Abstract
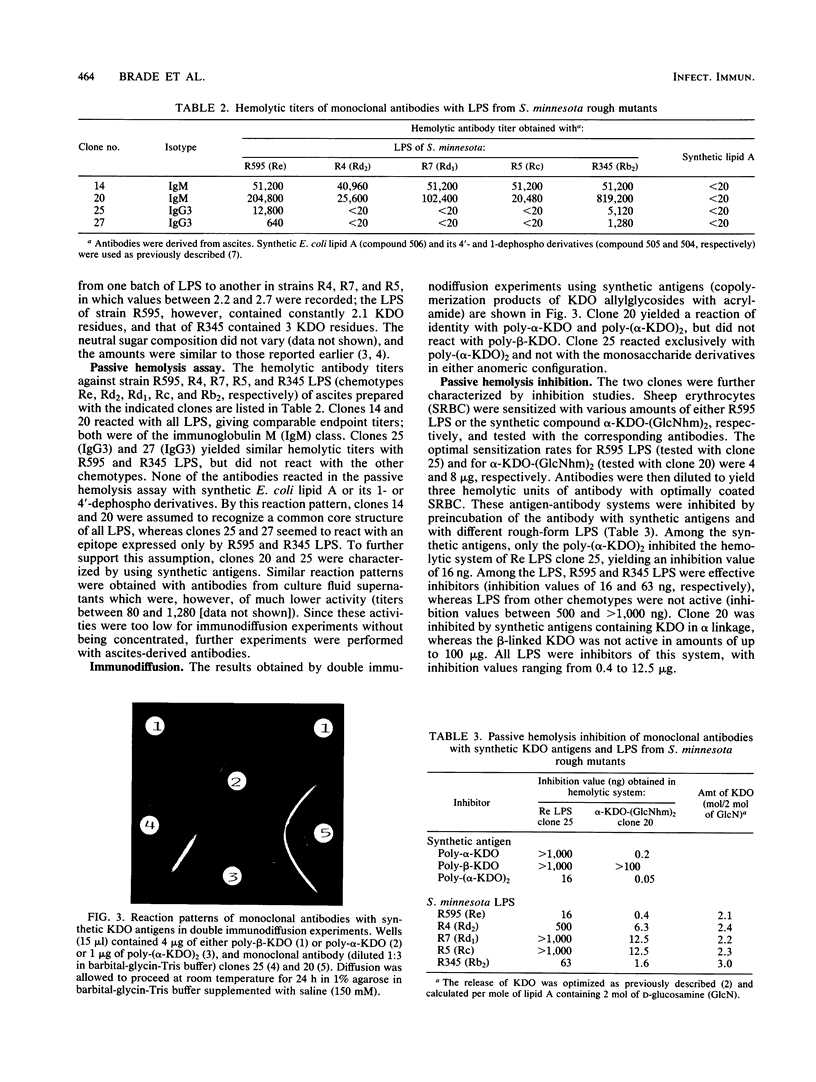
Mouse monoclonal antibodies were raised against heat-killed bacteria of the Re mutant R595 of Salmonella minnesota and characterized by the passive hemolysis and passive hemolysis inhibition tests and by double immunodiffusion experiments using lipopolysaccharide (LPS) from different rough mutants of S. minnesota and synthetic antigens. The latter were copolymerization products of acrylamide with the alpha- and beta-allylglycosides of 3-deoxy-D-manno-octulosonic acid (KDO) and the alpha-2,4-linked KDO disaccharide [poly-alpha-KDO, poly-beta-KDO, and poly-(alpha-KDO)2, respectively], and sodium (3-deoxy-D-manno-octulopyranosyl)onate-(2----6)-(2-deoxy-2-[ (R)-3- hydroxytetradecanoylamino]- beta-D-glucopyranosyl)-(1----6)-(2-deoxy-2-[(R)-3-hydroxytetradecanoy lam ino]-D-glucose) [alpha-KDO-(GlcNhm)2], representing a part structure of Re LPS. One antibody (clone 20, immunoglobulin M) was found to recognize a terminal alpha-linked KDO residue, since it reacted in the passive hemolysis assay with alpha-KDO-(GlcNhm)2 and all LPS tested, it was inhibited by all synthetic antigens containing alpha-linked KDO residues, and it gave a reaction of identity with poly-alpha-KDO and poly-(alpha-KDO)2 in double immunodiffusion experiments. A second antibody (clone 25, immunoglobulin G3) was identified as specific for an alpha-2,4-linked KDO disaccharide, since it reacted in immunodiffusion exclusively with synthetic poly-(alpha-KDO)2 and not with the monosaccharide derivatives in either anomeric configuration, and it was inhibited only with poly-(alpha-KDO)2 and with LPS from S. minnesota R595 (Re) and R345 (Rb2). The reaction of this antibody with R345 LPS is attributed to the quantitative substitution with KDO disaccharide present as a side chain, which is not present in stoichiometric amounts in the other LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner J. D., Glauser M. P., McCutchan J. A., Ziegler E. J., van Melle G., Klauber M. R., Vogt M., Muehlen E., Luethy R., Chiolero R. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985 Jul 13;2(8446):59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Isolation of a 3-deoxy-D-mannooctulosonic acid disaccharide from Salmonella minnesota rough-form lipopolysaccharides. Eur J Biochem. 1983 Mar 1;131(1):201–203. doi: 10.1111/j.1432-1033.1983.tb07250.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Moll H., Rietschel E. T. Structural investigations on the inner core region of lipopolysaccharides from Salmonella minnesota rough mutants. Biomed Mass Spectrom. 1985 Oct;12(10):602–609. doi: 10.1002/bms.1200121007. [DOI] [PubMed] [Google Scholar]
- Brade H., Rietschel E. T. Alpha-2----4-interlinked 3-deoxy-D-manno-octulosonic acid disaccharide. A common constituent of enterobacterial lipopolysaccharides. Eur J Biochem. 1984 Dec 3;145(2):231–236. doi: 10.1111/j.1432-1033.1984.tb08543.x. [DOI] [PubMed] [Google Scholar]
- Brade L., Rietschel E. T., Kusumoto S., Shiba T., Brade H. Immunogenicity and antigenicity of synthetic Escherichia coli lipid A. Infect Immun. 1986 Jan;51(1):110–114. doi: 10.1128/iai.51.1.110-114.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., Douglas H., McCutchan J. A. Antibody to cell wall glycolipid of Gram-negative bacteria: induction of immunity to bacteremia and endotoxemia. J Infect Dis. 1977 Aug;136 (Suppl):S167–S173. doi: 10.1093/infdis/136.supplement.s167. [DOI] [PubMed] [Google Scholar]
- Bruins S. C., Stumacher R., Johns M. A., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Comparison of the protective effect of immunization with lipid A and the Re mutant. Infect Immun. 1977 Jul;17(1):16–20. doi: 10.1128/iai.17.1.16-20.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak AYa, Levinsky A. B., Dmitriev B. A., Kochetkov N. K. A new type of carbohydrate-containing synthetic antigen: synthesis of carbohydrate-containing polyacrylamide copolymers having the specificity of O:3 and O:4 factors of Salmonella. Carbohydr Res. 1984 Jun 1;128(2):269–282. doi: 10.1016/0008-6215(84)85334-3. [DOI] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985 Jun;151(6):1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., DuBuy J. B., Woodward C. L. Experimental gram-negative bacterial sepsis: reevaluation of the ability of rough mutant antisera to protect mice. Proc Soc Exp Biol Med. 1978 Jul;158(3):482–490. doi: 10.3181/00379727-158-40231. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Tanamoto K., Galanos C., McKenzie G. R., Brade H., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Lipopolysaccharides: structural principles and biologic activities. Rev Infect Dis. 1984 Jul-Aug;6(4):428–431. doi: 10.1093/clinids/6.4.428. [DOI] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A. K., Chen C. L., Chang C. M., Nowotny A. Relationship of structure to function in bacterial endotoxins: serologically cross-reactive components and their effect on protection of mice against some gram-negative infections. J Gen Microbiol. 1976 May;94(1):107–116. doi: 10.1099/00221287-94-1-107. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Siber G. R., Kania S. A., Warren H. S. Cross-reactivity of rabbit antibodies to lipopolysaccharides of Escherichia coli J5 and other gram-negative bacteria. J Infect Dis. 1985 Nov;152(5):954–964. doi: 10.1093/infdis/152.5.954. [DOI] [PubMed] [Google Scholar]
- Tacken A., Rietschel E. T., Brade H. Methylation analysis of the heptose/3-deoxy-D-manno-2-octulosonic acid region (inner core) of the lipopolysaccharide from Salmonella minnesota rough mutants. Carbohydr Res. 1986 Jul 1;149(2):279–291. doi: 10.1016/s0008-6215(00)90051-x. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- de Jongh-Leuvenink J., Vreede R. W., Marcelis J. H., de Vos M., Verhoef J. Detection of antibodies against lipopolysaccharides of Escherichia coli and Salmonella R and S strains by immunoblotting. Infect Immun. 1985 Dec;50(3):716–720. doi: 10.1128/iai.50.3.716-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



