implemented for MA on control and experimental nights. The presentation procedure of auditory stimuli for MA in the 30-sec experimental condition was unchanged from Experiment 1, as were the arousal identification protocols. The MA entered SWS during phase 5 on 16 of 20 nights, a frequency not significantly different from the 20 of 21 nights for the YA.
Equipment and Materials
The auditory stimuli equipment, assessment of thoracoabdominal efforts, measurement of sleep/wake state and assessment of the dependent variables were identical to Experiment 1. Similarly, the same digital recording and data acquisition settings were used, and the same sleep scoring and data editing procedures were followed.
Data Reduction and Statistical Analyses
The initial analysis of the data determined whether there were any age differences in the effect of experimental arousals from sleep on the fall in BP and HR over SO. Statistically, the pattern of BPsys, BPdia, and HR was assessed by comparing age differences in the phase averages in a 5 phases by 2 conditions (control and experimental) by 2 groups (YA and MA) ANOVA incorporating 2 within-subjects factors (phases and condition) and one between-subjects factor (age group). As in Experiment 1, the relevant information was contained in the change in cardiovascular activity over time, and the statistical outcomes of interest in this study were the interactions between phase, condition, and age group, as these indicated the effect of sleep fragmentation over SO in the different conditions and as a function of age. A second analysis compared 2-min epochs across time in phase 5 in each condition and age group, as the data during this period reflected both the accumulated effect of experimentally induced arousal from sleep in the experimental condition and the end of spontaneous arousals in the control condition. Thus, BPsys, BPdia, and HR were analyzed in a 10 epochs by 2 conditions (control and experimental) by 2 age groups (YA and MA) ANOVA incorporating 2 within-subjects factors (epochs and condition) and one between-subjects factor (age group).
Lastly, age effects in the difference in BP and HR between phase 5 means and the pre-arousal levels were examined in each condition. Statistically, the pre-arousal values used to represent the underlying sleep level in each condition were initially compared between age group in a 2 conditions (control and experimental) by 2 groups (YA and MA) ANOVA. The phase 5 prearousal levels of BP and HR were then compared to the phase average for each age group in the experimental condition via a 2 sleep levels (phase average v pre-arousal) by 2 age groups (YA and MA) ANOVA with one within-subjects factor (sleep level) and one between-subjects factor (age group). The statistical outcomes of interest in these analyses were the effect of sleep level and the age group by sleep level interaction effect.
RESULTS
The latency to the onset of theta (phase 3) was shorter for MA than YA in both conditions (MA: 8.6, 5.8 min; YA: 11.4, 11.9 min for the control and 30-sec conditions, respectively). Experimental arousals from sleep in MA did not delay the onset of stage 2 sleep (phase 4) but almost doubled the phase 3 duration in YA (MA: 10.2, 10.9 min; YA: 7.7, 15.1 min). Despite the magnitude of these differences, a 3-way group by condition by phase ANOVA was not significant (F1,18 = 0.36, P > 0.05), nor were the phase by group (F1,18 = 0.48, P > 0.05) or phase by condition (F1,18 = 3.40, P > 0.05) interaction effects.
Preliminary analyses to assess that auditory arousals elicited cardiovascular activation showed that arousals from sleep caused transient increases in BP and HR that were independent of age. Aside from the finding that experimentally induced arousals produced greater cardiovascular activation responses for BPsys than spontaneous arousals (spontaneous arousals: 13.20 and 14.95 mm Hg; auditory arousals: 17.17 and 18.03 mm Hg for YA and MA, respectively), there were no other significant magnitude effects for any variable. There were significant differences in arousal durations depending on arousal type and age; for YA, spontaneous arousal durations were on average 7 sec shorter in phase 4 (15 sec) than phase 3 (22 sec), while for MA, they were 6 sec longer (22 v 16 sec, respectively). Also, tone-induced arousals were approximately 10 sec shorter in phase 4 than phase 3 for both age groups (13 v 23 sec).
Age Effects on the Influence of Arousal From Sleep on the Fall in BP & HR Over the Initial Sleep Period
Figure 5 illustrates that BP decreased during both conditions in MA and YA. Overall, the falls in BPsys and BPdia from phase 1 to phase 5 were 7 and 4 mm Hg, respectively. The fall in BP, independent of condition and age group, was significant across phases (BPsys: F4,72 = 5.38, P < 0.01; BPdia: F4,72 = 15.63, P < 0.001). Figure 5 indicates a greater fall in the control conditions than in the experimental conditions. Consistent with this, the statistical analysis indicated that the interaction between condition and phase was significant (BPsys: F4,72 = 2.65, P < 0.05; BPdia: F4,72 = 3.30, P < 0.05). The greater fall in BP in the control condition compared to the experimental condition occurred similarly in MA and YA, as indicated by a non-significant 3-way interaction effect between condition, group, and phase (BPsys: F4,72 = 0.91, P > 0.05; BPdia: F4,72 = 1.15, P > 0.05). However, the phase by age group interaction effect was borderline significant for BPdia, showing a tendency for BPdia to decrease more in YA than MA, although this effect was not significant for BPsys (BP-sys: F4,72 = 1.18, P > 0.05; BPdia: F4,72 = 2.50, P = 0.05).
Figure 5.
% change from nightly mean values in BPsys, BPdia, and HR over SO in MA and YA during the control and 30-sec experimental condition as a function of time (2-min epochs) in phases 1 (30 min) and 5 (20 min), and 10% epochs in phases 2, 3, and 4. Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate; MA, middle-aged; YA, young adults; LO, lights out; X̄, overall raw score phase average values across conditions and groups in mm Hg (BPsys and BPdia) and bpm (HR). Labeled values on the right of the graph in phase 5 are the % changes from phase 1 to phase 5 for each condition. N=10 per age group. Standard error bars indicate within-subject variability (variance in the change within subjects over time).
As highlighted in Figure 6, during phase 5 (stable sleep in the control condition), BP was lower in the control condition compared to the experimental condition. Although a 3-way ANOVA (10 epochs by 2 conditions by 2 age groups) indicated a nonsignificant main effect of conditions for BPsys, it approached significance (F1,18 = 4.30, P = 0.053), and the interaction effect between condition and time was significant (F9,162 = 4.37, P < 0.001). As seen in Figure 6, for BPsys, there was a larger fall across time in phase 5 in the control conditions than in the experimental conditions. For BPdia the condition main effect was significant (F1,18 = 7.81, P < 0.05), while the condition by time interaction effect was not (F9,162 = 1.28, P > 0.05). Further, the overall fall in BPsys but not BPdia was significant across epochs in phase 5 (BPsys: F9,162 = 15.48, P < 0.001; BPdia: F9,162 = 0.54, P > 0.05).
Figure 6.
% change from nightly mean values in BPsys, BPdia, and HR in MA and YA during the control and 30-sec experimental condition during phase 5
Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate. N=10 per age group. Standard error bars indicate within-subject variability (variance in the change within subjects over time).
There were no significant age group main effects (BPsys: F1,18 = 0.001, P > 0.05; BPdia: F1,18 = 0.43, P > 0.05), nor were there any significant 2-way interaction effects between age group and condition (BPsys: F1,18 = 0.002, P > 0.05; BPdia: F1,18 = 0.93, P > 0.05) or age group and time (BPsys: F9,162 = 1.64, P > 0.05; BPdia: F9,162 = 0.31, P > 0.05). Lastly, the 3-way condition by age group by time interaction effect was not significant for BP (BPsys: F9,162 = 1.64, P > 0.05; BPdia: F9,162 = 1.56, P > 0.05).
As illustrated in Figure 5, HR decreased from pre-sleep wakefulness to stable sleep in both conditions and in MA and YA. It can also be seen that the transient increase at LO and more progressive fall in HR than BP over phase 3 and 4 was present in all conditions of the study. The total fall in HR across the 5 phases of SO approximated 7 bpm (F4,72 = 71.13, P < 0.001). There was a significant condition by phase interaction effect for HR (F4,72 = 3.97, P < 0.01) although as indicated in Figure 5, it is unclear whether this reflected differences within phase 4 or phase 5. As with BP, the greater fall in HR in the control condition compared to the experimental condition occurred in both MA and YA, as indicated by a non-significant 3-way condition by age group by phase interaction effect (F4,72 = 1.69, P > 0.05). Figure 5 also shows that the fall in HR was suspended by spontaneous arousals during phase 4 in MA, then decreased in phase 5. Unlike BPdia, there was no suggestion of a phase by age group interaction effect (F4,72 = 1.56, P > 0.05).
As indicated in Figure 6, HR was not significantly lower in the control condition compared to the experimental condition during phase 5 of SO (F1,18 = 3.49, P > 0.05). Further, the condition by time interaction effect was not significant (F9,162 = 0.86, P > 0.05). Also as shown in Figure 6, the overall fall in HR was not significant across epochs in phase 5 (F9,162 = 1.07, P > 0.05). There were no significant age group main effects (F1,18 = 0.47, P > 0.05), nor were there significant 2-way interaction effects between age group and condition (F1,18 = 0.33, P > 0.05) or age group and time (F9,162 = 1.00, P > 0.05). Finally, the 3-way condition by age group by time interaction effect was not significant for HR (F9,162 = 0.67, P > 0.05).
Age Effects on the Influence of Arousals on Underlying Sleep Levels of BP and HR
The pre-arousal levels in each condition were initially compared in a 2-way condition by age group ANOVA. This analysis confirmed that the pre-arousal values in phase 5 were not significantly different between the control and experimental conditions for any variable (BPsys: F1,18 = 3.91, P > 0.05; BPdia: F1,18 = 1.40, P > 0.05; HR: F1,18 = 1.00, P > 0.05). Further, the 2-way interaction effect between condition and age group was not significant for any variable (BPsys: F1,18 = 0.23, P > 0.05; BPdia: F1,18 = 2.02, P > 0.05; HR: F1,18 = 0.28, P > 0.05).
Subsequently, the phase 5 pre-arousal level was compared to the phase average level for each age group in the experimental condition. The pre-arousal levels were significantly lower for all variables, as indicated by the main effect of sleep level in the 2-way ANOVA (BPsys: F1,18 = 145.11, P < 0.001; BPdia: F1,18 = 16.69, P < 0.01; HR: F1,18 = 18.37, P < 0.001). The age group by sleep level interaction effect was not significant for any variable (BPsys: F1,18 = 1.82, P > 0.05; BPdia: F1,18 = 1.49, P > 0.05; HR: F1,18 = 2.85, P > 0.05), and there were no significant main effects of age group for any variable (BPsys: F1,18 = 0.12, P > 0.05; BPdia: F1,18 = 0.15, P > 0.05; HR: F1,18 = 0.82, P > 0.05). Thus, arousals from sleep affected average values but not the underlying sleep level between arousals. There was therefore no evidence to suggest that experimental arousal from sleep retarded the underlying sleep related fall in BP or HR in MA.
DISCUSSION
In summary, the results of Experiment 2 identified significant interaction effects between phase and condition for all variables but not a significant age group by condition by phase interaction effect for any variable. Subsequent analyses specifically within phase 5 supported the overall analysis for BP but not for HR, suggesting that the overall HR effect was influenced by earlier changes, particularly an increase in the control condition in phase 4. In addition, there was a tendency for BPdia to show a greater fall in YA, although statistically this was only borderline significant. Finally, the pre-arousal levels of both BP and HR, which represented the underlying sleep level, did not differ as a function of age or condition, indicating that experimental arousal from sleep did not retard the underlying sleep related fall in BP and HR in either age group.
Figure 7.
Comparison of phase average values with pre-arousal values in BPsys, BPdia and HR in MA and YA during the control and 30-sec experimental condition in phase 5 of SO
Legend: BPsys, systolic blood pressure; BPdia, diastolic blood pressure; HR, heart rate; MA, middle-aged; YA, young adults. N=10 per age group. Error bars indicate standard errors.
GENERAL DISCUSSION
The 2 studies demonstrated that experimental sleep fragmentation retards the progressive falls in BP, and to a lesser extent HR, over SO in normal healthy individuals, and that the effect occurs to a similar degree in YA and MA. The observed effects were small in magnitude and did not become apparent until stable sleep had been achieved in the control condition. Therefore, consistent with an earlier study,10 the absence or presence of arousals from sleep was a determinant of the extent to which cardiovascular activity fell over SO. There was some suggestion in the present experiments that more frequent sleep disruption caused a greater effect, although this was offset by less frequent arousals being associated with larger cardiovascular responses. This work is the first to demonstrate that repetitive arousals from sleep produce cardiovascular activation that delays the fall in BP in normal healthy individuals.
In both experiments, HR continued to fall across SO and was not affected by arousals from sleep to the same degree that arousals influenced BP. The absence of this effect may be caused by the HR arousal response being more transient than the BP arousal response.33–37 The return to baseline for HR occurs within 10 beats of arousal onset, while for BP, the return to baseline occurs after about 15 beats post arousal onset. The sustained fall in HR over SO may also be interpreted as being due to circadian factors, especially as circadian influences on HR are particularly influential at the time of an individual's normal SO.1 However, it is recognized that the potential influence of circadian factors on HR in these studies cannot be determined from the data from these experiments alone.
There was little evidence to suggest that the sleep related levels of BPsys were affected by continuous sleep fragmentation. Indeed, if anything, BPsys fell slightly more during the brief sleep periods in the experimental conditions than in the sustained sleep of the control conditions, although this was not significant. Further, the difference between the average BPsys and the sleep related levels in the experimental conditions during phase 5 was highly significant. There was a tendency for the fall in BPdia sleep levels to be held up in the YA group, although this was not significant and was not apparent in the MA group. The data for HR were less relevant on this point, as the effect of arousals from sleep on average HR was weak at best. Thus, the data from these experiments suggest that retarding nighttime reductions in BP is primarily attributable to transiently heightened cardiovascular activation at arousals contributing to and augmenting BP averages, rather than representing a disturbing influence of arousals from sleep on underlying sleep values. If so, these data would suggest that once arousals from sleep cease, BP would decrease and the tendency for non-dipping would disappear, as indeed occurred with spontaneous arousals in the control condition. A decrease in underlying BP suggests that a sleep related mechanism affects BP, driving it down during the brief sleep periods.
While there were no major age differences, there were subtle variations which were tentatively attributed to the effect of normal ageing on ANS control and arterial baroreceptor reflex (BR) function in MA. Firstly, BP increased transiently at LO in this group, but not in YA. It is speculated that this was due to a weaker BR mechanism in MA compared to YA adults. This is in accord with an inverse relationship between age and BR responsiveness during sleep.38 Secondly, there was a tendency for the fall in BPdia across SO to be reduced in MA, although statistically this was borderline significant. This may be explained by a reduction in the influence of sleep on BP in MA. It is speculated that with age, sleep drive is less powerful resulting in a reduced impact on BP. A second explanation may be a deterioration of ANS function with age, particularly in view of previous studies identifying a decrease in HR variability, especially at night25,26,28,39,40 that may occur as a consequence of impaired BR sensitivity.23–28 Thirdly, the fall in HR was suspended by spontaneous arousals during phase 4 in MA. This was likely the result of spontaneous arousals being longer in phase 4 in MA, or it reflects a general agitation effect and apprehension about getting undisturbed sleep in the control condition given that spontaneous arousals produced a smaller cardiovascular activation response than experimentally induced arousals. Overall, while these data tentatively suggest that cardiovascular activity was beginning to be compromised in MA, this did not influence the effect of arousals from sleep on changes in BP or HR over SO.
It was not anticipated that there would be gender differences in the effect of sleep fragmentation and, as a consequence, both male and female subjects were run in the study. Analyses of gender differences using subjects from both groups confirmed the absence of gender effects.
In these 2 experiments, the frequency of arousals was manipulated in healthy individuals in an attempt to simulate varying degrees of OSA. However, it may be argued as to whether arousals produced by auditory stimulation provide a valid model of OSA. The intervention itself was somewhat minimal as it was only conducted for one sleep cycle and did not simulate the intensity of cardiovascular responses that are produced with the substantial build-up of endogenous stimuli as seen in OSA (e.g., hypoxia, hypercapnia, negative intrathoracic pressure). Further, the duration of arousals from sleep were longer than is typically observed in OSA patients. It was likely that this was due to the manipulations being conducted primarily during SO and to the absence of the excessive sleepiness that characterizes OSA patients.
Nevertheless, the finding that repetitive arousals from sleep over a single sleep cycle resulted in a small reduction in the sleep related fall in BP in normal young individuals lends support to the interpretation that in OSA patients, the repetitive arousals associated with apnea termination may independently contribute to elevations in BP at night. However, the speculation that frequent arousal is the primary cause of non-dipping in OSA should be treated cautiously, particularly as there are chronic changes in cardiovascular activity in OSA patients.
ACKNOWLEDGMENTS
This work was supported by the Australian Research Council (ARC) grant DP0209296.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Carrington M, Walsh M, Stambas T, Kleiman J, Trinder J. The influence of sleep onset on the diurnal variation in cardiac activity and cardiac control. J Sleep Res. 2003;12:213–21. doi: 10.1046/j.1365-2869.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 2.Degaute JP, Van de Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet. 1988;2:397. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 5.Noda A, Okada T, Hayashi H, Yasuma F, Yokota M. 24-hour ambulatory blood pressure variability in obstructive sleep apnea syndrome. Chest. 1993;103:1343–7. doi: 10.1378/chest.103.5.1343. [DOI] [PubMed] [Google Scholar]
- 6.Pankow W, Nabe B, Lies A, et al. Influence of sleep apnea on 24-hour blood pressure. Chest. 1997;112:1253–8. doi: 10.1378/chest.112.5.1253. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–7. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- 8.Garpestad E, Parker JA, Katayama H, et al. Decrease in ventricular stroke volume at apnea termination is independent of oxygen desaturation. J Appl Physiol. 1994;77:1602–8. doi: 10.1152/jappl.1994.77.4.1602. [DOI] [PubMed] [Google Scholar]
- 9.Ringler J, Basner RC, Shannon R, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1990;69:2143–8. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 10.Carrington MJ, Barbieri R, Colrain IM, Crowley KE, Kim Y, Trinder J. Changes in cardiovascular function during the sleep onset period in young adults. J Appl Physiol. 2005;98:468–76. doi: 10.1152/japplphysiol.00702.2004. [DOI] [PubMed] [Google Scholar]
- 11.Philip P, Stoohs R, Guilleminault C. Sleep fragmentation in normals: a model for sleepiness associated with upper airway resistance syndrome. Sleep. 1994;17:242–7. [PubMed] [Google Scholar]
- 12.ASDA. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. Washington, DC: National Institute of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [DOI] [PubMed] [Google Scholar]
- 14.Peñáz J. Photoelectric measurement of blood pressure, volume and flow in the finger. Digest 10th Int Conf Med Biol Engng; Dresden. 1973. p. 104. [Google Scholar]
- 15.Chaicharn J, Carrington M, Trinder J, Khoo MC. The effects on cardiovascular autonomic control of repetitive arousal from sleep. Sleep. 2008;31:93–103. doi: 10.1093/sleep/31.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imholz BP, Langewouters GJ, van Montfrans GA, et al. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertension. 1993;21:65–73. doi: 10.1161/01.hyp.21.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 18.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL. Sleep and aging. In: Pressman MR, Orr WC, editors. Understanding sleep: the evaluation and treatment of sleep disorders. 1st ed. Washington, DC: American Psychological Association; 1997. pp. 441–464. [Google Scholar]
- 20.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: Saunders; 2000. pp. 26–42. [Google Scholar]
- 21.Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–7. [PubMed] [Google Scholar]
- 22.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 23.Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol (Lond) 1969;202:45P–46P. [PubMed] [Google Scholar]
- 24.Floras JS, Hassan MO, Jones JV, Osikowska BA, Sever PS, Sleight P. Factors influencing blood pressure and heart rate variability in hypertensive humans. Hypertension. 1988;11:273–81. doi: 10.1161/01.hyp.11.3.273. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Ferrari A, Gregorini L, et al. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci. 1980;59(Suppl 6):401s–404s. doi: 10.1042/cs059401s. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Ferrari A, Gregorini L, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- 27.Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268(4 Pt 2):H1606–12. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 28.Veerman DP, Imholz BP, Wieling W, Karemaker JM, van Montfrans GA. Effects of aging on blood pressure variability in resting conditions. Hypertension. 1994;24:120–30. doi: 10.1161/01.hyp.24.1.120. [DOI] [PubMed] [Google Scholar]
- 29.Gosselin N, Michaud M, Carrier J, Lavigne G, Montplaisir J. Age difference in heart rate changes associated with micro-arousals in humans. Clin Neurophysiol. 2002;113:1517–21. doi: 10.1016/s1388-2457(02)00189-x. [DOI] [PubMed] [Google Scholar]
- 30.Noda A, Okada T, Yasuma F, Yamada Y, Nakashima N, Yokota M. Effect of aging on cardiac and electroencephalographic arousal in sleep apnea/hypopnea syndrome. J Am Geriatr Soc. 1995;43:1070–1. doi: 10.1111/j.1532-5415.1995.tb05585.x. [DOI] [PubMed] [Google Scholar]
- 31.Noda A, Yasuma F, Okada T, Koike Y, Nakashima N, Yokota M. Age related differences in electroencephalographic and cardiac arousal at the termination of sleep apnea/hypopnea. Intern Med. 2000;39:375–80. doi: 10.2169/internalmedicine.39.375. [DOI] [PubMed] [Google Scholar]
- 32.Okada T, Hanyu M, Noda A, Kayukawa Y, Ohta T. Differences in arousal response between aged and middle-aged patients with obstructive sleep apnea syndrome. Psychiatry Clin Neurosci. 1998;52:218–9. doi: 10.1111/j.1440-1819.1998.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 33.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 34.Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 35.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–36. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- 36.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000;111:1611–9. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 37.Trinder J, Allen N, Kleiman J, et al. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003;26:543–51. [PubMed] [Google Scholar]
- 38.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–31. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 39.Bonnemeier H, Richardt G, Potratz J, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003;14:791–9. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- 40.Drayer JI, Weber MA, DeYoung JL, Wyle FA. Circadian blood pressure patterns in ambulatory hypertensive patients: effects of age. Am J Med. 1982;73:493–9. doi: 10.1016/0002-9343(82)90327-8. [DOI] [PubMed] [Google Scholar]