Abstract
Study Objectives:
to estimate the prevalence of the most common sleep related symptoms (SRS) in the metropolitan areas of Mexico City, Montevideo (Uruguay), Santiago (Chile), and Caracas (Venezuela).
Methods:
The study consisted of a multistage cluster sampling of adults aged ≥ 40 years living in metropolitan areas. All participants completed a questionnaire on sleep related symptoms. Simplified respiratory polygraphy during sleep was conducted on 188 subjects from Mexico City. Obstructive sleep apnea syndrome was defined as Epworth Sleepiness Scale score ≥ 11 and respiratory disturbance index (RDI) ≥ 15 events/h; a cut-off of 15 was chosen because of its high sensitivity and specificity in association with the portable monitor used in the study.
Results:
The study included 4,533 subjects (1,062 in Mexico City, 941 in Montevideo, 1,173 in Santiago, and 1,357 in Caracas). Snoring was reported by 60.2% (95% CI 58.8% to 61.6%), excessive daytime sleepiness by 16.4% (15.3% to 17.5%), observed apneas by 12.3% (11.4% to 13.3%), insomnia by 34.7% (33.3% to 36%), sedative use by 15.1% (14.1% to 16.2%), daytime napping by 29.2% (27.7% to 30.6%), and a combination of snoring, sleepiness, and observed apneas by 3.4% (2.9% to 4%). Men had a higher frequency of snoring and daytime napping, whereas women reported more insomnia and sedative use. Prevalence of OSAS varied from 2.9% among subjects who denied snoring, excessive daytime sleepiness, and observed apneas, to 23.5% among those reporting these 3 symptoms.
Conclusions:
A high prevalence of sleep related symptoms and undiagnosed obstructive sleep apnea in Latin America was observed.
Citation:
Bouscoulet LT; Vázquez-García JC; Muiño A; Márquez M; López MV; Montes de Oca M; Talamo C; Valdivia G; Pertuze J; Menezes AMB; Pérez-Padilla R. Prevalence of Sleep Related Symptoms in Four Latin American Cities. J Clin Sleep Med 2008;4(6):579-585.
Keywords: Prevalence, sleep apnea, sleep related symptoms, epidemiology, PLATINO Study, sleepiness, insomnia, napping
Sleep disorders are frequent causes of morbidity and mortality. Among these disorders, obstructive sleep apnea syndrome (OSAS), which is characterized clinically by habitual snoring, excessive daytime sleepiness (EDS), and breathing pauses during sleep, has been associated with cardiovascular diseases,1 traffic accidents,2 and a decreased quality of life3; all of which generate a substantial burden on public health services.4
Understanding the prevalence of sleep related symptoms (SRS) is critical to anticipating healthcare needs and allocating appropriate resources. To the best of our knowledge, the population-based prevalence of SRS among Latin American groups has not yet been reported. Therefore, the objectives of this study were, first, to estimate the frequency of self-reported SRS, focusing primarily on sleep disordered breathing in adults ≥ 40 years living in the metropolitan areas of 4 Latin American cities; and second, to identify related risk factors. In addition, we evaluated the significance of clinically relevant questions used to diagnose sleep disordered breathing (SDB).
METHODS
This report is part of the PLATINO study.5 PLATINO is a cross-sectional survey, designed principally to estimate the prevalence of chronic obstructive pulmonary disease (COPD) in 5 Latin American cities: Mexico City, São Paulo (Brazil), Montevideo (Uruguay), Santiago (Chile), and Caracas (Venezuela). A total of 5,315 subjects were included in the PLATINO study, which has already published results on the prevalence of COPD6 and hypoxemia,7 as well as on reference values for spirometry.8,9
A similar multistage sampling strategy was used in all 5 areas. Metropolitan areas were first stratified into the main city and surrounding municipalities. These 2 subsets were further stratified by socioeconomic status. We selected 68 census tracts at each site, taking stratification into account, and using a probability of selection proportionate to the number of households in each tract. Within each tract, we counted the number of people in each household and every count was updated from the most recent census. We used simple sampling to choose an average of 15 households in each tract. All adults ≥ 40 years of age in selected households were invited to participate. The sample was self-weighted in each city. Sample size calculations suggested that 1,000 people would be needed in each area with a margin of error ranging between 2% and 4%. Thus, we aimed to locate about 1,200 eligible participants per site, with a predicted refusal rate of 20%.5 The institutional review board in each city approved the study, and all participants signed a written consent form.
All interviews and measurements took place at subjects' homes. The questionnaire included sections from the American Thoracic Society Division of Lung Diseases (ATS/DLD),10 the European Community Respiratory Health Survey II11 and the Lung Health Study instruments.12 Questions from the SF-12 were also included to assess overall health status.13 To evaluate the prevalence of the main SRS, we applied an additional section, a compilation of questions previously applied in surveys, through an agreement with 2 specialists in sleep medicine. Topics included self-reported duration of sleep (number of hours of sleep per night); estimated time to onset of sleep (minutes); number of awakenings per night. The responses to the following questions were “yes or no”: difficulty in falling asleep (insomnia) during the previous 6-month period ≥ 2 nights a week; sedative use in the last 6 months ≥ 2 night a week; alcohol consumption at least once a week; snoring all or most nights; observed breathing pauses during sleep time; EDS and daytime napping (and nap duration) all or most days; excessive tiredness during the day and previous adenotonsillectomy. Copies of the questionnaire are available at the PLATINO web site (www.platino-alat.org/docs/cuestionario_platino_mexico.pdf). The questionnaire was tested in a pilot phase to identify difficult to understand questions and improve them as needed. The city of São Paulo did not participate in the survey on sleep related symptoms.
We measured subjects' weight (solar scale HS-301 Tanita Corporation, Inc., USA) height (208 stadiometer Seca Corporation, USA) and neck circumference (fiberglass tape measure) on 2 occasions, and then used average values for analysis. Oxygen saturation (SpO2) and heart rate were ascertained using a pulse oximeter (Nonin Onyx 9500 Finger Pulse Oximeter, Nonin Medical, USA).14 Spirometric testing (Easy-One; ndd Medizintechnik AG, Zurich, Switzerland) was done in accordance with ATS/ERS recommendations.15
To estimate the prevalence of OSAS objectively, we studied a subsample of participants in Mexico City (n = 188) who completed a Spanish language validated Epworth Sleepiness Scale16 (ESS) and underwent a simplified respiratory polygraphy at their homes (Remmers Sleep Recorder, Sagatech, Calgary, Alberta, Canada), which records oximetry, airflow, snoring, heart rate, leg movement, and respiratory effort, and calculates the respiratory disturbance index (RDI) using an automatic algorithm.17 The algorithm sequentially scans each recorded oxygen saturation value (1 Hz). Whenever a drop in a sampled oxygen saturation value is detected, the program assigns an event marker to that reading. Because oxygen saturation values were sampled and recorded at 1 Hz, event markers were separated by no less than one second. When an increase in oxygen saturation was detected, the program determined if ≥ 3 consecutive event markers—that is, 3 consecutive falls in recorded oxygen saturation readings—are present prior to this rise. If this criterion was met and one of the event markers was associated with an oxygen saturation value > 4% lower than the baseline oxygen saturation, then a respiratory disturbance was designated.17 The quality of the simplified respiratory polygraphy was ascertained by a visual analysis performed by an experienced sleep technician. Studies with < 4 h of recording were not included for the analysis. The diagnosis of OSAS was confirmed when the following 2 criteria were present: ESS ≥ 11 points and RDI ≥ 15 events/h. An RDI of 15 events/h or greater, as measured by the Remmers Sleep Recorder, has a sensitivity of 98% and a specificity of 88% for diagnoses of moderate-to-severe obstructive sleep apnea, compared to polysomnography.17
The prevalence of the main SRS was estimated on the basis of the answers to the sleep questionnaire, taking into account the sampling strategy. Using 3 symptoms (habitual snoring, EDS, and observed apneas), we stratified the sample into 6 groups, considering a priori different risks for OSAS. The groups were set up as follows: (1) asymptomatic subjects; (2) primary snoring (no apneas, no EDS); (3) habitual snoring and apneas, but no EDS; (4) habitual snoring and EDS (no apneas); (5) habitual snoring, EDS and observed apneas; and (6) EDS (no apnea, no snoring). In the case of Mexico, the prevalence of OSAS was confirmed through a simplified respiratory polygraphy and ESS. For this part of the study, a random sample made up of 20% of the subjects from groups 1, 2, and 4; all subjects from groups 3 and 5; and 25% of subjects from group 6 were invited to participate.
In order to explore associations of SRS, multiple logistic regression models were constructed, which considered the following dependent variables: habitual snoring, EDS, the combination of snoring, EDS, and apneas; insomnia; daytime napping; and sedative use. The independent variables analyzed included gender, age, BMI, neck circumference, total scores on the SF-12 survey (and single questions from that instrument), alcohol consumption, smoking (> 10 pack-years), daytime napping, EDS, habitual snoring, observed apneas, insomnia, sedative use, and clinical or spirometric diagnoses of COPD (FEV1/FVC < 0.7).
Differences among groups with respect to the continuous variables were determined using ANOVA with Bonferroni correction. Differences between the 2 groups were probed using either the t-test for independent groups or the Mann-Whitney test, according to the distribution of data, with or without normality. Type 2 × 2 tables were used to calculate sensitivity, specificity, predictive values, and odds ratios. Analysis was performed using STATA release 9.2 (StataCorp, College Station, TX), taking the sampling strategy (“survey” commands) into account. The acceptable level of statistical significance based on a 2-sided value for each test was p < 0.05.
RESULTS
A total of 5,561 eligible subjects were identified in the 4 cities. The overall response rate was 81.5%, with 4,533 participants being included in the study—1,804 male (39.8%) and 2,729 female (60.2%). Mean age was 56.8 years (SE 12 years). Information about age, sex, and smoking were obtained from approximately 50% of the individuals who were identified but refused to respond to the questionnaire. On the basis of this information, we estimated that non-response was consistently higher for men, but only slightly higher among older people and current smokers. The distribution of participants according to city and general demographic characteristics is shown in Table 1.
Table 1.
Characteristics of Participants According to City
| Mexico City | Montevideo | Santiago | Caracas | |
|---|---|---|---|---|
| No. of eligible individuals (n) | 1,452 | 1,106 | 1,476 | 1,527 |
| No. of individuals interviewed (n) | 1,062 | 941 | 1,173 | 1,357 |
| Response rate (%) | 73.1 | 85.0 | 79.4 | 88.8 |
| Age (y)*† | 55.9 ± 0.5 | 60.2 ± 0.5† | 56.8 ± 0.5 | 55.1 ± 0.3 |
| Men n (%)† | 450 (42.3) | 399 (42.4) | 453 (38.6) | 502 (36.9) |
| BMI (kg/m2)*† | 28.7 ± 0.1 | 28.2 ± 0.2 | 28.4 ± 0.1 | 27.4 ± 0.1 |
| Neck circumference (cm)*† | 37.6 ± 0.1 | 36.9 ± 0.1 | 36.4 ± 0.1 | 35.5 ± 0.1 |
| Obesity n (%)† | 373 (35.1) | 320 (34) | 373 (31.7) | 343 (25.2) |
| Smoking (>10 pack-years) n (%)† | 75 (7) | 171 (18) | 116 (10) | 181 (13) |
BMI = body mass index; obesity = body mass index > 30 kg/m2.
Mean ± Standard error.
p < 0.05 for extreme value.
For the entire group, the prevalence of reported habitual snoring was 60.2% (95% CI 58.8 to 61.6), EDS 16.4% (95% CI 15.3to 17.5), insomnia 34.7% (95% CI 33.3 to 36), daytime napping 29.2% (95% CI 27.7 to 30.6), and sedative use 15.1% (95% CI 14.1 to 16.2). The prevalence of the combination of habitual snoring, EDS and observed apneas in the 4 cities was: 4.6% (95% CI 3.6 to 5.6) among men and 2.7% (95% CI 2.1 to 3.4) for women. Self-reported duration of sleep was 6.9 ± 1.5 h/night and was virtually equal among the 4 cities. In subjects with EDS, zelf-reported duration of sleep was 6.7 ± 1.7 h/night, whereas it was 7.0 ± 1.4 h/night among subjects who did not report EDS (p < 0.001).
Table 2 and Table 3 show the prevalence of the SRS according to city and gender, respectively. Statistically significant differences were observed in the frequency of the SRS among cities (Fig. 1, A–C). Santiago reported the highest prevalence of habitual snoring (66.4%), EDS (22.7%), insomnia (41.6%), and sedative use (23.7%), as well as of combinations of symptoms such as snoring/observed apneas (11%) and snoring/EDS/ observed apneas (6.4%). The highest frequency of daytime napping was observed in Caracas (35.2%). Even after adjusting for potential confounding factors, Santiago maintained the highest prevalence of the combination of habitual snoring, EDS, and observed apneas, while Caracas retained the highest frequency of daytime napping.
Table 2.
Prevalence of Sleep Related Symptoms According to City. Proportions and 95% Confidence Intervals are Shown.
| Mexico City n = 1,062 | Montevideo n = 941 | Santiago n = 1,173 | Caracas n = 1,357 | |
|---|---|---|---|---|
| Habitual snoring (%) | 54.8 (51.2–58.3) | 59.6 (56.3–62.8) | 66.4 (62.9–69.8) | 59.6 (57.0–62.1) |
| EDS (%) | 17.7 (15.3–20.2) | 9.5 (7.3–11.8) | 22.7 (19.7–25.7) | 14.7 (12.2–17.2) |
| Snoring + sleepiness + apneas (%) | 3.2 (2.3–4.2) | 1.9 (1.0–2.8) | 6.4 (4.9–7.9) | 2.1 (1.2–2.9) |
| Snoring + apneas (%) | 4.9 (3.7–6.2) | 8.1 (6.6–9.7) | 11.0 (9.2–12.9) | 6.6 (5.0–8.1) |
| Insomnia (%) | 35.0 (32–38) | 31.3 (28.3–34.4) | 41.6* (38.7–44.4) | 30.8 (27.9–33.6) |
| Sedative use (%) | 5.8 (4.0–7.6) | 20.9 (18.3–23.6) | 23.7 (21.3–26.2) | 11.0 (9.1–13.0) |
| Daytime napping (%) | 21.3 (18.5–24.1) | 30.0 (26.8–33.1) | 28.7 (26.0–31.3) | 35.2 (32.5–38.0) |
The statistically highest daytime napping frequency was found in Caracas, whereas all other symptoms were statistically more frequent in Santiago. EDS = excessive daytime sleepiness
Table 3.
Prevalence of Sleep Related Symptoms by City and Gender. Proportions and 95% Confidence Intervals are Shown.
| Mexico City |
Montevideo |
Santiago |
Caracas |
|||||
|---|---|---|---|---|---|---|---|---|
| Men n= 450 | Women n=612 | Men n=399 | Women n=542 | Men n=453 | Women n=720 | Men n=502 | Women n=855 | |
| Habitual snoring (%) | 61.7 | 49.6 | 66.4 | 54.6 | 72.6 | 62.5 | 65.3 | 56.2 |
| (56.6–66.8) | (45.0–54.3)* | (61.8–70.9) | (50.7–58.4)* | (67.8–77.3) | (58.6–66.3)* | (61.3–69.3) | (53.1–59.3)* | |
| EDS (%) | 16.6 | 18.6 | 9.7 | 9.4 | 21.6 | 23.4 | 12.9 | 15.7 |
| (13.3–20.0) | (15.0–22.2) | (6.5–12.9) | (6.7–12) | (17.5–25.6) | (19.7–27.1) | (8.9–16.9) | (13.1–18.4) | |
| Snoring + sleepiness + apneas (%) | 4.4 | 2.4 | 3.7 | 0.5 | 8.8 | 5.0 | 1.5 | 2.4 |
| (2.6–6.2) | (1.2–3.6) | (1.7–5.7) | (0.07–1.1)* | (6.2–11.4) | (3.3–6.6)* | (0.5–2.6) | (1.2–3.6) | |
| Snoring + apneas (%) | 8.0 | 2.7 | 12.0 | 5.3 | 16.5 | 7.6 | 10.7 | 4.2 |
| (5.6–10.3) | (1.2–4.2)* | (8.9–15.0) | (3.4–7.2)* | (13.1–19.9) | (5.4–9.8)* | (7.6–13.8) | (3.0–5.4)* | |
| Insomnia (%) | 25.7 | 41.8 | 21.5 | 38.6 | 28.0 | 50.1 | 24.3 | 34.6 |
| (21.9–29.5) | (37.7–45.9)* | (17.5–25.5) | (34.2–42.9)* | (23.7–32.2) | (46.1–54.1)* | (20.8–27.7) | (30.5–38.6)* | |
| Sedative use (%) | 3.3 | 7.7 | 12.5 | 27.1 | 11.6 | 31.3 | 8.1 | 12.7 |
| (1.2–5.4) | (5.2–10.1)* | (9.5–15.6) | (23.3–30.8)* | (8.6–14.7) | (27.9–34.8)* | (5.5–10.8) | (10.3–15.1) | |
| Daytime napping (%) | 24.3 | 19.1 | 37.3 | 24.5 | 33.1 | 25.9 | 39.2 | 32.9 |
| (20.3–28.2) | (15.6–22.6)* | (32.7–41.9) | (20.9–28.1)* | (28.9–37.3) | (22.6–29.3)* | (34.6–43.7) | (29.7–36.1)* | |
EDS = excessive daytime sleepiness;
p < 0.05 between genders.
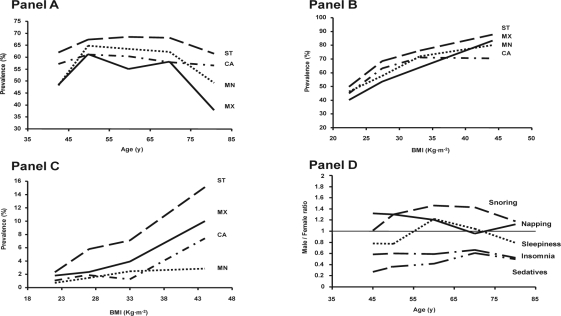
Figure 1.
Prevalence of habitual snoring according to age (Panel A) and body mass index (BMI) (Panel B). Panel C shows the prevalence of the combination of habitual snoring, excessive sleepiness, and observed apneas according to body mass index (BMI). Male to female ratios are shown in Panel D. ST = Santiago, Chile; CA = Caracas, Venezuela; MN = Montevideo, Uruguay; MX = Mexico City.
Univariate analysis revealed significant associations among snoring, EDS, observed apneas, insomnia, sedative use, and daytime napping, with odds ratios (ORs) that varied from 1.12 (p = 0.04) between snoring and insomnia, to 6.79 (p < 0.001) between insomnia and sedative use.
After adjusting for potential confounding factors by multivariate analysis (Table 4), the variables positively associated with snoring were: male gender, BMI, neck circumference, alcohol consumption, EDS, and observed apneas. For EDS, the associated variables were dyspnea, daytime napping, habitual snoring, observed apneas, and insomnia. Unlike men, women showed no association between observed apneas and EDS. For the combination of snoring, EDS, and observed apneas, the associated variables were male gender, BMI, residence in Santiago, alcohol consumption, smoking (> 10 pack-years), and daytime napping. For insomnia, the associated variables were female gender, EDS, sedative use, and clinical diagnosis of COPD. For daytime napping, the associated variables included male gender, age, neck circumference, residence in Caracas, EDS, and habitual snoring. The variables associated with sedative use were female gender, age, daytime napping, and insomnia. Excessive daytime sleepiness, insomnia, sedative use, and the combination of snoring, EDS, and apneas were significantly associated with a decreased quality of life (Table 4). Even after adjustment for potential confounding factors, women had a higher frequency than men of insomnia (41.1% vs. 25%, p < 0.001) and sedative use (19.3% vs. 8.8%, p < 0.001), whereas men more often reported snoring (66.6% vs. 56.1%, p < 0.0001), daytime napping (33.5 vs. 26.3%, p < 0.001), and the combination of snoring, EDS, and apneas (4.6% vs. 2.7%, p < 0.001) (Figure 1, panel D).
Table 4.
Adjusted Odds Ratios (95% CI) Considering Sleep Related Symptoms as Dependent Variables. Each Column is a Multivariate Logistic Model
| Habitual snoring | EDS | Snoring+EDS+apneas | Insomnia | Daytime napping | Sedative use | |
|---|---|---|---|---|---|---|
| Female | — | — | — | 1.46 (1.28–1.72) | — | 2.38 (1.75–3.12) |
| Male | 1.35 (1.08–1.68) | — | 2.37 (1.64–3.42) | — | 1.54 (1.34–1.75) | — |
| Age (y) | — | — | — | — | 1.03 (1.02–1.03) | 1.02 (1.01–1.03) |
| BMI (Kg·m2) | 1.48 (1.32–1.64) | — | 1.34 (1.16–1.56) | — | — | — |
| SF-12 Physical scale | — | 0.96 (0.95–0.97) | 0.94 (0.92–0.96) | 0.96 (0.95–0.97) | — | 0.97 (0.96–0.98) |
| SF-12 Mental scale | — | 0.96 (0.95–0.97) | 0.95 (0.94–0.96) | 0.94 (0.93–0.94) | — | 0.95 (0.94–0.96) |
| Residence in Mexico City | 0.65 (0.54–0.78) | — | — | — | 0.70 (0.57–0.86) | 0.20 (0.14–0.28) |
| Residence in Montevideo | — | 0.45 (0.34–0.59) | 0.52 (0.31–0.89) | 0.65 (0.52–0.78) | — | — |
| Residence in Santiago | — | — | 1.77 (1.19–2.64) | 0.83 (0.70–0.97) | — | — |
| Residence in Caracas | — | — | — | — | 1.58 (1.33–1.88) | 0.50 (0.40–0.64) |
| Alcohol use | 1.20 (1.04–1.39) | — | 1.55 (1.13–2.12) | — | — | — |
| Smoking (>10 p/y) | — | — | 1.67 (1.11–2.50) | — | — | — |
| Daytime napping | — | 2.34 (1.96–2.79) | 1.60 (1.15–2.23) | — | — | 1.34 (1.09–1.66) |
| Excessive sleepiness | 1.30 (1.07–1.59) | — | — | 1.49 (1.19–1.76) | 2.32 (1.95–2.75) | — |
| Snoring | — | 1.27 (1.05–1.54) | — | — | 1.20 (1.05–1.37) | — |
| Observed apneas | 3.82 (2.94–4.95) | 1.51 (1.22–1.88) | — | — | — | — |
| Insomnia | — | 1.48 (1.22–1.79) | — | — | — | 4.59 (3.70–5.68) |
| Neck circumference (cm) | 1.03 (1.00–1.07) | — | — | — | — | — |
| Sedative use | — | — | — | 4.62 (3.78–5.70) | 1.27 (1.05–1.52) | — |
| Clinical Dx COPD | — | — | — | 1.77 (1.17–2.57) | — | — |
| Dx COPD (spirometry) | 0.78 (0.64–0.96) | — | — | — | — | — |
From multivariable logistic regression models taking into account sampling strategy. EDS = excessive daytime sleepiness; BMI = body mass index (OR per every 5 units of change in BMI); SF12 = short form 12 quality-of-life questionnaire.
The final proportion of subjects having a simplified respiratory polygraphy in Mexico City were 16.8% for group 1, 12% for group 2, 34.3% for group 3, 18.8% for group 4, 48.5% for group 5, and 23.4% for group 6. There were 9 records that were not of acceptable quality, and therefore they were not included in the analysis. No differences were found in relation to age, gender, BMI, neck circumference, or frequency of snoring between participants and non-participants. The overall prevalence of OSAS (ESS ≥ 11 and RDI ≥ 15 events/h) was 10.1% (95% CI 5.7 to 14.4) and varied according to the symptoms mentioned in the questionnaire. OSAS was found in 2.9% (95% CI 1.1 to 7) of subjects with no reports of symptoms; in 12.2% (95% CI 3.1 to 21) of snorers (with no other symptoms); in 8.6% (95% CI 4 to 21.1) of subjects with snoring and observed apneas; and in 6.6% (95% CI 3.2 to 20.9) of those with EDS (but no other symptoms). In subjects who reported simultaneously snoring and EDS (with or without observed apneas), the prevalence was 23.5% (95% CI 10.4 to 46). In the subsample, the factors associated with OSAS were habitual snoring (OR 4.86, p < 0.001) and smoking (> 10 pack-years) (OR 5.25, p < 0.001).
Using OSAS as the gold standard (ESS ≥ 11 and RDI ≥ 15 events/h), the reports of snoring, EDS, and observed apneas showed a sensitivity of 21%, a specificity of 92.8%, a positive predictive value (PPV) of 11.1%, a negative predictive value (NPV) of 91.2%, a positive likelihood ratio of 2.91, and a negative likelihood ratio of 0.85.
In the subsample, we also analyzed the repeatability of the questionnaire at a 6-month interval after the initial interview, which produced a kappa statistic that ranged from 0.40 to 0.85. In addition, our question about EDS (“Is it difficult for you to stay awake during the daytime at least 3 days a week?”) was evaluated previously in 109 subjects; 52 were healthy people without SRS, and the other 57 were patients who had already been evaluated in our sleep clinic for suspected sleep disordered breathing. Subjects who answered “Yes” (n = 56) to the above question had a significantly higher score on the ESS (13.5 ± 5.6 vs. 6.9 ± 5.2, p < 0.001) and a higher score on the Stanford Sleepiness Scale (3.3 ± 1.2 vs. 2.1 ± 0.9, p < 0.001) compared to the group of 53 subjects who answered “No.” The question used in our study to evaluate EDS had a sensitivity of 77%, a specificity of 69%, a positive predictive value of 66%, and a negative predictive value of 79%, compared to an ESS ≥ 11. When we compared the presence of EDS in the answers to our question (“Yes” or “No”) against ESS (≥ 11 or < 11), the kappa agreement statistic was 0.45 (p < 0.001).
DISCUSSION
This study confirms a high frequency of sleep related symptoms in the general Hispanic population residing in the 4 participating cities. Habitual snoring was the most common symptom (60%), followed by insomnia, which was reported by a third of the population sample. The prevalence of combined reports of snoring, EDS, and observed apneas was 4.6% among men and 2.7% among women. Men reported more frequent snoring and daytime napping, and had the highest prevalence of the combination of snoring, EDS, and observed apneas. Women, in contrast, reported more frequent insomnia and sedative use. Of the 4 cities, the highest prevalence of the combination of snoring, EDS, and observed apneas was found in Santiago, whereas the highest frequency of daytime napping occurred in Caracas. These differences regarding gender and cities remained even after adjusting for potential confounding factors. The reasons for the differences in prevalence of SRS among cities are not clear, but it is likely that mild racial and cultural diversity might be involved in this finding. More studies are needed to identify unknown factors involved in the prevalence of SRS among countries with similar race and cultural background.
Previous studies have reported prevalences of snoring in adults that range from a low of 2% to a high of 85%, depending on the method of measurement used.18–24 In our study, two-thirds of the sample population reported habitual snoring, more frequently among men than women (66% vs. 56%, p < 0.0001), a finding that was consistent in all cities; however, the prevalence of snoring among women was higher than has been reported previously, a result that may be related to the high prevalence of obesity we observed in our female sample (34.7%).20,23,24 After adjusting for potential confounding factors, habitual snoring was associated with such well-known factors as male gender, obesity, and alcohol consumption, but also with EDS and observed apneas.
Excessive daytime sleepiness—a risk factor for traffic accidents2—was reported by 16.5% of participants, a result that concurs with Hara et al25 and Duran et al,20 who reported similar prevalences of EDS in population-based studies. The factors that we found to be significantly associated with EDS were snoring, observed apneas, insomnia, and daytime napping. Men reported EDS as often as women; nonetheless, the primary sleep disorder causing EDS may well be different for the 2 genders, with insomnia being more frequent among women and snoring among men. In contrast to men, women showed no association between observed apneas and EDS. Subjects with EDS also self-reported a shorter duration of sleep than those with no EDS (6.7 vs. 7.0 h/ night, p < 0.001), though the difference was only 20 min, which is unlikely to be the cause of the high prevalence of EDS. Interestingly, EDS, insomnia, sedative use, and the combination of snoring, EDS, and observed apneas were all associated with low quality of life, underscoring the need to implement effective prevention and intervention programs in the area of public health as a means of promoting healthy sleep habits.
The figures in the 4 cities for the prevalence of prolonged symptoms of insomnia (≥ 2 nights/week during the previous 6 months) and sedative use were 34% and 15%, respectively, similar to those reported by other researchers.26 Complaints of insomnia by women were almost twice as common as among men (41% vs. 25.5%), a finding that may explain, at least in part, the higher indices of sedative use (19.8% vs. 8.8%) by the former group. However, this increased prevalence of insomnia and sedative use among women may also be associated with anxiety or depression.27 Although we did not investigate depression in a formal manner,26 women reported a greater frequency of “depressed moods” than did men (11% vs. 6%). In addition to female gender, other factors associated with insomnia included EDS, sedative use, dyspnea, and clinical diagnosis of COPD. As reported by George et al,28 patients with COPD experience poor sleep for several reasons, including coughing, excessive mucous production, and frequent arousals from sleep caused by hypercapnia and to the side effects of the medications used to treat lung disease.
Interestingly, almost one-third of the studied population reported daytime napping at least 3 days per week. Daytime napping has traditionally been deemed a Latin American custom, and it is not yet clear whether it represents a risk factor for the cardiovascular system or, alternatively, a protective measure. Research by Naska et al.29 suggests that daytime napping in apparently healthy individuals is inversely associated to coronary mortality, though other researchers, have reported daytime napping to be associated with increased cardiovascular risk.30–32 Taken together, what these results seem to suggest is that among apparently healthy people, daytime napping may be a protective factor, whereas in subjects with comorbid conditions, it may be a clinical expression of fatigue or EDS. In our study, male gender, age, EDS, snoring, and sedative use were the variables associated with daytime napping, which supports the finding that for our population, napping should be interpreted mainly as a symptom and not as a habit.
The subsample of participants in Mexico who completed an ESS and a simplified respiratory polygraphy at home showed a prevalence of OSAS of 10.1%, a figure higher than those reported in other studies;19,33 however, we were unable to evaluate this subsample using polysomnography, which is the gold standard. As expected, the prevalence of OSAS (ESS ≥ 11 and RDI ≥ 15 events/h) was different when self-reported symptoms were considered: 2.9% in subjects who did not report sleep symptoms vs. 23.5% among those who reported the combination of snoring and EDS with or without observed apneas. The risk factors for OSAS in our study were habitual snoring and smoking (> 10 pack-years). Other risk factors recognized in previous studies,4 such as male gender, age, BMI, and smoking, were not identified in the subsample, though they were associated with the combination of snoring, EDS, and apneas in the complete study group. If we consider an ESS ≥ 11 and an RDI ≥ 15 events/h as the gold standard for OSAS, then the combination of snoring and EDS (with or without observed apneas) had a low sensitivity and PPV, but a high specificity and NPV; results that indicate a low rate of false positives.
Our study has limitations. The estimation of SRS prevalence was based on self-reported symptoms through a non-validated questionnaire, and therefore we are not able to affirm that even the coexistence of sleepiness, observed apneas, and habitual snoring means that the patients have OSAS. However, we believe that patients with perception of those symptoms need further evaluation to rule out OSAS, regardless of whether the questionnaire had been previously validated. Indeed, the logistic models presented in this study were built with an exploratory purpose, and we recognize that other factors that are not well known could be involved. On the other hand, even though our indicator of EDS correlated with the Epworth and Stanford Sleepiness Scales, we did not use those scales due to the difficulty of applying them in a house-by-house survey such as ours. Moreover, we evaluated only adults aged 40 years or older; this is precisely the age group that is at high risk for OSAS and its cardiovascular consequences and must, therefore, have priority in terms of identification and treatment.1 Finally, simplified respiratory polygraphy was conducted on only a subsample in México City as consequence limited access to portable monitors. Nonetheless, the main strength of our study is that it presents information based on a population sample taken from the metropolitan areas of 4 Latin American cities, where we applied the same sampling strategy and questionnaire, took the same measurements, and achieved a high response rate (81%).
In summary, SRS are frequent in the general adult population of the 4 participating cities, with habitual snoring as the most common symptom. A better utilization of resources is necessary to evaluate and treat patients with SRS and, in this way, minimize the detrimental health outcomes associated with the sleep disorders.
DISCLOSURE STATEMENT
This study was proposed by ALAT (Asociación Latinoamericana del Tórax) and supported by a research grant from Boehringer-Ingelheim. The authors have indicated no financial conflicts of interest. This study was also supported by a grant from the Consejo Nacional de Ciencia y Tecnología in Mexico (CONACYT SALUD-2004-CO1-72).
ACKNOWLEDGMENTS
The PLATINO group also included Pedro Curi Hallal – Universidad Federal de Pelotas, Brasil; Jose Jardim – Universidade Federal de São Paulo, Brasil; Elizabeth Chávez-Plascencia, Raúl Peñuelas-Baldenebro, Franco-Marina, and Alexander Corcho – Instituto Nacional de Enfermedades Respiratorias, México, D.F.; Dolores Moreno – Universidad de la República, Facultad de Medicina, Montevideo, Uruguay; Jorge Corcuera and Carmen Lisboa – Pontificia Universidad Católica de Chile, Santiago, Chile.
The authors would like to thank Dr. Joaquín Durán for his critical reading of the manuscript and Mr. Paul Kersey for correcting language use.
Footnotes
See Acknowledgments.
REFERENCES
- 1.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 2.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin CM, Griffith KA, Nieto FJ, et al. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Menezes AM, Victora CG, Perez-Padilla R. The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15. doi: 10.1186/1471-2288-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Padilla R, Torre-Bouscoulet L, Muino A, et al. Prevalence of oxygen desaturation and use of oxygen at home in adults at sea level and at moderate altitude. Eur Respir J. 2006;27:594–9. doi: 10.1183/09031936.06.00075005. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Padilla R, Valdivia G, Muino A, et al. [Spirometric reference values in 5 large Latin American cities for subjects aged 40 years or over] Arch Bronconeumol. 2006;42:317–25. doi: 10.1016/s1579-2129(06)60540-5. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Padilla R, Torre Bouscoulet L, Vazquez-Garcia JC, et al. Spirometry reference values after inhalation of 200 microg of salbutamol. Arch Bronconeumol. 2007;43:530–4. doi: 10.1016/s1579-2129(07)60123-2. [DOI] [PubMed] [Google Scholar]
- 10.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 11.The European Community Respiratory Health Survey II. Eur Respir J. 2002;20:1071–9. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 12.BC Cancer Research Centre. Lung Health Study Questionnaire. Vancouver: BC Cancer Research Centre; 2004. [Google Scholar]
- 13.Ware JE, Kosinski M, Keller SD. How to score the SF12 Physical and Mental Health Summary Scales. 2nd edition. Boston: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 14.Beall CM, Almasy LA, Blangero J, et al. Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900-4,000 m. Am J Phys Anthropol. 1999;108:41–51. doi: 10.1002/(SICI)1096-8644(199901)108:1<41::AID-AJPA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Chiner E, Arriero JM, Signes-Costa J, et al. [Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome] Arch Bronconeumol. 1999;35:422–7. doi: 10.1016/s0300-2896(15)30037-5. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugaresi E, Cirignotta F, Coccagna G, et al. Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep. 1980;3:221–4. doi: 10.1093/sleep/3.3-4.221. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Palta M, Dempsey J, et al. The occurrence of sleepdisordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 20.Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 21.Norton PG, Dunn EV, Haight JS. Snoring in adults: some epidemiologic aspects. Can Med Assoc J. 1983;128:674–675. [PMC free article] [PubMed] [Google Scholar]
- 22.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 23.Liu SA, Liu CY. Prevalence of snoring in Taichung area: an epidemiological study. J Chin Med Assoc. 2004;67:32–36. [PubMed] [Google Scholar]
- 24.Larsson LG, Lindberg A, Franklin KA, et al. Gender differences in symptoms related to sleep apnea in a general population and in relation to referral to sleep clinic. Chest. 2003;124:204–11. doi: 10.1378/chest.124.1.204. [DOI] [PubMed] [Google Scholar]
- 25.Hara C, Lopes Rocha F, Lima-Costa MF. Prevalence of excessive daytime sleepiness and associated factors in a Brazilian community: the Bambui study. Sleep Med. 2004;5:31–6. doi: 10.1016/j.sleep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Becker PM. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Psychiatr Clin North Am. 2006;29:855–70. doi: 10.1016/j.psc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.George CF. Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep. 2000;23(Suppl 1):S31–35. discussion S36–38. [PubMed] [Google Scholar]
- 29.Naska A, Oikonomou E, Trichopoulou A, et al. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 30.Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–7. [PubMed] [Google Scholar]
- 31.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29:429–37. [PubMed] [Google Scholar]
- 32.Masa JF, Rubio M, Perez P, et al. Association between habitual naps and sleep apnea. Sleep. 2006;29:1463–8. doi: 10.1093/sleep/29.11.1463. [DOI] [PubMed] [Google Scholar]
- 33.Olson LG, King MT, Hensley MJ, et al. A community study of snoring and sleep-disordered breathing. Prevalence. Am J Respir Crit Care Med. 1995;152:711–6. doi: 10.1164/ajrccm.152.2.7633731. [DOI] [PubMed] [Google Scholar]