Abstract
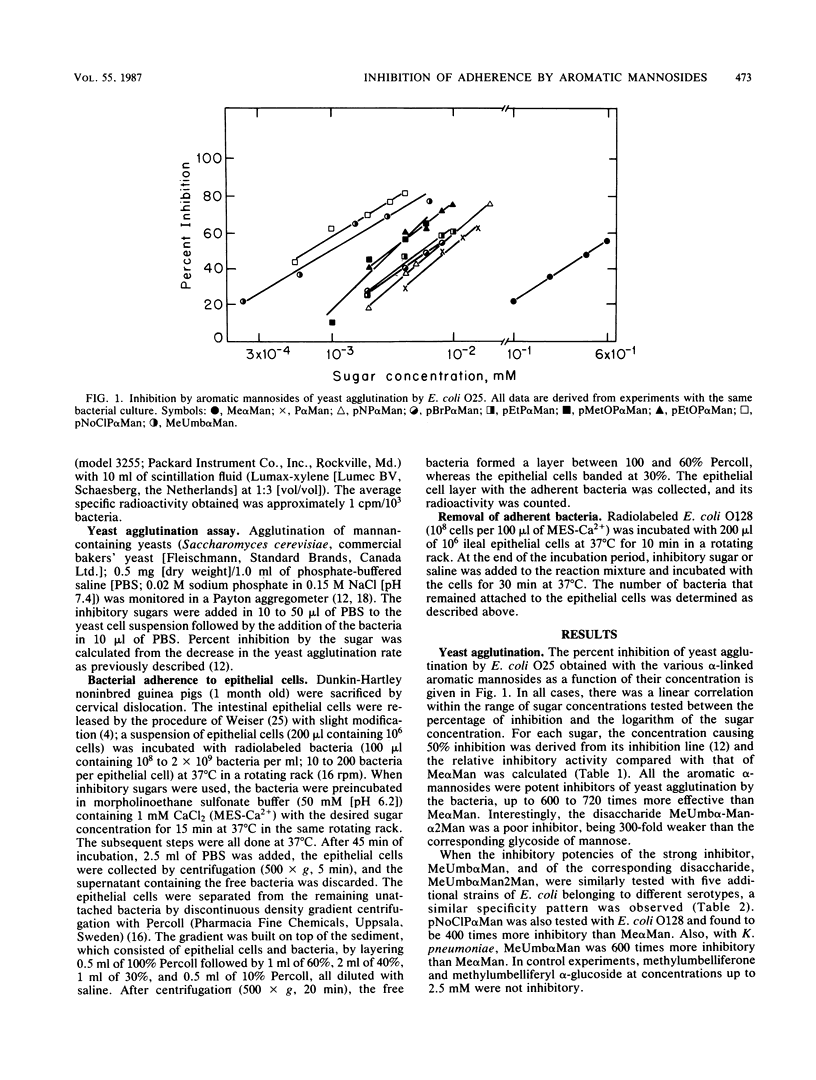
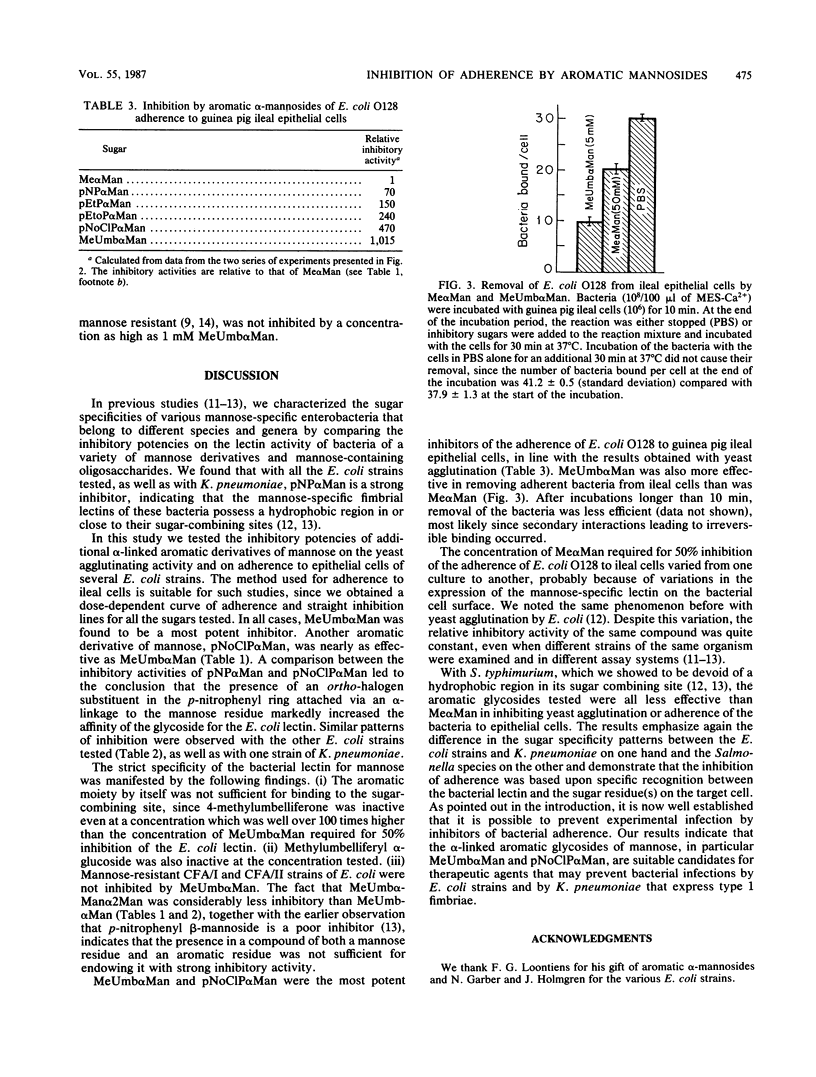
Adherence of bacteria via their surface lectins to host epithelial cells is considered an important initial event in bacterial pathogenesis. Mannose-specific (type 1) fimbriae are among the most commonly found lectins in enterobacteria. We studied the effect of aromatic alpha-glycosides of mannose on the agglutination of mannan-containing yeasts by different strains of Escherichia coli and on the adherence of the bacteria to guinea pig ileal epithelial cells. In both systems these compounds were considerably more effective inhibitors than methyl alpha-mannoside, with 4-methylumbelliferyl alpha-mannoside and p-nitro-o-chlorophenyl alpha-mannoside being the strongest inhibitors. Both compounds were approximately 400-times stronger inhibitors of yeast agglutination by E. coli O128 than was methyl alpha-mannoside and 1,000- and 470-fold stronger, respectively, than was methyl alpha-mannoside in inhibiting the adherence of the bacteria to ileal epithelial cells. 4-Methylumbelliferyl alpha-mannoside was 540 to 1,000 times more effective in inhibiting yeast agglutination by four additional strains of mannose-specific E. coli. It was also more efficient than methyl alpha-mannoside in removing adherent E. coli O128 from ileal epithelial cells. Our results provide further evidence that type 1 fimbriae of E. coli possess a hydrophobic region next to the mannose-binding site. The results suggest that 4-methylumbelliferyl alpha-mannoside and p-nitro-o-chlorophenyl alpha-mannoside are good candidates for the design of therapeutic agents that may prevent adherence in vivo and infection by E. coli strains that express type 1 fimbriae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Babu J. P., Giampapa C. S., Hasty D. L., Simpson W. A., Beachey E. H. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985 Jun;48(3):625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Mirelman D. The effect of postnatal age on the adherence of Shigella flexneri, Escherichia coli 0124, and E. coli 0128 to guinea pig intestinal cells. Pediatr Res. 1984 Dec;18(12):1366–1371. doi: 10.1203/00006450-198412000-00030. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Brauner A., Leissner M., Wretlind B., Julander I., Svenson S. B., Källenius G. Occurrence of P-fimbriated Escherichia coli in patients with bacteremia. Eur J Clin Microbiol. 1985 Dec;4(6):566–569. doi: 10.1007/BF02013396. [DOI] [PubMed] [Google Scholar]
- Daifuku R., Stamm W. E. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986 May 8;314(19):1208–1213. doi: 10.1056/NEJM198605083141902. [DOI] [PubMed] [Google Scholar]
- Elo J., Tallgren L. G., Väisänen V., Korhonen T. K., Svenson S. B., Mäkelä P. H. Association of P and other fimbriae with clinical pyelonephritis in children. Scand J Urol Nephrol. 1985;19(4):281–284. doi: 10.3109/00365598509180270. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ahrén C. Nonimmunoglobulin fraction of human milk inhibits bacterial adhesion (hemagglutination) and enterotoxin binding of Escherichia coli and Vibrio cholerae. Infect Immun. 1981 Jul;33(1):136–141. doi: 10.1128/iai.33.1.136-141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Porter T. N., Schaeffer A. J., Duncan J. L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985 Nov;50(2):370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loontiens F. G., Van Wauwe J. P., De Gussem R., De Bruyne C. K. Binding of para-substituted phenyl glycosides to concanavalin A. Carbohydr Res. 1973 Sep;30(1):51–62. doi: 10.1016/s0008-6215(00)82172-2. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A., Kaack B., Källenius G., Möllby R., Winberg J., Svenson S. B. Receptors for pyelonephritogenic Escherichia coli in primates. J Urol. 1984 Jan;131(1):163–168. doi: 10.1016/s0022-5347(17)50251-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. A. Urinary tract infections. Am J Kidney Dis. 1984 Sep;4(2):103–117. doi: 10.1016/s0272-6386(84)80057-8. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Weinstein R., Rene P. Protection against experimental pyelonephritis by antibodies to pili. Scand J Infect Dis Suppl. 1982;33:79–82. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]



