Abstract
Objectives:
Obstructive sleep apnea often results in a wide range of comorbid conditions. Although some conditions have been clearly identified as comorbid, a full clinical pattern of associated diseases has not been systematically documented. This research aimed to reveal the full pattern of comorbid conditions associated with OSA by employing a data mining technique.
Methods:
A large data repository (the New South Wales Inpatient Data Collection) collected between 1999 and 2004 was mined, and all clinical diagnoses were coded with ICD-10-AM codes.
Results:
A total of 60,197 cases (4% of total records) were identified as related to OSA (72.2% males, 27.8% females). OSA occurrence showed 2 peaks at 0–4 years and 55–59 years. A strikingly low occurrence was observed for the adolescent years. Conditions comorbid with OSA in adults by descending frequency were essential hypertension, obesity, hypercholesterolemia, type 2 diabetes, past or current tobacco use, and ischemic heart conditions. Obesity and OSA showed a similar time course of onset, with a latent period of 5 years for hypertension and type 2 diabetes and 15 years for chronic ischemic heart conditions. Comorbid conditions were predominantly of the cardiovascular, endocrine/metabolic and respiratory systems. The data also indicated OSA patients are high users of health services.
Conclusions:
The data mining technique confirms the prevalence of the disease, describes the age distribution patterns and time courses of disease development from obesity and OSA to comorbid conditions, and implicates possible interrelationships among these conditions and high cost of treating OSA patients.
Citation:
Huang QR; Qin Z; Zhang S; Chow CM. Clinical patterns of obstructive sleep apnea and its comorbid conditions: a data mining approach. J Clin Sleep Med 2008;4(6):543–550.
Keywords: Obstructive sleep apnea, comorbidity, data mining, obesity, cardiovascular conditions, ICD-10, disease patterns
Obstructive sleep apnea is a sleep disorder common in males with a male to female ratio of 2–3:1.1 It is characterized by intermittent upper airway collapse occurring during sleep.2 Complete collapse of the airway leads to apneic episodes, whereas partial collapse leads to hypopneic episodes. The most frequent symptoms of OSA are snoring, transient cessation of breathing, and excessive daytime sleepiness. Discomfort during sleep, sore throat, headache, and exhaustion in the mornings constitute the rest of the symptoms. Epidemiological studies have shown that OSA is associated with obesity and a broad range of cardiovascular morbidities including hypertension, coronary artery disease, myocardial infarction, congestive heart failure, and stroke.3
Epidemiological studies have provided important information about demographic characteristics of OSA patients and comorbid conditions. However prevalence, risk factors, comorbid conditions, their patterns by age distribution and time course in relation to OSA are not well described. Recently, a different approach, data mining, an integral component of Knowledge Discovery in Databases, has been used in medical research.4 This technique is able to identify potentially useful knowledge and ultimately understandable disease patterns and associated comorbid conditions from large clinical data sources.5 Because the data mining method assumes no prior knowledge, it has the potential to uncover previously unknown relationships or clusters of associated conditions and generate new knowledge about particular diseases in question. This approach has demonstrated success in exploring clinical patterns and associations in many clinical applications such as cesarean delivery,6 coronary artery bypass graft,7 and cancer risk for non-aspirin NSAID users.8 The aim of the study was to apply a data mining technique to a clinical data repository in order to identify new, unexpected, and interesting patterns or disease associations in OSA patients.
MATERIALS AND METHODS
Data Source
The data repository for this study was sourced from the New South Wales (NSW is the most populated Australian state) Inpatient Data Collection (also called Health Information Exchange). A random dataset (1999–2004) was extracted using a Health Outcomes and Information Statistical Toolkit (HOIST). The data set is a representative collection of hospital records in NSW, since it is compulsory for public and some private hospitals to collect all inpatient data and report it on a monthly basis to the Department of Health. Data collected for this study were inpatient data from 278 public and 180 private hospitals. Data items de-identified for this study were gender, age group, diagnosis, and procedure. All diagnostic and procedural data were coded with the International Classification of Diseases (ICD)10-Australian Modification (AM) codes.9
Disease Classification and Definition of OSA
ICD-10, developed by World Health Organization in 1992, uses an alphanumeric coding scheme for diseases, as opposed to numerical coding scheme in ICD-9-CM (Clinical Modification). It is structured by body system and etiology, and comprises 3, 4, and 5 character categories. Its main purpose is to collect statistics of morbidity data and has been used by many countries throughout the world for coding the cause of death and for hospital diagnoses since 1994. One of the major advantages of ICD-10 is that it is more detailed with a total of 12,420 codes compared to ICD-9, which uses a numerical coding scheme with 6,969 codes. The ICD-10 thus permits a richer capture of clinical information.10 In Australia all administrative data are coded with the ICD-10-AM classification. The core classification of ICD-10 employs a 3-character (alphanumeric) code, plus 4th and 5th digits for sub-classifications. For example: J38 is for disease of “vocal cords and larynx (not elsewhere classified),” J38.0 is “paralysis of vocal cords and larynx,” and J38.01 is “paralysis of vocal cords or larynx, unilateral, partial.”
OSA was considered the principal diagnosis when it was the reason for admission, or a secondary diagnosis where another condition was the reason for admission. Each of the OSA cases represents an admission. Age is defined as the age at presentation on admission and it is not related to the age at the onset of OSA. According to the classification, G37.32 is the code for obstructive sleep apnea syndrome. Patients who had a diagnosis of OSA were initially retrieved using this code. When comorbid conditions were subsequently explored, the 4th and 5th digits for sub-classification were ignored. For example, J38.0 and J38.01 were both regarded as J38.
In NSW hospitals, clinical codes are assigned to diagnoses and procedures of each episode of care by clinical coders, who extract information from patients' clinical notes (written by doctors, nurses, and allied health workers). The principal diagnosis is usually decided upon by the discharging medical officer. The number of codes assigned to each episode of care is unlimited and can be from 5–20. For some patients, especially those with chronic diseases such as diabetes mellitus, the number of codes could be well over 20 and even as many as 50. Therefore the data were unstructured.
Data Mining Technique
Association rule analysis is a well-known data mining technique that aims to quickly discover the statistical inferential relationship of 2 sets of items in a very huge data repository.11,12 In brief, the method is explained as follows: I ={i1, i2, …, iN} is a set of N distinct literal called items and a subset of I is called an itemset. T = {i1, i2, …, iN } is a database with N records i1, i2, …, iN. Each record is called a transaction (t) which contains a set of items {i1, i2, …; iN} and each transaction could be regarded as an itemset. Association rule is an implication of the form X → Y, where X and Y are itemsets and X∩Y=Φ. The rule X → Y can be used to predict that “if X (a disease in a clinical setting) occurs in a transaction (an episode of care), then Y (another disease) will likely also occur in the same transaction.” Each rule has 2 measures of its strength: The support of a rule is the ratio of the number of transactions satisfying both X and Y over the total number of transactions (i.e., the frequency of causality); the confidence is the ratio of the number of transactions satisfying both X and Y over the number of transactions satisfying X (i.e., the strength of the causality).
Transactions were identified from whole dataset as containing a diagnosis of OSA and the identified data is called focused dataset. Frequency patterns discovered by association mining were noted as focused patterns, while patterns found in the whole dataset were called global patterns. In this paper, focused patterns as against the global patterns of age and gender distribution were studied. Any unexpected comorbid conditions were identified with domain knowledge from the frequency patterns, such as associated conditions of essential hypertension and obesity.
RESULTS
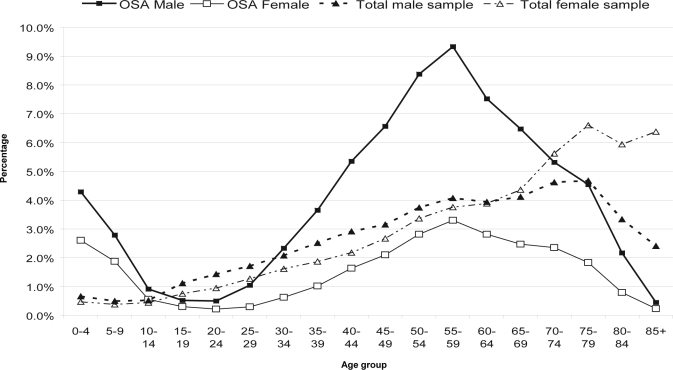
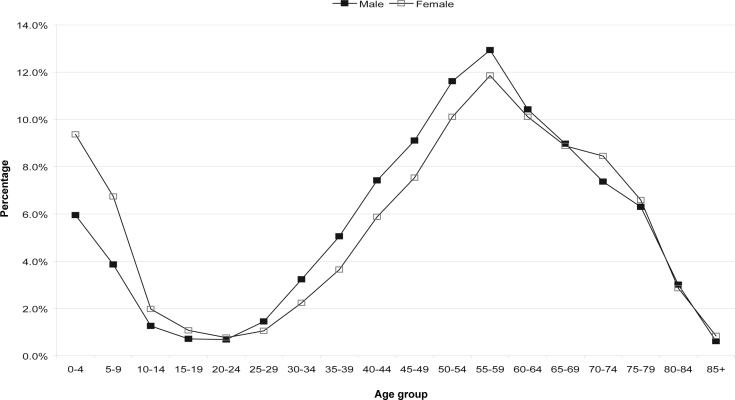
The extracted dataset contained a total of 1,511,078 hospital records. Of these records, there were 47.5% male and 52.5% female admissions. A total of 60,197 (4% of all records) had a diagnosis of OSA, either as a principal or a secondary diagnosis. Of these OSA cases, 72.2% were males and 27.8% were females. Figure 1 shows the distribution of the OSA cases broken down by gender and age, compared to that for the entire dataset. The distribution was bimodal with peaks at 0–4 years and 55–59 years. A low occurrence was seen during the adolescent years. The number of cases rose from 25–29 years to a peak at 55–59 years in both males and females before a decline. The number of cases in males superseded that of females in all age groups and the difference was greatest at the peak occurrence. When the occurrence at different age groups was stratified by gender, the rates were slightly higher for girls than boys in the 0–4 (9.4% and 6.7%) and 5–9 year age (6.0% and 3.9%) groups (Figure 2). However, occurrence rate in males was higher than females after age 20–24 years.
Figure 1.
Age and gender distributions of OSA and total sample
Figure 2.
Age distribution of OSA cases in males and females
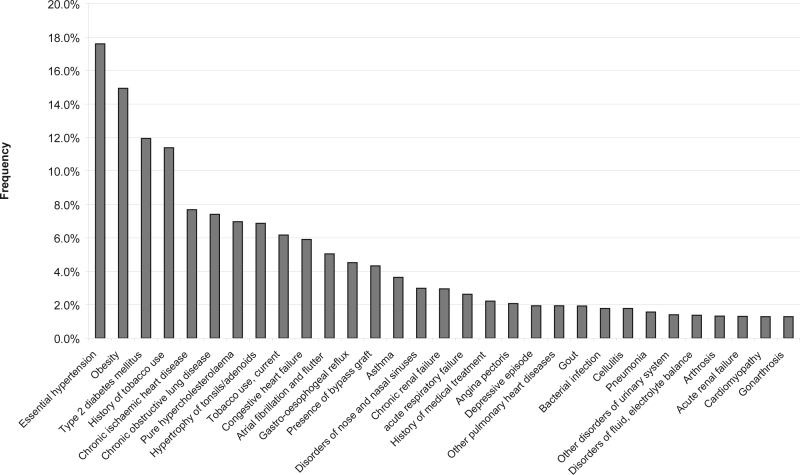
The comorbid conditions with at least 1% frequency occurrence are shown in Figure 3. Essential hypertension (I10), obesity (E66), and type 2 diabetes mellitus (IDDM2) (E11) were found to be most frequently associated with OSA. Conditions such as use of tobacco (past or present) (Z86), ischemic heart diseases (IHD) (I25), hypercholesterolemia (E78), congestive heart failure (I50), atrial fibrillation and flutter (I48), and bypass graft (Z95) were all common in the OSA patients. Diseases of the respiratory system (obstructive lung diseases [J44] and other pulmonary heart diseases) also were commonly associated with OSA. Other associations with OSA were hypertrophy of tonsils/adenoids (J35), disorders of nose and nasal sinuses (J34), and joint problems of gout (M10), arthrosis (degenerative disease of the joints) (M19), gonarthrosis (inflammation of the knee) (M17), and osteoporosis with fracture (M81).
Figure 3.
Common comorbid conditions associated with OSA (% of each code is the number of count of a code over the total count of all codes in these 60,197 cases)
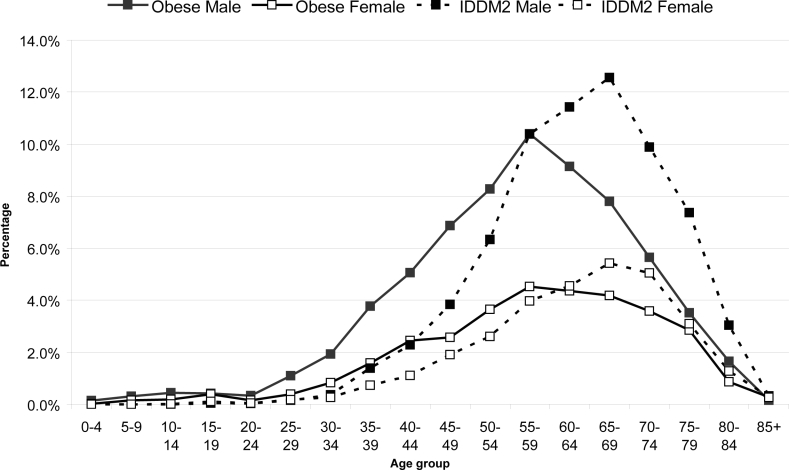
The age distribution pattern of the four most common comorbid diseases, namely obesity, IDDM2, hypertension and IHD, are shown in Figures 4 and 5. These conditions were more common in males than in females. Obesity was noted from age 25 years, and hypertension and diabetes from age 30–34. Obesity occurrence peaked at 50–59 years, whereas IDDM2 peaked nearly 10 years later (65–69 years) for both genders. Hypertension cases remained relatively steady at a peak level over the age range of 55 to 69 years in males and 55–74 years in females.
Figure 4.
The age distribution of obesity and IDDM2 in OSA patients
Figure 5.
Age distribution of hypertension and ischemic heart diseases (IHD) in OSA patients
Figure 6 shows that when all comorbid conditions were categorized into disease systems according to the ICD-10 classification, 3 main clusters were identified. These were cardiovascular diseases (heart diseases predominantly), endocrine/ metabolic diseases (mainly diabetes), and respiratory diseases. Other associated conditions included gastrointestinal diseases (e.g., gastroesophageal reflux [K21]) and musculoskeletal diseases (reflected by high occurrence of gout, arthrosis, and gonarthrosis). There was also a high clustering of Z codes (Figure 6), which coded for factors influencing health status and contact with health services.
Figure 6.
Clusters of comorbid conditions by disease system/category. A-B: infectious and parasitic diseases; C&D: neoplasms, blood and immune system; E: endocrine – metabolic diseases; F: mental &behavioural disorders; G: nervous diseases; H: diseases of the eye, ear and mastoid process; I: circulatory diseases; J: respiratory diseases; K: digestive diseases; L: dermatological diseases; M: musculoskeletal system – connective tissue diseases; N: genitourinary diseases; Q: congenital malformations – chromosomal abnormalities; S-T: injury, poisoning – other consequence of external causes, V-X: External causes of morbidity and mortality; and Z: Factors influencing health status and contact with health services. O codes for “pregnancy, childbirth and the puerperium”, P codes for “certain condition originating in the perinatal period,” and R codes for “symptoms not elsewhere specified” were not included for this analysis.
DISCUSSION
To our knowledge, this is the first study that has employed the novel approach of data mining to reveal the complex clinical patterns of OSA in a large clinical dataset. Previous knowledge of disease associations with OSA was fragmented and did not explain possible interrelationships between comorbid diseases. Because inpatient data tend to have a more complete and accurate collection of clinical records, the data mining technique was a useful tool in providing the full clinical picture of comorbid conditions associated with OSA. Our results confirmed the prevalence of OSA noted in earlier studies.13–15 This study has provided novel data on the age and gender distribution and frequency of conditions comorbid with OSA.
In the dataset of 1999–2004, OSA cases represented 4% of total hospital patient records. It is worth noting that this occurrence rate is comparable to the prevalence data observed in the Sleep Heart Health Study of 4.1% from 6 study cohorts,16 and of 3% to 15% in another general population study.13 Of the 4% diagnosed with OSA, the male to female ratio was 2.6:1, which was consistent with the population-based studies,17,1 although considerably lower than in clinical studies (ratio of 5 to 8:1).18,19 Although various mechanisms for gender difference have been investigated, such as the role of respiratory control of upper airway dilator muscles of the genioglossus, of upper airway cross-sectional area, of increased airway resistance during wakefulness and sleep, and of progesterone-estrogen/testosterone, conclusive evidence is not apparent.20 The authors suggest that females have a more stable upper airway than males. Two recent papers appear to support this contention. After puberty, boys have a longer upper airway length than girls, even when normalized for body height, as an increased airway length predisposes to pharyngeal collapse.21 In addition, Tsai showed a greater pharyngeal length, hyoid bone position, oropharyngeal dimension, and soft palate length in males than females at stage 3 complete permanent dentition.22
The age distribution for OSA occurrence showed 2 peaks: at 0–4 years and at 55–59 years in both genders. High occurrence at a young age has been reported in earlier studies that found obesity and adenotonsillar hypertrophy were the main cause.23 Indeed, Tsai showed that rapid growth of the lymphatic system during the first 7 years of life could overwhelm the small space provided by the shallow sockets for the tonsils, prompting susceptibility to obstructive apnea during sleep.22 This study lends support to our observation that the relatively low occurrence in the adolescent years could be related to the increased nasopharynx dimensions with dentition age (from stage 2 early permanent to stage 3 dentition) as the size of the adenoids decreases with the progress of age to adulthood. The rise in number of OSA after age 25–29 years coincides with the rate of obesity onset (Figures 1 and 4). The decline after age 60 years likely reflects an increased mortality related to comorbid conditions especially of cardiovascular diseases.
In line with previous studies, we confirm that hypertension, obesity, IDDM2, and chronic ischemic heart conditions, COPD, hypercholesterolemia, and hypertrophy of tonsils and adenoids are conditions frequently associated with OSA. Our data also revealed a higher dominance of males than females for these comorbid conditions (Figures 1, 3, and 4). In support of these findings is the lower prevalence of obesity in females.24 Other comorbid conditions are congestive heart disease, atrial fibrillation, asthma, nasal abnormalities, and gastroesophageal reflux.
Our finding of cardiac arrhythmias such as fibrillation and flutter are common in OSA patients suggests an increased risk of chronic ischemia heart disease, acute myocardial infarction and even stroke in these patients. Earlier studies have suggested that stroke is a leading cause of morbidity among OSA patients.25–27 It is logical to assume that obesity and hypercholesterolemia will lead to an increased chance of developing stroke or cerebrovascular disorder. However, our data show that stroke is not frequently associated with OSA compared to other cardiovascular events. Many OSA patients were current smokers or had a past history of smoking, making it a contributory factor to ischemic heart and pulmonary diseases.28
Not previously described is that joint diseases such as arthrosis and gout show a high occurrence in OSA. It is known that intermittent hypoxia during the sleep period increases purine catabolic products of adenosine and uric acid, which may be causative in the arthritic sequelae in OSA.29 Braghiroli et al. showed high levels of uric acid in OSA patients and this level was restored by continuous positive airway pressure therapy.30
Hypoxia may also influence cartilage damage by increasing cytokine induced pro-inflammatory mediator production.31 We further postulate that the extra body load of obesity that imposes substantial mechanical stress on joints and bones, together with the ageing process, may all culminate in joint damages.
Previous studies have also suggested that OSA patients are at risk of developing mental problems such as anxiety, depression, psychosis because of low oxygen saturation levels during apneic events, severe sleep fragmentation, and excessive daytime sleepiness.32 Our data indicated that although depressive disorder is associated with OSA (Figure 3), its frequency is lower than that of insomnia, which is a common condition in mental disorders.33
The results generated from this analysis cannot determine the cause-effect relationship between comorbid conditions and OSA as the administrative data do not shed light on whether the comorbid conditions occurred before or after the diagnosis of OSA. However, it is known that the obesity associated with peripharyngeal infiltration of fat and increased size of the soft palate and tongue is a major risk factor for OSA, whereas cardiovascular conditions are likely the complication of OSA or obesity itself.18,19,34 Replotting the data from Figures 1, 4, and 5, the time course of OSA, obesity, IDDM2, hypertension, IHD, and their relationships become clear (Figure 7). The onset and peak occurrences of obesity and OSA are identical. There is a latent period of 5 years for hypertension and IDDM2 development and 15 years for chronic ischemic heart conditions from obesity onset. This observation is consistent with the time course of pathogenesis for these comorbid conditions.35–37 This finding lends further support for obesity or OSA being key risk factors for the development of comorbid conditions. It is worth noting that Sharma et al. reported that obesity but not OSA is responsible for the metabolic derangement in diabetes.38
Figure 7.
Time courses of obesity, OSA, IDDM2, hypertension, and ischemic heart diseases (numbers on the Y axis are arbitrary, 1: onset at 1% occurrence, 10: peak occurrence)
All OSA patients from this clinical dataset were a result of their admission for polysomnography testing and treatment or for associated comorbid conditions. Because of chances of re-admission, it is possible that the observed associations and trends may be overestimated. However, given the large sample (~60,000) employed in this study, multiple admissions by the same persons are mathematically insignificant. Importantly, the results of this clinical data repository analysis remain useful. Its significance lies in the revelation of the complex clinical patterns, which provides a useful prediction of longer term consequences of OSA/ obesity, a course that would be followed if not for treatment intervention. Our study also clearly indicates that OSA patients are heavy users of health services. Given an array of comorbid conditions associated with OSA, this finding is not surprising. The high Z codes are directly translated into a high cost of health care and services.
In conclusion, we have employed the powerful tool of data mining in discovering the disease pattern of OSA. This approach identifies obesity as a key factor associated with OSA, and for the first time, shows the full clinical picture of comorbid conditions, quantifies their frequency of occurrence, and reveals their possible interrelationships. Novel findings from the dataset have revealed the following: low occurrence in adolescent years; a distinct occurrence peak noted at 55–59 years; comorbid conditions involving the cardiovascular, endocrine/metabolic and respiratory systems; and OSA patients being high users of health care services. These findings warrant further research with case-control studies and standard statistical analyses to have a better understanding of interrelationships of these comorbid conditions.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
We would like to thank the Centre for Epidemiology and Research, NSW Department of Health and the Demand and Performance Evaluation Branch, NSW Department of Health, for providing the dataset.
REFERENCES
- 1.Redline S, Kump K, Tishler PV, Browner I, Ferretter V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–26. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 2.Krieger J, McNicholas W, Levy P, et al. Public health and medicolegal implications of sleep apnoea. Eur Respir J. 2002;20:1594–1609. doi: 10.1183/09031936.02.00404502. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 4.Fayyad U, Piatetsky-Shapiro G, Smyth P. From data mining to knowledge discovery: an overview. In: Fayyad U, Piatetsky-Shapiro G, Smyth P, Uthurusamy R, editors. Advances in knowledge discovery and data mining. Menlo Park: Am Assoc Artificial Intelligence; 1996. pp. 37–54. [Google Scholar]
- 5.Brossette SE, Sprague AP, Jones WT, et al. A data mining system for infection control surveillance. Methods Inf Med. 2000;39:303–10. [PubMed] [Google Scholar]
- 6.Lin HC, Xirasagar S. Institutional factors in cesarean delivery rates: policy and research implications. Obstet Gynecol. 2004;103:128–36. doi: 10.1097/01.AOG.0000102935.91389.53. [DOI] [PubMed] [Google Scholar]
- 7.Bock BJ, Dolan CT, Miller GC, et al. The data warehouse as a foundation for population-based reference intervals. Am J Clin Pathol. 2003;120:662–70. doi: 10.1309/W8J8-5AG4-WDG6-JGJ9. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen HT, Friis S, Norgard B, et al. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88:1687–92. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Classification Centre. Australian Modification (ICD-10-AM) Sydney: 2000. The international statistical classification of disease and related health problems, 10th revision. [PubMed] [Google Scholar]
- 10.De Coster C, Quan H, Finlayson A, et al. Identifying priorities in methodological research using ICD-9-CM and ICD-10 administrative data: report from an international consortium. BMC Health Serv Res. 2006;6:7. doi: 10.1186/1472-6963-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal R, Imielinski T, Swami A. Database mining: A performance perspective. IEEE Trans Knowl Data Eng. 1993;5:914–25. [Google Scholar]
- 12.Brossette SE, Sprague AP, Hardin JM, Waites KB, Jones WT, Moser SA. Association rules and data mining in hospital infection control and public health surveillance. J Am Med Inform Assoc. 1998;5:373–81. doi: 10.1136/jamia.1998.0050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 14.Guilleminault C, Abad V. Obstructive sleep apnea. Curr Treat Options Neurol. 2004;6:309–17. doi: 10.1007/s11940-004-0030-7. [DOI] [PubMed] [Google Scholar]
- 15.Rollheim J, Osnes T, Miljeteig H. The relationship between obstructive sleep apnoea and body mass index. Clin Otolaryngol Allied Sci. 1997;22:419–22. doi: 10.1046/j.1365-2273.1997.00048.x. [DOI] [PubMed] [Google Scholar]
- 16.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S communities. Sleep Breath. 2002;22:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(Suppl 4):S122–6. [PubMed] [Google Scholar]
- 18.Guillemninault C, Quera-Salva M, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–9. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 19.Crocker BD, Olson LG, Saunders NA, et al. Estimation of the probability of disturbed breathing during sleep before a sleep study. Am Rev Respir Dis. 1990;142:14–8. doi: 10.1164/ajrccm/142.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–89. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 21.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:1028–34. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HH. Developmental changes of pharyngeal airway structures from young to adult persons. J Clin Pediat Dent. 2007;31:219–21. doi: 10.17796/jcpd.31.3.023h753711p24273. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngology Head Neck Surg. 2007;137:43–8. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obesity Rel Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 25.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. New Eng J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [see comment] [DOI] [PubMed] [Google Scholar]
- 26.McArdle N, Riha RL, Vennelle M, et al. Sleep-disordered breathing as a risk factor for cerebrovascular disease: a case-control study in patients with transient ischemic attacks. Stroke. 2003;34:2916–21. doi: 10.1161/01.STR.0000103748.31609.4E. [DOI] [PubMed] [Google Scholar]
- 27.Wessendorf TE, Teschler TH, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 28.Shepard JW., Jr. Cardiopulmonary consequences of obstructive sleep apnea. Mayo Clin Proc. 1990;65:1250–9. doi: 10.1016/s0025-6196(12)62749-9. [DOI] [PubMed] [Google Scholar]
- 29.Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [comment] [DOI] [PubMed] [Google Scholar]
- 30.Braghiroli A SC, Erbetta M, Ruga V, Donner CF. Overnight urinary uric acid: creatinine ratio for detection of sleep hypoxemia. Validation study in chronic obstructive pulmonary disease and obstructive sleep apnea before and after treatment with nasal continuous positive airway pressure. Am Rev Respir Dis. 1993;148:173–8. doi: 10.1164/ajrccm/148.1.173. [DOI] [PubMed] [Google Scholar]
- 31.Cernanec J, Guilak F, Weinberg JB, Pisetsky DS, Fermor B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002;46:968–75. doi: 10.1002/art.10213. [DOI] [PubMed] [Google Scholar]
- 32.Baran AS, Richert AC. Obstructive sleep apnea and depression. CNS Spectr. 2003;8:128–34. doi: 10.1017/s1092852900018356. [DOI] [PubMed] [Google Scholar]
- 33.Costa e Silva JA. Sleep disorders in psychiatry. Metabolism. 2006;55:S40–4. doi: 10.1016/j.metabol.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Naughton MT. The link between obstructive sleep apnea and heart failure: underappreciated opportunity for treatment. Curr Heart Fail Rep. 2006;3:183–8. doi: 10.1007/s11897-006-0020-z. [DOI] [PubMed] [Google Scholar]
- 35.Galal W, van Domburg RT, Feringa HH, et al. Relation of body mass index to outcome in patients with known or suspected coronary artery disease. Am J Cardiol. 2007;99:1485–90. doi: 10.1016/j.amjcard.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–50. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 37.Nanchahal K, Morris JN, Sullivan LM, et al. Coronary heart disease risk in men and the epidemic of overweight and obesity. Int J Obesity. 2005;29:317–23. doi: 10.1038/sj.ijo.0802877. [DOI] [PubMed] [Google Scholar]
- 38.Sharma SK, Kumpawat S, Goel A, et al. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–7. doi: 10.1016/j.sleep.2006.06.014. [see comment] [DOI] [PubMed] [Google Scholar]