Abstract
Study Objectives:
Polysomnographic respiratory events in children should be scored using pediatric respiratory rules. However, due to a lack of data on adolescents, recently revised rules allow children aged 13–18 years to be scored by adult or pediatric criteria. To clarify which criteria to use, we describe the evolution of respiratory events with Tanner stage, and we compare events in children aged 13–18 years with the new American Academy of Sleep Medicine adult and pediatric respiratory rules.
Design:
Cross-sectional
Setting:
Academic hospital
Participants:
Healthy subjects aged 8–18 years recruited for research purposes
Interventions:
Physical examination to determine Tanner stage, overnight polysomnogram, and determination of sex hormones.
Results:
Sixty-eight subjects (Tanner 1–5) were studied, mean age [SD] = 13 ± 3 years, median apnea hypopnea index (AHI) = 0.1 (range: 0–1.2)/h. The median percentages of total sleep time (TST) with SpO2 < 92% were 0.1 (0–4.2)%, and with end-tidal CO2 > 50 torr was 0.1 (0–88.6)%. Thirty-two subjects were aged 13–18 years, (Tanner 3–5). The difference between AHI scored by pediatric (median = 0 [0–0.9]/h) and adult (median = 0 [0 – 0.5]/h) criteria was statistically significant (P = 0.043), but not clinically relevant.
Conclusions:
Respiratory events in normal children aged 8–18 years are rare and unrelated to Tanner stage. Adult or pediatric respiratory rules can be used for scoring polysomnograms in asymptomatic subjects approaching adulthood. Further studies are needed in symptomatic children within this age group.
Citation:
Tapia IE; Karamessinis L; Bandla P; Huang J; Kelly A; Pepe M; Schultz B; Gallagher P; Brooks LJ; Marcus CL. Polysomnographic values in children undergoing puberty: pediatric vs. adult respiratory rules in adolescents. SLEEP 2008;31(12):1737–1744.
Keywords: Adolescents, apnea, hypopnea
POLYSOMNOGRAPHY IS THE GOLD STANDARD FOR THE ASSESSMENT OF CHILDREN WITH SUSPECTED SLEEP DISORDERED BREATHING.1–3 BECAUSE OF wellrecognized differences in epidemiology4 and pathophysiology5–9 of sleep disordered breathing in children and adults, respiratory events in children should be scored using pediatric respiratory criteria.10 However, there is little information as to which respiratory rules should be used for scoring polysomnograms in adolescents between 13 and 18 years of age. Even the recently published “American Academy of Sleep Medicine manual for the scoring of sleep and associated events” states that there is no consensus for this age group.3
Adolescence is a dynamic transition process from childhood to adulthood. It is characterized by simultaneous but asynchronous development that starts with puberty, the onset of which is variable,11–14 and ends with adulthood.15 Therefore, it is reasonable to infer that respiratory polysomnographic scoring criteria and normal reference values would transition from pediatric to adult during adolescence.
We present this cross-sectional study primarily aimed at examining the evolvement of respiratory events with Tanner stage in a population of 68 children aged 8–18 years, recruited as part of a research study directed at investigating the effects of puberty on upper airway dynamics during sleep.16 Because of the lack of data regarding which respiratory rules to use in children aged 13–18 years, we further analyzed a subset of 32 subjects within this age group to identify a possible rationale for the use of pediatric or adult scoring criteria in children approaching adulthood. We rescored the respiratory events of these subjects according to the new American Academy of Sleep Medicine guidelines for children and adults.
MATERIALS AND METHODS
Subjects
Subjects were selected from a population of asymptomatic, healthy children recruited by means of advertisement to investigate the effects of puberty on upper airway dynamics during sleep at The Children's Hospital of Philadelphia during a 2-year period (2004–2006).16 Healthy, non-snoring males between 9 and 18 years old and females between 8 and 18 were included. The age criteria were designed to recruit adolescents with Tanner stages 1–5.11–13,15 Exclusion criteria included significant medical conditions, significant medications, history of upper airway surgery, family history of obstructive sleep apnea syndrome, and a body mass index > 95th percentile. The Institutional Review Board of The Children's Hospital of Philadelphia approved the study. Informed consent was obtained from 18-year-old subjects and the parents guardians of subjects younger than 18, as well as assent from those subjects younger than 18 years.
All subjects underwent a physical examination to determine Tanner stage, followed by a polysomnogram. Tanner stage was corroborated with serum hormone levels, which were obtained first thing in the morning following the polysomnogram, to minimize circadian variability. Ultra-sensitive luteinizing hormone was obtained in all subjects, follicular stimulating hormone and estradiol in females, and testosterone in males.
Polysomnogram
Subjects were studied in the sleep laboratory at The Children's Hospital of Philadelphia as previously described.17 Polysomnography data were digitally recorded using Somnostar Alpha (SensorMedics, Yorba Linda, CA) or Rembrandt (Medcare, Buffalo, NY). The electroencephalogram was recorded using 4 scalp electrodes (C3/A2, C4/A1, O1/A2, and O2/A1), and the right and left electrooculograms were recorded via 2 electrodes at the lateral canthi. Muscle tone of the chin was measured by submental electromyography. Oxyhemoglobin saturation (SpO2) was measured by pulse oximetry (Masimo Rad-9, Irvine, CA), set at 2-sec averaging. Nasal pressure was measured by cannula (Pro-Tech, Mukilteo, WA). As a back-up, oronasal airflow was measured by an oronasal thermistor (Pro-Tech, Mukilteo, WA). End-tidal CO2 was measured by sidestream sample (Novametrix Medical Systems, Wallingford, CT). Movements of the chest and abdomen were measured by respiratory inductive plethysmography (SensorMedics, Yorba Linda, CA). The electrocardiograph was recorded by a modified lead 1. Leg movements were recorded by electromyographic leads placed over the anterior tibialis muscles of both legs.
Sleep architecture, arousals from sleep, respiratory variables, and periodic limb movements were determined using the American Academy of Sleep Medicine new criteria.3 In brief, arousals were defined as an abrupt shift in EEG frequency ≥3 sec duration, preceded by a period ≥ 10 sec of stable sleep. During REM sleep, a concurrent increase in submental electromyography ≥ 1 second was required. The arousal index represented the number of arousals per hour of sleep. Respiratory events were scored with pediatric respiratory rules. For each type of respiratory event (central apnea, obstructive apnea, mixed apnea, hypopnea, and respiratory effort related arousal), an index that represented the number of events per hour of sleep was calculated. Respiratory effort related arousals were scored using a nasal pressure sensor, as recommended by The AASM Manual for the Scoring of Sleep and Associated Events.3 The apnea/hypopnea index (AHI) included the sum of all obstructive and mixed apneas, hypopneas, and respiratory effort related arousals divided by total sleep time (TST). The total duration of time with SpO2 below 92% was expressed as a percentage of TST. The total duration of time with end-tidal CO2 > 50 torr was expressed as a percentage of TST. We excluded SpO2 and endtidal CO2 measurements associated with poor waveforms or artifacts. The periodic limb movement index was the number of periodic limb movements per hour of sleep.
In addition, all the respiratory events of subjects with ages between 13 and 18 years old were rescored by one of the authors (I.T.) using the new respiratory rules for adults of The AASM Manual for the Scoring of Sleep and Associated Events,3 and results were compared. The main difference between the apnea scoring criteria is the duration of the event. Specifically, an event must last ≥ 10 sec to be scored as an apnea using adult rules. The respiratory rules for children mandate that an event must last ≥ 2 missed breaths to meet the apnea criteria, regardless of its duration in seconds. The adult rules give 2 options for scoring hypopneas; both require the duration of the event to be ≥ 10 sec. The recommended option establishes that at least a 30% drop in nasal pressure and at least 4% in oxyhemoglobin saturation from baseline are necessary to meet the hypopnea criteria. The alternative is identical to pediatric criteria, except for the duration of the event. The pediatric rules dictate that a ≥ 50% drop in nasal pressure and a ≥ 3% in oxyhemoglobin saturation from baseline or an associated arousal are needed to score an event as a hypopnea. We used both adult hypopnea rules.
Data Analysis
The statistical analysis was performed with SigmaStat 3.0 (Systat Software Inc., San Jose, CA). The distribution of continuous data was tested with the Kolmogorov-Smirnov test. The data that passed this normality test are presented as mean ± standard deviation (SD). The data that failed this normality test are presented as median and range. Differences in AHI, central apnea index, hypopnea index, and obstructive apnea index between pediatric and adult respiratory scoring rules were examined using the paired signed rank test. One-way ANOVA was used to compare ≥ 3 groups with a normal distribution (i.e., arousal index distribution according to Tanner stages). A post hoc analysis, when appropriate, was performed with the Bonferroni t-test. The Kruskal-Wallis One-way ANOVA Ranks test was utilized to compare ≥ 3 groups without a normal distribution (i.e., AHI distribution according to Tanner stages). A post hoc analysis, when appropriate, was performed with the Dunn's test. Associations between 2 variables were examined using Pearson or Spearman correlation coefficients, depending on normalcy of distribution of the data. A P value < 0.05 was required for significance.
RESULTS
All subjects
Study Group Characteristics
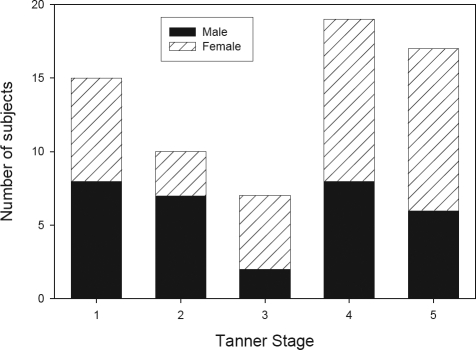
Sixty-eight subjects (37 female, 54%) were recruited. The mean age of the group was 13.2 ± 2.8 years, with a range between 8.1 and 18.7 years. The racial distribution, by Tanner stage, is shown in Table 1. Most subjects were African American or Caucasian. Three of the Caucasian subjects (of Tanner stages 1, 4, 5) were of Hispanic ethnicity. Tanner staging was consistent with hormone levels in 97% of subjects (the remaining 3% were assigned using clinical Tanner staging). The sex distribution according to Tanner stage is shown in Figure 1. As expected, older subjects had more advanced Tanner stages (Table 2).
Table 1.
Racial Distribution by Tanner Stage
| Tanner Stage |
Total | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| African American (N) | 5 | 6 | 3 | 9 | 9 | 32 |
| Caucasian (N) | 6 | 1 | 3 | 7 | 7 | 24 |
| Asian (N) | 2 | 2 | 0 | 3 | 0 | 7 |
| Mixed (N) | 2 | 1 | 1 | 0 | 1 | 5 |
| Total | 15 | 10 | 7 | 19 | 17 | 68 |
Figure 1.
Sex distribution according to Tanner stage. Each bar represents the number of subjects within each Tanner stage. The black portion represents the males and the lined portion the females.
Table 2a.
Sleep Architecture and Periodic Limb Movements During Sleep (PLMS) According to Tanner stage. Data with Normal Distribution. Values are Displayed as mean ± SD.
| Tanner Stage |
P value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Number of patients | 15 | 10 | 7 | 19 | 17 | |
| Age (years) | 10.1 ± 0.9 | 10.9 ± 0.9 | 12.3 ± 1.3 | 14.7 ± 1.8 | 16.1 ± 1.8 | <0.001 |
| Arousal index (N/hour) | 11.5 ± 4.4 | 9.4 ± 3.4 | 10.4 ± 3.2 | 9.5 ± 3.4 | 10.6 ± 4.0 | 0.532 |
| Stage N1 (% TST) | 6.8 ± 3.7 | 4.5 ± 2.3 | 5.3 ± 2.9 | 6.7 ± 4.0 | 6.9 ± 5.6 | 0.545 |
| Stage N2 (% TST) | 48.2 ± 4.2 | 49.7 ± 7.3 | 52.3 ± 8.6 | 56.6 ± 7.2 | 59.7 ± 7.9 | <0.001 |
| Stage N3 (% TST) | 26.1 ± 4.9 | 27.8 ± 7.8 | 25.4 ± 8.4 | 18.9 ± 7.3 | 15.7 ± 6.0 | <0.001 |
| Stage R (% TST) | 18.9 ± 4.4 | 17.4 ± 5.0 | 17.0 ± 6.4 | 17.7 ± 4.5 | 17.8 ± 5.7 | 0.922 |
TST, total sleep time.
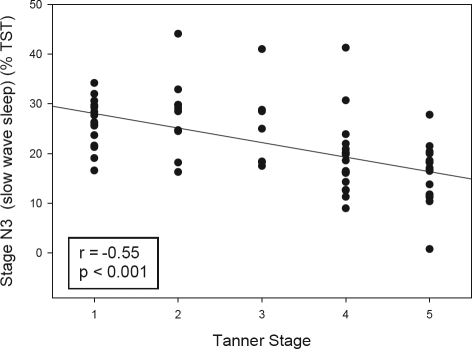
Stage N3 (slow wave sleep) decreased with increased Tanner stage. Consequently, stage N2 increased with Tanner stage. Since older subjects were in more advanced Tanner stages, this represents the well recognized reduction of stage N3 with age.
Table 2b.
Sleep Architecture and Periodic Limb Movements During Sleep (PLMS) According to Tanner Stage. Data Without Normal Distribution. Values are Displayed as Median (Range).
| Tanner Stage |
P value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Number of patients | 15 | 10 | 7 | 19 | 17 | |
| Sleep effi ciency (%) | 85.0 (71–94) | 86.0 (74–95) | 87.0 (66–92) | 89.0 (43–97) | 89.0 (56–98) | 0.719 |
| Sleep latency (minutes) | 19.0 (0–42) | 22.5 (3.5–76) | 14.0 (5.0–33.5) | 14.5 (1.0–83.5) | 16.5 (2.5–72.0) | 0.862 |
| REM latency (minutes) | 154.5 (60.5–267.0) | 102.0 (53–325) | 194.5 (133.5–329) | 136.5 (69.0–392.0) | 116.5 (44.5–287.0) | 0.106 |
| PLM index (N/hour) | 0.0 (0.0–7.2) | 0.0 (0.0–0.9) | 0.6 (0.0–2.4) | 0.2 (0.0–13.1) | 0.0 (0.0–4.1) | 0.025 |
REM, rapid eye movement; PLM, periodic limb movements.
No significant differences were observed between the different Tanner stages in regards to sleep effi ciency, sleep latency, and REM latency. There was a statistically signifi cant difference between the groups in regards to the PLM index. However, after adjusting the P value to account for the multiple pairwise comparisons none of the pairwise tests were statistically significant.
Sleep Architecture
Sleep architecture according to Tanner stage is shown in Tables 2a and b. Adolescents of Tanner stages 4–5 had less percentage of stage N3 (slow wave sleep) than subjects with Tanner stages 1–2 (Figure 2). Conversely, subjects of Tanner stages 4–5 had a greater percentage of stage N2 sleep than subjects of Tanner stages 1–2. There were no differences in regards to sleep efficiency, sleep latency, REM latency, arousal index, or percentage of stage R (REM) among Tanner stages.
Figure 2.
Duration of stage N3 (slow wave sleep) according to Tanner Stage. The correlation between stage N3 (slow wave sleep, expressed as percentage of total sleep time [TST]) according to Tanner stage is shown. Pearson's correlation coefficient (r).
Respiratory Events
Respiratory events according to Tanner stage are presented in Table 3. Central apneas were rare across the Tanner stages. The central apnea index for all the groups ranged between 0–1.5/ hour. Only 1 subject had a mixed apnea. Obstructive events were scarce, and did not differ between Tanner stages. The median apnea/hypopnea index for the entire study group was 0.1/ hour. Respiratory effort related arousals were determined in the 66 of 68 subjects who had accurate nasal pressure measurements throughout the night. Only 1 subject (a 10-year-old male, Tanner stage 2) had 1 respiratory effort related arousal.
Table 3.
Respiratory Rate During Sleep Stage N1, N2, N3, and REM, According to Tanner Stage.
| Respiratory Rate (breaths/min) | Tanner Stage |
P value | ||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||
| Stage N1 | 17.7 ± 2.3 | 17.2 ± 1.9 | 16.7 ± 2.1 | 18.0 ± 3.1 | 16.5 ± 2 | 0.38 |
| Stage N2 | 16.0 ± 1.9 | 17.1 ± 1.8 | 16.1 ± 1.8 | 16.3 ± 2.8 | 15.5 ± 1.9 | 0.43 |
| Stage N3 | 16.6 ± 1.9 | 17.7 ± 1.8 | 16.7 ± 2.3 | 17.1 ± 2.7 | 16.6 ± 2.3 | 0.75 |
| Stage R | 16.3 ± 1.4 | 17.9 ± 1.7 | 15.9 ± 2.3 | 17.5 ± 3.1 | 16.6 ± 2.1 | 0.24 |
Values are displayed as mean ± SD. There were no significant differences in respiratory rate between the different Tanner stages.
The respiratory rate during sleep stage N1, N2, N3, and R is shown in Table 3. The respiratory rate during NREM and REM sleep did not change with Tanner stage. The mean respiratory rate for the entire group was 16.5 ± 2.1 breaths/min during NREM sleep, and 16.9 ± 2.3 breaths/min during REM sleep.
Sixty-four of 68 subjects had accurate end-tidal CO2 measurements during the study. The median percentage of total sleep time > 50 torr and peak end-tidal CO2 for the entire group was 0.1% and 49 torr, respectively. Five outliers had end-tidal CO2 > 50 torr more than 25% of the total sleep time; their percentage of total sleep time > 50 torr ranged between 39% and 88.6% with peak end-tidal CO2 between 52 and 59 torr.
The median percentage of total sleep time < 92% for the entire group was 0.1%, and the median nadir SpO2 was 93%.
There were no significant gas exchange differences among Tanner stages.
Periodic Limb Movements
Periodic limb movements during sleep were infrequent. Only 22 subjects had periodic limb movements, and of those, only 2 (a 14-year-old female [Tanner stage 4] and a 10-year-old male [Tanner stage 1]) had a periodic limb movement index > 5/h. The periodic limb movement index for the group ranged between 0 and 13.1/h. The median periodic limb movement index was 0/hour. There was a statistically significant difference between Tanner stages in regard to the periodic limb movement index. However, after adjusting the P value to account for the multiple pairwise comparisons, none of the pairwise tests were statistically significant.
13–18 Year Old Subset
Respiratory Events
We further analyzed the respiratory events of the subjects between 13–18 years old (N = 32; Tanner stages 3–5, mean age 15.8 ± 1.4 years). The results are shown in Table 5. Sixteen subjects had central apneas, with a median duration of 12.2 (5.7–21) sec; the median central apnea index was 0.06 (0–0.6)/h. Since all of our subjects were normal and asymptomatic, only 15 of them had obstructive events. All 15 subjects had hypopneas, with a median duration of 15.5 (6–24.8) sec. Two subjects also had obstructive apneas, with a mean duration of 13.7 sec. The median apnea hypopnea index was 0 (0–0.9)/h. Of 32 subjects, 31 had accurate nasal pressure measurements; no subject in this age group had respiratory effort related arousals.
Table 5.
Respiratory Events with Pediatric Criteria in the Subset of Subjects Aged 13-18 Years (32 Patients)
| Median | Range | Limit of normality | |
|---|---|---|---|
| Age (years) | 15.8 | 13.3–18.7 | |
| Apnea hypopnea index (N/h) | 0.0 | 0.0–0.9 | 0.8 |
| Obstructive apnea index (N/h) | 0.0 | 0.0–0.5 | 0.1 |
| Hypopnea index (N/h) | 0.0 | 0.0–0.9 | 0.8 |
| Central apnea index (N/h) | 0.06 | 0.0–0.6 | 0.4 |
| % TST with end-tidal CO2 > 50 torr | 0.2 | 0.0–64.6 | 24.0 |
| Peak end-tidal CO2 (torr) | 50.0 | 45.0–54.0 | 53.0 |
| % TST with SpO2 <92% | 0.0 | 0.0–1.5 | 1.0 |
| SpO2 Nadir (mm Hg) | 93.0 | 88.0–97.0 | 89.0 |
TST, total sleep time; SpO2, oxyhemoglobin saturation.
The limit of normality was calculated as the 95th percentile for all the parameters, except for SpO2 nadir that was calculated as the 5th percentile.
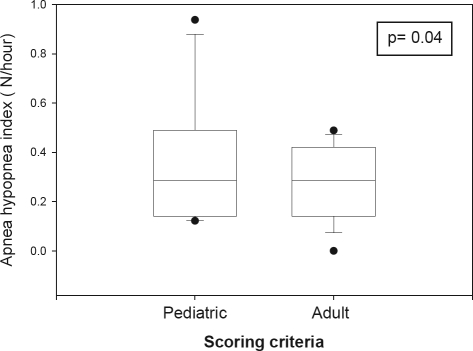
After rescoring the events with the new adult rules,3 there was no significant difference in the median central apnea index with pediatric (0.05, range: 0–0.6/h) and adult ( 0, range: 0–0.4/h) criteria (P = 0.13). However, the difference between AHI index values as scored by pediatric and adult respiratory rules did attain statistical significance (P = 0.04, Figure 3), which was caused by a decrease in the distribution range from 0.1–0.9/h with pediatric rules to 0–0.5/h with adult rules, since short hypopneas did not meet the adult criteria. The median AHI however, did not change (pediatric criteria = 0, adult criteria = 0). This observed difference (mean difference = 0.048, SD = 0.14, 95% confidence interval = –0.001 to 0.098) is so small that it would not be of clinical relevance. We did not find differences between the 2 adult hypopnea rules. Specifically, 15 subjects had 36 hypopneas with pediatric rules, but only 23 hypopneas lasted ≥ 10 sec. Those 23 hypopneas were characterized by drops in nasal pressure ≥ 50% and ≥ 4% desaturation from pre-event baseline. Therefore, the described events met the 2 adult hypopnea rules. In addition, 15 hypopneas were associated with arousal.
Figure 3.
Apnea hypopnea index in subset of subjects aged 13–18 years scored by pediatric and adult criteria. Apnea hypopnea index results using pediatric and adult respiratory scoring criteria. The boundaries of the boxes represent the 25th and 75th percentiles. The line within the boxes marks the medians. The whiskers indicate the 10th and 90th percentiles, and the points represent the range. The apnea hypopnea index obtained with adult respiratory rules was significantly lower than with pediatric respiratory rules. However, the observed difference (mean difference = 0.048, SD = 0.14, 95% confidence interval = −0.001 to 0.098) is so small, that it would not be of clinical relevance.
Twenty-nine of 32 subjects had accurate end-tidal CO2 measurements. The 3 outliers with high CO2 levels from this subset were described in the previous section. Gas exchange data are shown in Table 3. Since the percentages of total sleep time with end-tidal CO2 >50 torr and SpO2 <92% failed the normality test, the cut-off values were calculated at the 95th percentile (Table 4).
Table 4.
Respiratory Events According to Tanner Stage. Values are Displayed as Median (Range)
| Tanner Stage |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Apnea hypopnea index (N/h) | 0.1 (0.0–0.9) | 0.0 (0.0–0.5) | 0.1 (0.0–0.5) | 0.1 (0.0–1.2) | 0.0 (0.0–0.7) |
| Obstructive apnea index (N/h) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) | 0.0 (0.0–0.3) | 0.0 (0.0–0.5) |
| Hypopnea index (N/h) | 0.1 (0.0–0.9) | 0.0 (0.0 –0.5) | 0.0 (0.0–0.4) | 0.1 (0.0–1.0) | 0.0 (0.0–0.3) |
| Central apnea index (N/h) | 0.1 (0.0–1.3) | 0.0 (0.0 –1.5) | 0.1 (0.0–1.3) | 0.0 (0.0–0.4) | 0.1 (0.0–0.6) |
| % TST with end-tidal CO2 > 50 torr | 0.1 (0.0– 88.6) | 0.5 (0.0 –79.0) | 0.0 (0.0–0.2) | 0.4 (0.0–59.4) | 0.0 (0.0–64.6) |
| Peak end-tidal C2 (torr) | 50.0 (47–59) | 51.5 (43 –59) | 47.0 (45–55) | 50.0 (51–59) | 48.5 (45–53) |
| % TST with SpO2 <92% | 0.4 (0.0–2.3) | 0.4 (0.0 –4.2) | 0.0 (0.0–1.0) | 0.0 (0.0–1.5) | 0.0 (0.0–1.0) |
| Nadir SpO2 | 93.0 (90–97) | 93.5 (88 –98) | 93.0 (89–96) | 94.0 (88–96) | 93.0 (89–97) |
TST, total sleep time; SpO2, oxyhemoglobin saturation.
No significant differences in respiratory events between the different Tanner stages were observed.
DISCUSSION
The American Academy of Sleep Medicine published The AASM Manual for the Scoring of Sleep and Associated Events3 in 2007 to encourage standardized polysomnogram scoring based on the medical literature. Since there was no study comparing adult and pediatric criteria in adolescents,3 the recent rules allow children aged 13–18 years to be scored with either pediatric or adult criteria, based on empiric observations.
This is the first study analyzing the polysomnographic respiratory parameters of healthy non-obese asymptomatic adolescents between 13 and 18 years old, comparing the new pediatric and adult scoring criteria. We have shown no clinically significant apnea hypopnea index differences using either set of criteria in normal, asymptomatic subjects. We have also confirmed that healthy, asymptomatic, non-obese children and adolescents between 8 and 18 years rarely have obstructive events (including respiratory effort related arousals) during sleep. This result is independent of the Tanner stage. Additionally, this study has also corroborated that slow wave sleep (stage N3) decreases during puberty.18,19
Adolescents have been underrepresented in the sleep literature.3 They have been analyzed either with younger children20,21 or with young adults,22 but rarely as a solo group. Acebo et al. described sleep architecture, respiratory variables and cephalometrics in healthy boys and girls aged 9.7–16.8 years along with young adults.22 The subjects were characterized by Tanner stage, but the results were analyzed according to age. Uliel et al. reported 70 healthy children and adolescents between 1 and 15 years,21 and Verhulst reported 60 subjects between 6–16 years.20 However, Tanner staging was not included in their methods. Therefore, the main difference between our study and previous publications describing normal populations20–23 is that we focused our analysis on the changes in respiratory parameters with Tanner stage to clarify whether there was a rationale for the use of pediatric or adult scoring criteria in children approaching adulthood. In addition, we included data on respiratory rate during sleep, respiratory effort related arousals, and periodic limb movements, and used the recommended nasal pressure signal rather than thermistor flow as the primary signal for determining respiratory events.
Sleep Architecture
We found that slow wave sleep stage decreased significantly with Tanner stage, with a concomitant increase in stage N2. Since our older subjects were at a more advanced puberty stage, the result is concordant with previous studies18,24 showing that stage N2 increases and stage N3 (slow wave sleep) decreases with age. Feinberg et al.25 have demonstrated in a longitudinal study that slow wave sleep decline starts at age 12 years, and is independent of Tanner stage.
Our results on sleep latency and sleep efficiency are similar to other publications.22,24,26 We did not find stage R (REM sleep) differences with Tanner stage, which is also concordant with other publications.19,27
Arousal Index
The arousal index increases linearly from childhood to old age28 but remains unchanged during adolescence.29 Similarly, we did not find differences in the arousal index across the different puberty stages.
Respiratory Parameters According to Tanner Stage
We add to the field further data that normal adolescents have infrequent respiratory events, and no gas exchange abnormality. Our results are concordant with the published data in younger children30–32 and provide evidence that biological maturation expressed by Tanner staging is not associated with respiratory events. As previously reported,20 hypopneas were the most frequent obstructive event observed.
In addition, this is the first study, to our knowledge, reporting respiratory rates during sleep in association with Tanner stage. Carskadon et al previously studied 22 children aged 9–13 years, but without Tanner staging, and found similar mean respiratory rate values.33 The respiratory rate during sleep is the cause of the main difference in scoring criteria between children and adults.3 Since children have faster respiratory rates than adults and a lower functional residual capacity that could lead to rapid desaturation with short respiratory events, the AASM pediatric committee considered that the 10-second rule would overlook many respiratory events in children. We expected older and more biologically mature children to have a respiratory rate during sleep close to 12 breaths/minute, since with that rate the 10-second adult apnea scoring rule would be appropriate (2 missed breaths = 10 seconds). However, we found that the respiratory rate did not change significantly with biological maturation during adolescence. Few studies have analyzed age-related changes in respiratory rate during sleep in adults.34–36 Shore et al studied 12 healthy young adult subjects (19–29 years) and 12 elderly (> 65 years) subjects during sleep, and found that that the elderly had a significantly lower respiratory rate during wakefulness (13.1 breaths/min) and sleep stages N1 and N2 (12.4 breaths/min) compared to the younger group (16.4 and 14.9 breaths/min respectively).35 However, the results from the younger adults were similar to the adolescents reported in the current study. Data from middle-aged adults reported in the literature34,36 and summarized by Krieger,37 also suggest that adults have a respiratory rate during sleep (mean respiratory rate of 15 breaths/min) that is similar to that of the adolescents in the current study. Thus, in these adults, an apnea that is 2 missed breaths in duration would last for 8 seconds.
Our SpO2 results are in agreement with Gries and Brooks,38 Verhulst,20 Poets,30 and Marcus.23 Virtually all our subjects had SpO2 > 92% throughout the night.
Similar to Uliel,21 the majority of the subjects did not have any period of total sleep time with end-tidal CO2 > 50 torr. This result is also in agreement with previous data in younger children,31,32 and our previous data measuring end-tidal CO2 with mass spectrometry.23 This observation did not change with Tanner stage. However, the 95th percentile for total sleep time with end-tidal CO2 > 50 torr was 24%. Therefore, we recommend that patients with values above this cut-off point should be assessed to rule out clinical hypoventilation. The outliers in this study spent most of the night with end-tidal CO2 in the low 50s.
Respiratory Parameters in Subjects 13–18 Years Old
We did not find significant differences in the central apnea index after rescoring the respiratory events with pediatric and adult respiratory rules. The statistically significant difference observed in the AHI after scoring with adult respiratory rules was due to a decrease in the number of hypopneas since short hypopneas did not meet adult criteria. Despite attaining statistical significance, this difference is so small that it would not be of clinical relevance. However, the results in adolescents with the obstructive sleep apnea syndrome may be very different. Further studies are warranted.
Limitations
Study limitations include a relatively small number of subjects per Tanner stage, but larger than most other normative studies.20,22,23 Our research was based on normal children, who we know rarely have respiratory events.21–23,39 The subjects were mostly African American or Caucasian, with only a few Asians and Hispanics. Thus, these data may not be applicable to other racial ethnic groups. The results are also limited by our crosssectional study design. Future longitudinal studies are desirable.
CONCLUSIONS
This study has confirmed that apneas and hypopneas in normal adolescents are rare. We add to the field evidence that respiratory effort related arousals are virtually non-existent in normal subjects, that the respiratory rate and the frequency of respiratory events does not change with Tanner stage, which is a marker of biological maturation, in normal subjects.
Since we did not find a rationale for the scoring of respiratory events with pediatric or adult criteria in children, we recommend using either rule for scoring respiratory events in polysomnograms of normal adolescents between 13 and 18 years old recruited for research purposes. However, further studies are needed in patients with respiratory conditions, such as the obstructive sleep apnea syndrome, within this age group.
In addition, we suggest that adult apnea duration criteria should be reconsidered, based on the fact that the respiratory rate during sleep in adults is similar to that of adolescents. We suggest that 2 missed breaths, regardless of the duration in seconds, may be a more accurate criterion to score respiratory events at any age.
ACKNOWLEDGMENTS
The authors thank the children participating in this study and their families, the sleep laboratory technicians, and Mary Tate, the sleep laboratory scheduler, for their valuable help. This research was supported by the National Institutes of Health R01 HL 58585, MO1-RR-000240 and UL1 RR 024134.
ABBREVIATIONS
- AHI
Apnea hypopnea index
- SpO2
Oxyhemoglobin saturation
- TST
Total sleep time
- REM
Rapid eye movement
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Marcus has received research support and the use of equipment from Respironics. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 3.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 4.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 5.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JL. Obstructive sleep-disordered breathing in children: new controversies, new directions. Clin Chest Med. 2003;24:261–82. doi: 10.1016/s0272-5231(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 7.Marcus CL. Obstructive sleep apnea syndrome: differences between children and adults. Sleep. 2000;23(Suppl 4):S140–1. [PubMed] [Google Scholar]
- 8.Marcus CL. Pathophysiology of childhood obstructive sleep apnea: current concepts. Respir Physiol. 2000;119:143–54. doi: 10.1016/s0034-5687(99)00109-7. [DOI] [PubMed] [Google Scholar]
- 9.Bandla P, Brooks LJ, Trimarchi T, Helfaer M. Obstructive sleep apnea syndrome in children. Anesthesiol Clin North America. 2005;23:535–49. doi: 10.1016/j.atc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Rosen CL, D'Andrea L, Haddad GG. Adult criteria for obstructive sleep apnea do not identify children with serious obstruction. [see comment] Am Rev Respir Dis. 1992;146(5 Pt 1):1231–4. doi: 10.1164/ajrccm/146.5_Pt_1.1231. [DOI] [PubMed] [Google Scholar]
- 11.Herman-Giddens ME, Kaplowitz PB, Wasserman R. Navigating the recent articles on girls' puberty in Pediatrics: what do we know and where do we go from here? [see comment] Pediatrics. 2004;113:911–7. doi: 10.1542/peds.113.4.911. [DOI] [PubMed] [Google Scholar]
- 12.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. [see comment] Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 13.Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988-1994. [see comment] Arch Pediatr Adolesc Med. 2001;155:1022–8. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- 14.Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25:535–50. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- 15.Rosen DS. Physiologic growth and development during adolescence. Pediatr Rev. 2004;25:194–200. doi: 10.1542/pir.25-6-194. [DOI] [PubMed] [Google Scholar]
- 16.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karamessinis L, Galster P, Schultz B, et al. Relationship between REM density, duty cycle, and obstructive sleep apnea in children. Sleep. 2007;30:837–43. doi: 10.1093/sleep/30.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep. 2005;28:637–43. doi: 10.1093/sleep/28.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 20.Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol. 2007;42:159–67. doi: 10.1002/ppul.20551. [DOI] [PubMed] [Google Scholar]
- 21.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. [see comment] Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 22.Acebo C, Millman RP, Rosenberg C, Cavallo A, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Part I -- Normative values. Chest. 1996;109:664–72. doi: 10.1378/chest.109.3.664. [DOI] [PubMed] [Google Scholar]
- 23.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. [see comment] Am Rev Respir Dis. 1992;146(5 Pt 1):1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 24.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. [see comment] Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palm L, Persson E, Elmqvist D, Blennow G. Sleep and wakefulness in normal preadolescent children. Sleep. 1989;12:299–308. doi: 10.1093/sleep/12.4.299. [DOI] [PubMed] [Google Scholar]
- 27.Jenni OG, van Reen E, Carskadon MA. Regional differences of the sleep electroencephalogram in adolescents. J Sleep Res. 2005;14:141–7. doi: 10.1111/j.1365-2869.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 28.Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–7. [PubMed] [Google Scholar]
- 29.Carskadon MA. The second decade. In: Guillminault C, editor. Sleep and waking disorders: indications and techniques. Menlo Park, CA: Addison Wesley; 1982. pp. 99–125. [Google Scholar]
- 30.Moss D, Urschitz MS, von Bodman A, et al. Reference values for nocturnal home polysomnography in primary schoolchildren. Pediatr Res. 2005;58:958–65. doi: 10.1203/01.PDR.0000181372.34213.13. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 32.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. [see comment] Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 33.Carskadon MA, Harvey K, Dement WC, Guilleminault C, Simmons FB, Anders TF. Respiration during sleep in children. West J Med. 1978;128:477–81. [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger J, Maglasiu N, Sforza E, Kurtz D. Breathing during sleep in normal middle-aged subjects. Sleep. 1990;13:143–54. [PubMed] [Google Scholar]
- 35.Shore ET, Millman RP, Silage DA, Chung DC, Pack AI. Ventilatory and arousal patterns during sleep in normal young and elderly subjects. J Appl Physiol. 1985;59(5):1607–15. doi: 10.1152/jappl.1985.59.5.1607. [DOI] [PubMed] [Google Scholar]
- 36.Stradling JR, Chadwick GA, Frew AJ. Changes in ventilation and its components in normal subjects during sleep. Thorax. 1985;40:364–70. doi: 10.1136/thx.40.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger J. Respiratory physiology: breathing in normal subjects. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 232–44. [Google Scholar]
- 38.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. 1996;110:1489–92. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 39.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. [comment] Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]