Abstract
The oxysterol receptor LXR is a key transcriptional regulator of lipid metabolism. LXR increases expression of SREBP-1, which in turn regulates at least 32 genes involved in lipid synthesis and transport. We recently identified 25-hydroxycholesterol-3-sulfate (25HC3S) as an important regulatory molecule in the liver. We have now studied the effects of 25HC3S and its precursor, 25-hydroxycholesterol (25HC), on lipid metabolism as mediated by the LXR/SREBP-1 signaling in macrophages. Addition of 25HC3S to human THP-1-derived macrophages markedly decreased nuclear LXR protein levels. 25HC3S administration was followed by dose- and time-dependent decreases in SREBP-1 mature protein and mRNA levels. 25HC3S decreased the expression of SREBP-1-responsive genes, acetyl-CoA carboxylase-1, and fatty acid synthase (FAS) as well as HMGR and LDLR, which are key proteins involved in lipid metabolism. Subsequently, 25HC3S decreased intracellular lipids and increased cell proliferation. In contrast to 25HC3S, 25HC acted as an LXR ligand, increasing ABCA1, ABCG1, SREBP-1, and FAS mRNA levels. In the presence of 25HC3S, 25HC, and LXR agonist T0901317, stimulation of LXR targeting gene expression was repressed. We conclude that 25HC3S acts in macrophages as a cholesterol satiety signal, downregulating cholesterol and fatty acid synthetic pathways via inhibition of LXR/SREBP signaling. A possible role of oxysterol sulfation is proposed.
Keywords: 25-hydroxycholesterol, sulfated 25-hydroxycholesterol, cholesterol and triglyceride metabolism, oxysterol sulfation, oxysterol metabolism
macrophages are key cellular players in the pathophysiology of atherosclerosis. Macrophages in arterial walls ingest oxidized low-density lipoproteins and accumulate cholesterol esters and triglycerides. These lipid-laden macrophages, termed foam cells, are characteristic of the reversible early cellular phase of the atherosclerotic plaque. Physiological or pharmacological maneuvers that reduce macrophage lipids may be effective for preventing or reversing atherosclerosis.
Nuclear receptors are ligand-activated transcription factors that regulate the expression of target genes to affect processes as diverse as reproduction, development, and general metabolism (6). A number of these nuclear receptors, such as the nuclear receptors for oxysterols [liver X receptors (LXRs)], bile acids (farnesoid X receptors), and retinoic acids [retinoid X receptors (RXRs)], function as sensors of cellular cholesterol and lipid levels (24), eliciting gene expression that maintains lipid homeostasis and protects cells from lipid overload (33). Sterol regulatory element-binding proteins (SREBPs) are a family of transcription factors that have been established as key regulators of cholesterol and fatty acid synthesis by directly activating the expression of more than 32 genes involved in the regulation of lipid metabolisms (15, 16). In the liver, the nuclear receptor LXR has been shown to regulate SREBP-1 expression (37).
Recently, we identified a novel oxysterol, 25-hydroxycholesterol-3 sulfate (25HC3S), that accumulates in hepatocyte nuclei following overexpression of the mitochondrial cholesterol delivery protein StarD1 (26, 29, 30). This oxysterol appears to be synthesized by sterol sulfotransferase SULT2B1b (23). Of note is that overexpression of SULT2B1b inactivates the response of LXR to multiple oxysterol ligands (8). The addition of 25HC3S to primary human hepatocytes decreased cholesterol synthesis and downregulated expression of key enzymes such as 3-hydroxy-3-methylglutaryl-coenzyme A (CoA) reductase (HMGR), acetyl-CoA carboxylase-1 (ACC1), and fatty acid synthase (FAS) by blocking the activation of SREBP-1 and inhibiting its expression in hepatocytes (31). These results suggest that 25HC3S plays an important role in maintenance of hepatic lipid homeostasis.
In the present study, we demonstrate that 25HC3S is also a potent downregulator of the cholesterol and fatty acid biosynthetic pathways in macrophages. We provide strong evidence that its effects appear to be mediated via inhibition of LXR/SREBP signaling. These findings suggest that 25HC3S and its analogs may represent a new therapeutic approach to the treatment of atherosclerosis.
MATERIALS AND METHODS
Materials
Cell culture reagents and supplies were purchased from GIBCO BRL (Grand Island, NY), and the reagents for real-time RT-PCR were obtained from AB Applied Biosystems (Warrington, UK). Antibodies against human SREBP-1, FAS, ACC1, and LXR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against human ATP-binding cassette transporter A1 (ABCA1) and SREBP-2 were from Abcam (Cambrige, MA). The enhanced chemiluminescence reagents were purchased from Amersham Biosciences (Piscataway, NJ). FuGENE HD transfection reagents were obtained from Roche Applied Science (Indianapolis, IN). The Dual-Glo Luciferase Assay System was purchased from Promega (Wisconsin, WI). LXR agonist T0901317 was from New Cayman Chemical (Ann Arbor, MI). [14C]cholesterol, 25-hydroxycholesterol, and [1-14C]acetate were from New England Nuclear (Boston, MA). Free cholesterol C, cholesterol E assay, and Wako NEFA-HR(2) assay kits for free fatty acid were from Wako Bioproducts (Richmond, VA). Infinity triglyceride assay kit was purchased from Thermo Electron (Arlington, TX). The chemicals used in this research were obtained from Sigma Chemical (St. Louis, MO) or Bio-Rad Laboratories (Hercules, CA). All solvents were obtained from Fisher (Fair Lawn, NJ) unless otherwise indicated. LK6 20 × 20-cm thin-layer chromatography (TLC) plates were purchased from Whatman (Clifton, NJ).
Methods
Plasmids.
Receptor expression plasmids encoding full-length human LXRα (CMX-hLXRα), LXRβ (CMX-LXRβ), and a luciferase reporter gene plasmid [TK-CYP7a-LXRE(X3)-LUC] containing three tandem copies of the sequence (gcttTGGTCActcaAGTTCAagtta) from the rat Cyp7a gene were prepared as described previously (9, 13).
Cell culture.
Human THP-1 monocytes were purchased from American Type Culture Collection (Manassas, VA) and maintained according to the supplier's protocols. Cells were differentiated to macrophages by adding 100 nM phorbol 12-myristate 13-acetate. When cells reached ∼90% confluence, 25HC3S in DMSO or 25HC in ethanol (the final concentration of DMSO or ethanol in medium was 0.1%) was added. The cells were harvested at the times indicated. Nuclear and cytosolic fractions were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL).
Human lung adenocarcinoma epithelial cell line H441 cells were kindly provided by Dr. S. J. Chu (Veterans Affairs Medical Center, Richmond, VA) (10) and maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and 2 mM glutamine.
Synthesis of 5-cholesten-3β,25-diol 3-sulfate (25HC3S).
25HC3S was synthesized as described previously (31). Briefly, a mixture of 25-hydroxycholesterol (25HC; 402 mg, 1 mmol) and triethylamine-sulfur trioxide pyridine complex (160 mg, 1 mmol) in 5 ml of chloroform was stirred at 25°C for 7 days. After the solvent was evaporated at reduced pressure, products were purified by HPLC using a silica gel column and methylene chloride and methanol (5%) as the mobile phase. The product was further purified by reverse-phase HPLC using C18 column as a white powder. The structure of the product was characterized by mass spectrum and nuclear magnetic resonance spectroscopy analysis, as described previously (31).
Determination and Oil Red O staining of total intracellular neutral lipids.
THP-1-derived macrophages were plated on 22 × 22-mm glass coverslips in six-well plates. The medium was replaced after 24 h. Cells were loaded with acetylated LDL (50 μg/ml) and treated with 25HC3S or vehicle control for 48 h as indicated. Cells were fixed with 3.7% formaldehyde in PBS for 30 min followed by two washes with phosphate-buffered saline (PBS). The cells were stained with 0.2% Oil Red O in 60% of 2-propanol for 10 min and washed three times with PBS. The images were taken with the use of a microscope (Olympus, Tokyo, Japan) equipped with image recorder under ×40 lenses.
Determination of cholesterol biosynthesis.
Following the treatment of THP-1-derived macrophages with different concentrations of 25HC or 25HC3S for 6 h, the cells in 60-mm dishes were fed with 3 ml of the same fresh medium containing 3 μCi of [1-14C]acetate. After incubation at 37°C for 2 h, the medium was removed and the cells were washed twice with cold PBS, harvested with rubber police, and collected to microcentrifuge tubes. The cells were centrifuged and the pellets washed three times through resuspension and sedimentation. The cellular pellets were resuspended in 0.3 ml of PBS. The total lipids were extracted and separated by Folch partitioning after five volumes of chloroform-methanol (2:1) were added. [14C]cholesterol and hydroxycholesterols extracted to chloroform phase were separated on TLC (toluene-acetyl acetate, 2:3, vol/vol), as described previously (31). [1-14C]acetate derivatives were visualized by Image Reader, Fujifilm BAS-1800 II.
Determination of cholesterol, cholesterol esters, free fatty acids, and triglycerides.
THP-1 macrophages were plated on 100-mm tissue culture dishes and treated with control vehicle and 25HC3S (3–25 μM) for 48 h. The cells were collected with 0.5 ml of PBS and sonicated. Total lipids were extracted by addition of 10 ml of chloroform-methanol (2:1, vol/vol) mixture. The extract, 0.5 ml, was evaporated to dry and resolved in 100 μl of isopropanol containing 10% of Triton X-100 for cholesterol assay, isopropanol only for triglyceride, or NEFA solution (0.5 g of EDTA-Na2, 2 g of Triton X-100, 0.76 ml of 1 N NaOH, and 0.5 g of sodium azide/l, pH 6.5) for free fatty acid assay. The intracellular total and free cholesterol, triglyceride, and free fatty acid assays were performed according to the manufacturer's instructions.
Cell proliferation and cytotoxicity assay.
THP-1 cells were plated in 96-well plates with a density of 1 × 104/well. The medium was replaced after 24 h. The cells were differentiated to macrophages by the addition of 100 nM phorbol 12-myristate 13-acetate and culturing for 7 days. After treatment of 25HC or 25HC3S for 48 h, 10 μl/well of CellTiter 96Aqueous One Solution Reagent [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reagent] was added. After incubation at 37°C for 1 h in a humidified 5% CO2 atmosphere, the absorbance at 490 nm was recorded with an ELISA plate reader. Control refers to incubations in the presence of vehicle only (0.1% of DMSO or ethanol) and was considered as 100% viable cells (40).
Analysis of apoptosis by annexin V and propidium iodine staining.
THP-1 cells and THP-1-derived macrophages were treated with oxysterols (0–50 μM) for 48 h and stained with Annexin V-FITC and propidium iodine using Vybrant Apoptosis Assay Kit No. 3 (Molecular Probes, Eugene, OR). The percentiles of dead, apoptotic, and living cells of 1 × 104 from 1 × 106 cells/ml were analyzed by flow cytometry.
Determination of mRNA levels by real-time RT-PCR.
Total RNA was isolated from THP-1-derived and oxysterol-treated macrophages using SV Total RNA Isolation Kit (Promega, Madison, WI). Two micrograms of total RNA were used for first-strand cDNA synthesis as recommended by the manufacturers (Invitrogen, Carlsbad, CA). Real-time RT-PCR was performed using SYBR Green as an indicator on the ABI 7500 Fast Real-Time PCR system. The final reaction mixture contained 10 ng of cDNA, 100 nM of each primer, 10 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), and RNase-free water to complete the reaction mixture volume to 20 μl. All reactions were performed in triplicate. The PCR was carried out for 40 cycles of 95°C for 15 s and 60°C for 1 min. The fluorescence was read during the reaction, allowing a continuous monitoring of the amount of PCR product. The data were normalized to internal control GAPDH mRNA. The sequences of primers are as shown in Table 1.
Table 1.
Primer sets for real-time RT-PCR analysis of gene expression
| Name | GenBank No. | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| GAPDH | NM_002046 | CAATGACCCCTTCATTGACC | TTGATTTTGGAGGGATCTCG |
| HMGR | NM_000859 | GTCATTCCAGCCAAGGTTGT | GGGACCACTTGCTTCCATTA |
| FAS | NM_004104 | TGTGGACATGGTCACGGAC | GGCATCAAACCTAGACAGGTC |
| ACC1 | NM_198834 | TCGCTTTGGGGGAAATAAAGTG | ACCACCTACGGATAGACCGC |
| SREBP-1 | NM_004176 | CAGCCCCACTTCATCAAGG | ACTGTTGCCAAGATGGTTCCG |
| SREBP-2 | NM_004599 | AACGGTCATTCACCCAGGTC | GGCTGAAGAATAGGAGTTGCC |
| LDLR | NM_002335 | AGTTGGCTGCGTTAATGTGAC | TGATGGGTTCATCTGACCAGT |
| ABCA1 | NM_005502 | GGTGATGTTTCTGACCAATGTGA | TGTCCTCATACCAGTTGAGAGAC |
| ABCG1 | NM_207629 | CCAGAAGTCGGAGGCCATC | AAGTCCAGGTACAGCTTGGCA |
HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; FAS, fatty acid synthase; ACC1, acetyl-CoA carboxylase-1; SREBP, sterol regulatory element-binding protein; LDLR, LDL receptor; ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1.
Western Blot Analysis
Fifty micrograms of total or nuclear protein, otherwise as indicated, were separated on 7.5 or 10% SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane using a Bio-Rad Mini-Blot transfer apparatus, as described previously (28). Membranes were blocked in Tris-buffered solution (TBS) containing 5% nonfat dried milk for 1 h. The immunoblotting was performed at 4°C overnight with shaking and using antibodies against human ABCA1, ACC1, FAS, SREBP-1, SREBP-2, or LXR, respectively. After being washed, the membrane was incubated in a 1:3,000 dilution of a secondary antibody (goat anti-rabbit or anti-mouse IgG-HRP conjugate) at room temperature for 1 h in TBS washing buffer containing 0.5% of Tween-20. Protein bands were visualized using Western Lightning Chemiluminescence Reagent (PerkinElmer, Waltham, MA).
Transfection and Promoter Reporter Gene Luciferase Assays
H441 cells were seeded into 96-well plates. When cell density reached 90–95%, cells were transfected by using a lipid-based FuGENE HD transfection reagent according to the manufacturer's instructions (Roche, Indianapolis, IN). A synthetic renilla luciferase reporter, phRG-TK (Promega), was used as a luciferase internal standard. Fifty nanograms of luciferase reporter gene, 50 ng of expression plasmid pCMX-hLXRα or pCMX-LXRβ, and 50 ng of phRG-TK vector (internal standard) were cotransfected. Twenty-four hours following the transfection, different concentrations of 25HC3S and/or 25HC or T0901317 were added as indicated and incubated for another 24 h. Luciferase activities were determined using the Dual-Glo Luciferase Assay system according to the manufacturer's protocol (Promega). The amount of luciferase activity was measured using a TopCount NXT Microplate Scintillation and Luminescence Counter (Packard, Meriden, CT) and normalized to the amount of phRG-TK luciferase activity. Transfections were carried out in triplicate for each sample, and each experiment was repeated three times.
Statistics
Data are reported as means ± SD. Where indicated, data were subjected to t-test or ANOVA analysis and determined to be significantly different at P < 0.05. An asterisk represents significant difference (P < 0.05) compared with the control (0 group) as otherwise indicated.
RESULTS
25HC3S Decreases Intracellular Lipid Levels in Macrophages
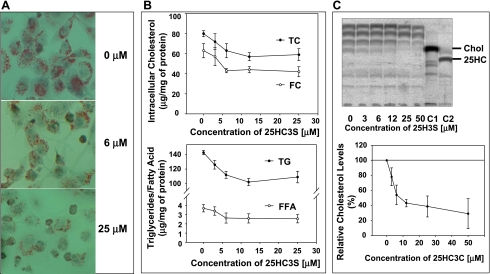
To examine the intracellular lipids levels, total neutral lipids were examined by Oil Red O staining. As shown in Fig. 1A, addition of 25HC3S to macrophages led to a concentration-dependent decrease in the intracellular neutral lipid levels. Quantitative analysis showed that addition of 25HC3S at 6 μM decreased intracellular total cholesterol by 20% (P < 0.05), free cholesterol by 30% (P < 0.01), triglycerides by 20% (P < 0.01), and free fatty acids by 30% (P < 0.01), as shown in Fig. 1B. 25HC3S addition did not change cholesterol ester levels significantly (total cholesterol minus free cholesterol; Fig. 1B). To examine the effects of 25HC3S on cholesterol biosynthesis, the rates of cholesterol synthesis were determined. After addition of 25HC3S in THP-1-derived macrophages in culture, the cells were cultured for 6 h and then with [1-14C]acetate for an additional 2 h, after which total lipids were extracted and partitioned. TLC analysis showed that the newly synthesized [14C]cholesterol was significantly decreased in a concentration-dependent manner following addition of 25HC3S (Fig. 1C). The decreased amounts of [14C]cholesterol bands on the TLC were confirmed by HPLC analysis (data not shown). These results were consistent with those in human hepatocytes, as reported previously (31).
Fig. 1.
25-Hydroxycholesterol-3 sulfate (25HC3S) decreases intracellular lipids. Following THP-1, macrophages were treated with 25HC3S at the indicated concentration and at 50 μg/ml of acetylated LDL for 48 h. A: the total intracellular neutral lipids were stained with 0.2% Oil Red O. B: total lipids were extracted, and individual lipid was analyzed. Effects of 25HC3S on cholesterol biosynthesis were analyzed by thin-layer chromatography (TLC), using [1-14C]acetate as substrate. After incubation of the 25HC3S-treated macrophages with [1-14C]acetate, the total neutral lipids were extracted. C: TLC analysis of the [14C]acetate derivatives extracted from the equivalent of 5 × 106 cells/lane. Relative cholesterol levels were determined by quantifying the densities of each band using Image Reader, Fujifilm BAS-1800 II. Each %value represents the mean of 3 experiments ± SD. The data represent a typical result from 1 of 3 independent experiments. TC, total cholesterol; FC, free cholesterol; TG, triglycerides; FFA, free fatty acids; 25HC, 25-hydroxycholesterol.
25HC3S Increases Cell Proliferation and Decreases Apoptosis in Macrophages
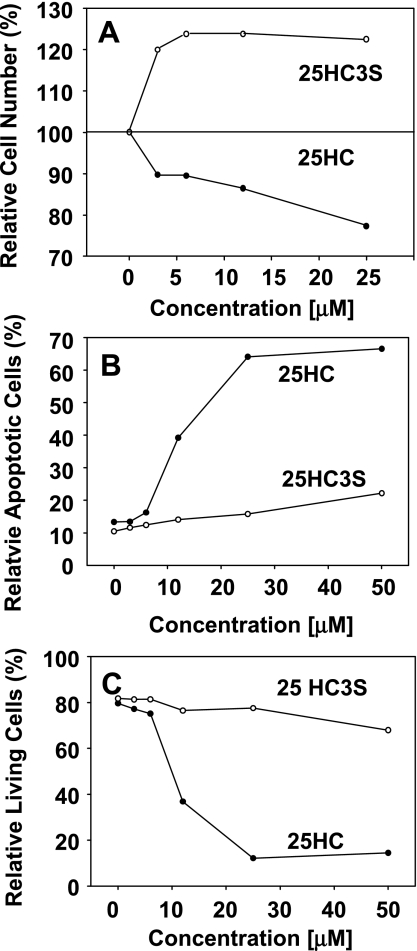
To study whether lowering intracellular lipid level can decrease cell toxicity, the effects of 25HC and 25HC3S on the cell proliferation and cytotoxicity were compared using MTT reagent. As shown in Fig. 2A, at 3 μM the percentages of relative cell number after treatment for 48 h were 120% (P < 0.01) for 25HC3S and 90% for 25HC; at 25 μM the percentages were 120% for 25HC3S and only 75% (P < 0.01) for 25HC. The difference in cell viability between different treatments was statistically significant (P < 0.001). These results indicate that 25HC3S induces cell proliferation and 25HC induces cell death.
Fig. 2.
Effects of 25HC and 25HC3S on cell proliferation, apoptosis, and viability. THP-1-derived macrophages or THP-1 cells were treated with vehicle control or various concentrations of 25HC or 25HC3S (0–25 μM) for 48 h. A: the macrophage proliferation was measured using CellTiter 96AQueous One Solution Reagent. Control refers to incubations in the presence of vehicle only (0.1% DMSO) and was considered as 100% of viable cells. B and C: apoptotic and dead THP-1 cells were analyzed by flow cytometry using Annexin V-FITC/propidium iodide staining. The data represent a typical result from 1 of 3 independent experiments.
Lipid accumulation in macrophages has been shown to induce cell apoptosis and cell death via the endoplasmic reticulum stress signal pathway (3, 20, 21). To examine whether the decreases in intracellular lipid levels are correlated with decreased THP-1 cell apoptosis, the cells were treated with 25HC or 25HC3S at different concentrations for 48 h, and the apoptotic, dead, and live cells were stained with Annexin V-FITC and propidium iodine and determined by a flow cytometry. 25HC3S did not significantly affect the numbers of apoptotic or live cells, as shown in Fig. 2, B and C. The induction of apoptosis by 25HC was concentration dependent (Fig. 2B). Furthermore, 25HC markedly decreased the live cell counts but 25HC3S did not (Fig. 2C).
25HC3S Inhibits Expression and Activation of SREBP-1
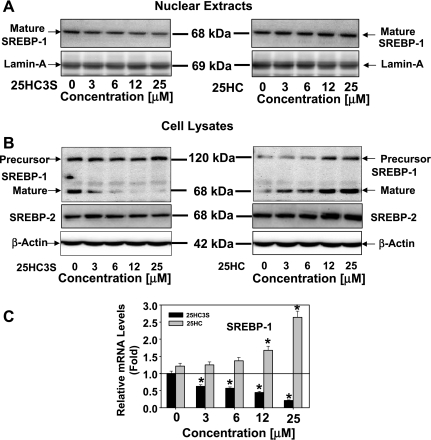
To study whether the SREBP-1 regulatory pathway is involved in the decreases of intracellular lipid levels regulated by 25HC3S, its protein and mRNA levels were analyzed in THP-1-derived macrophages following treatment with 25HC3S or 25HC for 6 h. Total cellular cytosol and nuclear proteins were extracted from the cells treated with 25HC3S or 25HC at different concentrations for 6 h. The precursor and mature forms of SREBPs were determined by Western blot analysis, using specific antibodies (38, 39). As expected, decreases of the mature forms of SREBP-1 (P < 0.01) in both nuclear (Fig. 3A) and cytosol (Fig. 3B) fractions were concentration dependent. There was no significant change of the precursor form of SREBP-1 following addition of 25HC3S (Fig. 3B). However, 25HC substantially increased SREBP-1 protein levels of mature and precursor forms in cytosol fractions (Fig. 3B). It was observed that the activation of SREBP-1 was much more sensitive to the treatment with 25HC3S than with SREBP-2 (Fig. 3B). The inhibition of SREBP-1 activation is consistent with the decreases in intracellular lipid levels. Thus, 25HC3S may inhibit the activation of SREBP-1 and subsequently inhibit intracellular lipid biosynthesis.
Fig. 3.
Effects of 25HC3S on expression and activation of sterol regulatory element-binding protein (SREBP)-1 and SREBP-2. After 6 h following addition of 25HC3S or 25HC at the indicated concentration, total protein and mRNA were isolated from the macrophages. SREBP-1 precursor or mature form levels in the nuclear (A) and SREBP-1 and SREBP-2 in cell lysate (B) fractions were analyzed by Western blot. Effects of 25HC3S and 25HC on SREBP-1 mRNA levels were determined by real-time RT-PCR. C: the expression levels were normalized to GAPDH. The values represent means of triplicates ± SD. The data represent 1 of 3 experiments. *Statistical significance (P < 0.01).
To further confirm 25HC3S functions in a different manner from 25HC, expression of SREBP-1 was determined in the THP-1-derived macrophages, as shown in Fig. 3C. At 3 μM, cultured for 6 h, addition of 25HC3S decreased SREBP-1 mRNA by 45%. In contrast with 25HC3S, 25HC significantly increased SREBP-1 mRNA levels (P < 0.01). The regulation of the gene expression was concentration dependent (Fig. 3C). These results were consistent with the previous data from primary human hepatocytes (31).
Effects of 25HC3S on LXR and SREBPs Targeting Gene Expressions
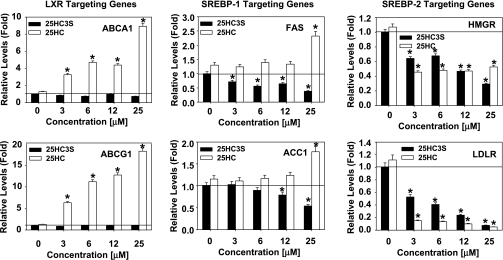
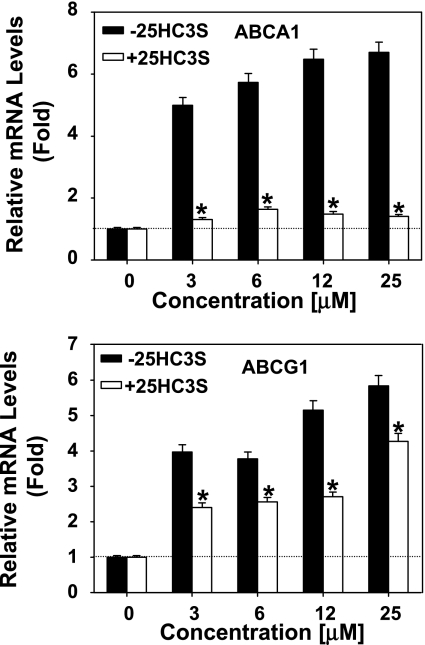
To investigate how 25HC3S inhibits lipid synthesis, total RNA was isolated from the treated THP-1-derived macrophages. The mRNA levels of ABCA1 and ATP-binding cassette transporter G1 (ABCG1) (LXR targeting genes), FAS and ACC1 (SREBP-1 targeting genes), and HMGR and LDLR (SREBP-2 targeting genes) were determined by real-time RT-PCR. As shown in Fig. 4, those decreases in ACC1, FAS, HMGR, and LDLR mRNA levels following 25HC3S treatment and increases in ABCA1, ABCG1, FAS, and ACC1 mRNA levels following 25HC were all concentration dependent.
Fig. 4.
Effects of 25HC3S and 25HC on the expression of liver X receptor (LXR) and SREBP response genes. mRNA levels of LXR and SREBP response genes in 25HC3S- or 25HC-treated macrophages were determined by real-time RT-PCR analysis. The expression levels were normalized to GAPDH. Left: effects of 25HC3S and 25HC on the expression of LXR targeting. Middle: SREBP-1 targeting genes. Right: SREBP-2 targeting genes. Each value represents mean of triplicates ± SD. The data represent 1 of 3 experiments. *Statistical significance (P < 0.01). ABCA1, ATP-binding cassette transporter A1; FAS, fatty acid synthase; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; ABCG1, ATP-binding cassette transporter G1; ACC1, acetyl-CoA carboxylase-1; LDLR, LDL receptor.
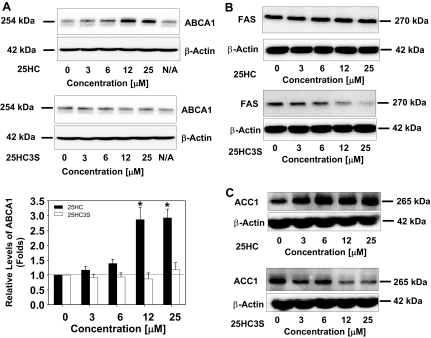
To evaluate effect of 25HC3S on the expression at the protein levels, ABCA1, FAS, and ACC1 were selected as the targeting gene expressions for the Western blot analysis. As shown in Fig. 5, 25HC significantly increased ABCA1 and ACC1, whereas 25HC3S significantly decreased FAS and ACC1 protein levels. These results are consistent with the mRNA data in Fig. 4.
Fig. 5.
Effects of 25HC3S and 25HC on the expression of ABCA1, FAS, and ACC1 at protein levels. A–C: following the treatment of macrophages with the indicated concentrations of 25HC3S and 25HC for 6 h, ABCA1, FAS, and ACC1 protein levels were analyzed by Western blot analysis. Each positive band was quantified by the Image Data Analyzer and normalized to β-actin. The data represent 1 of 3 experiments.
25HC3S Prevents 25HC-Mediated Upregulation of LXR Targeting Gene Expression
25HC is considered as a regulatory oxysterol by serving as an LXR ligand (8, 19, 34). The present study shows that 25HC can increase ABCA1 and ABCG1 (LXR targeting genes) expression but 25HC3S cannot. A competitive assay was performed to study whether both bind the same molecule. Cells were pretreated with 12 μM 25HC3S for 10 min prior to addition of 25HC at different concentrations for 6 h. Total mRNA was isolated, and levels of the specific mRNAs coding for ABCA1 and ABCG1 were analyzed by real-time RT-PCR. As shown in Fig. 6, 25HC increased ABCA1 and ABCG1 mRNA levels in control cells but failed to increase the ABCA1 and could not stimulate ABCG1 mRNA to the maximal levels in the 25HC3S-pretreated cells. The different responses of ABCA1 and ABCG1 expression to 25HC3S suggest that a different signal transduction system in addition to the LXR signal pathway may be involved in the regulation.
Fig. 6.
25HC3S inhibits expression of ABCA1/G1 induced by 25HC. Following the treatment of macrophages with the indicated concentration of 25HC in the presence or absence of 12 μM 25HC3S for 6 h, total mRNA was extracted. ABCA1 (top) and ABCG1 (bottom) mRNA levels were analyzed by real-time RT-PCR. The expression levels were normalized to GAPDH. The values represent means ± SD. *Statistical significance (P < 0.01).
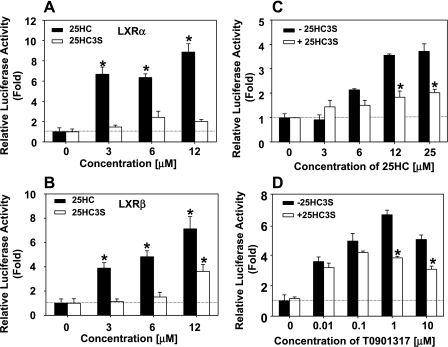
25HC3S Inhibits the LXRE-Luciferase Reporter Gene Expression Induced by 25HC and LXR Agonist T0901317
To further confirm that 25HC and 25HC3S responses are through LXR signaling pathway, H441 cells were cotransfected with the luciferase reporter gene plasmid [TK-CYP7a-LXRE(X3)-LUC] and LXRα or LXRβ recombinant plasmid and treated with 25HC, T0901317, and/or 25HC3S. As shown in Fig. 7, 25HC treatment increased luciferease activities six- to eightfold, whereas 25HC3S only slightly increased its activities (Fig. 7, A and B). Interestingly, in the presence of 12 μM 25HC3S, 25HC and T0901317 failed to induce the activities to the maximal levels (Fig. 7, C and D). These results suggested that 25HC3S is a competitor to 25HC and T0901317 in LXR activation.
Fig. 7.
25HC3S inhibits the activities of LXRα promoter reporter gene expression induced by 25HC and T0901317. After 24 h following the cotransfection of luciferase promoter reporter gene and the expression plasmid full-length human LXR (pCMX-hLXR)α or pCMX-LXRβ and/or phRG-TK vector, H411 cells were treated with the indicated concentrations of 25HC and T0901317 in the presence or absence of 12 uM 25HC3S and incubated for other 24 h. Luciferase activities were determined. A: effects of 25HC and 25HC3S on LXRα activity. B: effects of 25HC and 25HC3S on LXRβ activity. C: 25HC3S inhibits activation of LXRα induced by 25HC. D: 25HC3S inhibits activation of LXRα induced by T0901317. Transfections were carried out in triplicate for each sample in each experiment. The data represent the mean of 3 independent experiments ± SD. *Statistical significance (P < 0.01).
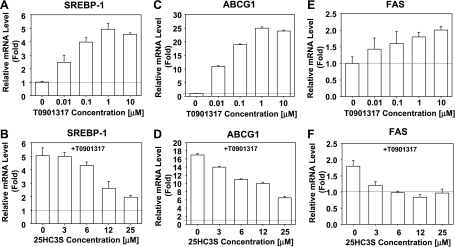
25HC3S Blocks Induction of mRNA Levels of ABCG1, SREBP-1, and FAS by LXR Agonist T0901317
To further demonstrate that 25HC3S affects lipid metabolism by LXR/SREBP signaling pathway, LXR nonsteroid activator T0901317 was used. Like 25HC, T0901317 increased LXR target gene ABCA1 and SREBP-1 transcriptional expressions in a concentration-dependent manner. T0901317 at 1 μM increased SREBP-1, ABCG1, and FAS mRNA expressions to maximal levels (Fig. 8, A, C, and E), but 25HC3S repressed the T0901317-induced increases (Fig. 8, B, D, and F).
Fig. 8.
25HC3S blocks stimulation of LXR targeting gene expression by T0901317. THP-1-derived macrophages were incubated with the indicated concentrations of T0901317 or with the indicated concentration of 25HC3S in the presence of 1 uM T0901317 for 6 h. Cells were harvested, and mRNA levels of SREBP-1 (A and B), ABCG1 (C and D), and FAS (E and F) were determined by real-time PCR analysis. The expression levels were normalized to GAPDH. The data represent 1 of 3 experiments. Each data point represents the mean ± SD. ANOVA showed that T0901317 significantly increases SREBP-1, ABCG1, and FAS expressions (P < 0.01; A, C, and E, respectively), and 25HC3S significantly blocks T0901317 activation (P < 0.05; B, D, and F).
25HC3S Inhibits Activation of Liver Oxysterol Receptor LXRα
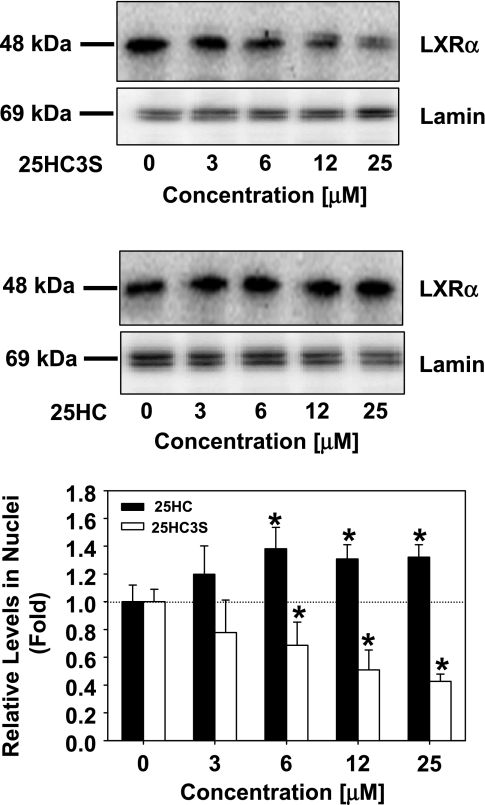
LXRs are nuclear receptors activated by oxysterols that are recognized to play an important role in the control of lipid metabolism (7, 15, 16). As shown in Fig. 3, 25HC3S is capable of decreasing SREBP-1 mRNA levels and inhibiting SREBP-1 activation, which are the key regulatory steps in lipid metabolism. To determine whether inhibition of SREBP-1 is via the LXR signaling pathway, we have evaluated the effects of 25HC3S on LXRα activation in macrophages. Administration of 25HC3S at 12 μM markedly decreased LXRα levels in the nuclei in a concentration-dependent manner (Fig. 9). These results suggest that the decreases in SREBP-1 are most likely via blocking macrophage LXR activation.
Fig. 9.
Effects of 25HC3S and 25HC on the activation of LXRα. Following the treatment of THP-1-derived macrophages with the indicated concentrations of 25HC3S (top) or 25HC (middle), LXR levels in nuclear extracts were detected by Western blot analysis. The extracted nuclear protein (50 μg) was loaded in each lane. Each positive band was quantified by the Image Data Analyzer and normalized to Lamin. Bottom: summary of LXRα levels is shown. The data represent the mean of 3 independent experiments ± SD.
DISCUSSION
LXRs are nuclear receptors activated by oxysterols that are recognized to play an important role in the control of lipid metabolism (5, 7). The role of LXRs in the treatment of atherosclerosis is controversial. LXR ligand oxysterols upregulate expressions of cholesterol reverse transporters such as ABCA1 and ABCG1 via activation of LXR/RXR heterodimers. ABCA1/ABCG1 is known to mediate efflux of cellular cholesterol and phospholipids, which has potential in the prevention of atherosclerosis (4, 36). However, administration of synthetic LXR ligands to mice triggers induction of the lipogenic pathway and elevates plasma triglyceride levels via SREBP-1, whose promoter is an LXR direct target (7, 12, 14, 32). In the present study, we show that administration of 25HC3S decreases intracellular lipids and increases cell proliferation. Coincidently, 25HC3S inhibits LXR activation and SREBP-1 expression and activation with subsequent decreases in ACC1, FAS, and HMGR mRNA levels. However, 25HC, as an LXR ligand and a direct precursor of 25HC3S, has opposite effects, increasing SREBP-1, FAS, ABCA1, and ABCG1 mRNA levels in the macrophages, increasing intracellular lipid levels, inducing apoptosis, and decreasing cell viability. These results are consistent with those of administration of LXR ligands in vivo in mice (7, 12, 14, 32), indicating that, under these conditions, ligand activation of LXRα increases SREBP-1 expression and is the major reason for their hyperlipidemia state. Interestingly, after sulfation of 25HC, the product 25HC3S inhibits LXRα activation and decreases SREBP-1 expression and activation, indicating that sulfation of 25HC dose more than simply inactivates 25HC, providing evidence for a new regulator of lipid metabolism.
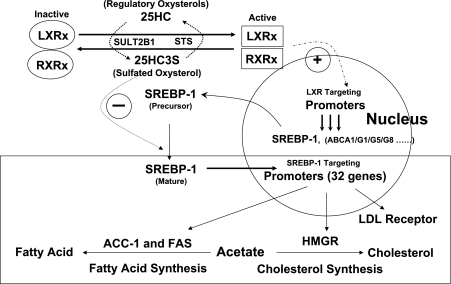
Although many oxysterols are reported to be LXR ligands and to regulate LXR targeting gene expression (2, 17, 18, 22, 25, 27, 35), the mechanism of the regulation is unclear. A recent report showed that overexpression of sulfotransferase SULT2B1b inactivates the response of LXR to multiple oxysterol ligands (8), suggesting that SULT2B1b is involved in a liver oxysterol receptor (LXR) signaling pathway. The present study provides evidence that oxysterol sulfation/desulfation is another regulatory pathway in intracellular lipid metabolism by regulating activation and inactivation of nuclear oxysterol receptors, similar to the role that protein phosphorylation/dephosphorylation plays in carbohydrate metabolism. It is possible that high intracellular cholesterol levels result in generating regulatory oxysterols that can be further sulfated to be another regulatory sulfated oxysterol. The findings of this study suggest that regulatory oxysterols activate LXRs and sulfated oxysterols inactivate LXRs. The levels of endogenous 25HC3S in cells are very low. We are assuming that 25HC3S functions much like intracellular hormone. We were barely able to detect 25HC3S in human liver tissues (∼0.1 μg/g of liver tissues) using the liquid chromatography-mass spectrometry-mass spectrometry system (29). These levels can be dramatically increased by overexpression of the mitochondrial cholesterol delivery protein (StAR), which presents in macrophages (Supplemental Figs. S1 and S2; Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site). We also used RT-PCR to prove the presence of oxysterol sulfotransferase and biochemical analysis of [14C]25-hydroxycholesterol derivatives to prove that the macrophages are able to biosynthesize this oxysterol (Supplemental Figs. S1 and S2). We have observed that 25HC3S can be biosynthesized in hepatocytes (31) and macrophages. The effects of 25HC3S and its precursor 25HC appear to be diametrically opposed and coordinately regulated. This regulation appears to play an important role in lipid metabolism. The present study provided evidence for both 25HC and 25HC3S as an activator and repressor, respectively, of LXR, a key nuclear receptor involved in intracellular lipid homeostasis. The ratio of 25HC to 25HC3S appears to play an important role in maintenance of intracellular lipid homeostasis. A possible physiological role of 25HC3S, oxysterol sulfation, in lipid metabolism was proposed as shown in Fig. 10.
Fig. 10.
Role of oxysterol sulfation in lipid metabolism. Oxysterols such as 25HC activate LXRα and -β. The activated LXRα and -β bind to LXR response elements in promoter regions and activate transcription of their targeting genes, including SREBP-1 expression, which increases the biosynthesis of fatty acids and cholesterol. Thus, the activation of LXRα and -β increases lipids in the circulation by increasing lipid synthesis and efflux. 25HC3S, the product of 25HC sulfation, inhibits SREBP-1 expression, and its activation subsequently inhibits lipid synthesis. LXRx and retinoid X receptor-x (RXRx) represent isomers (α and β). STS, steroid sulfatase.
Decreases in intracellular lipid levels by increasing elimination or by decreasing uptake and synthesis are critical in the prevention of atherosclerosis. The present study shows that administration of 25HC increases expression of the cholesterol efflux proteins ABCA1 and ABCG1 but also increases SREBP-1 expression and activation, which results in increasing intracellular neutral lipid levels, and decreases cell viability. On the other hand, 25HC3S does not increase ABCA1 and ABCG1 but decreases SREBP-1 expression and activation, which results in decreasing intracellular lipid levels and increases cell viability. From a drug development standpoint, the most desirable compound would appear to be one that is a strong inducer of the elimination of lipids but does not activate SREBP-1 and fatty acid synthase promoters. Although neither 25HC nor 25HC3S meets the drug development standpoint as shown, 25HC3S has a potential as a new medication for the treatment and the prevention of atherosclerotic diseases. The fact that increases in expression of ABCA1 and ABCG1 do not decrease intracellular neutral lipid levels indicates that the role of these cholesterol transport proteins in preventing atherosclerotic diseases is questionable.
Both 25HC and 25HC3S inhibit HMGR and LDLR most likely through inhibiting SREBP-2 activation. However, the inhibition of cholesterol biosynthesis by 25HC and 25HC3S might be through a different mechanism. The inhibition of HMGR by 25HC is much stronger than that of 25HC3S (data not shown). It is likely that 25HC inhibits the cholesterol biosynthesis by increasing HMGR fast degradation (ubiquitination) (1, 11), whereas 25HC3S inhibits HMGR expression only. Thus, the pair of the molecules, 25HC and 25HC3S, may play an important role in maintenance of intracellular lipid homeostasis.
In summary, both the precursor 25HC and the product 25HC3S are important in the regulation of macrophage lipid homeostasis. Their potentially contrasting regulatory effects demonstrate the importance of their physiological ratio and their synthesis.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL-078898) and Veterans Administration (VA merit review).
Acknowledgments
We acknowledge excellent technical help from Dalila Marques and Kaye Redford.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem 279: 52772–52780, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alberti S, Steffensen KR, Gustafsson JA. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene 243: 93–103, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Aronis A, Madar Z, Tirosh O. Lipotoxic effects of triacylglycerols in J774.2 macrophages. Nutrition 24: 167–176, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Beyea MM, Heslop CL, Sawyez CG, Edwards JY, Markle JG, Hegele RA, Huff MW. Selective up-regulation of LXR-regulated genes ABCA1, ABCG1, and APOE in macrophages through increased endogenous synthesis of 24(S),25-epoxycholesterol. J Biol Chem 282: 5207–5216, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 282: 743–751, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 294: 1866–1870, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA 101: 11245–11250, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab 5: 73–79, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang JY, Stroup D. Identification and characterization of a putative bile acid-responsive element in cholesterol 7 alpha-hydroxylase gene promoter. J Biol Chem 269: 17502–17507, 1994. [PubMed] [Google Scholar]
- 10.Chu S, Xu H, Ferro TJ, Rivera PX. Poly(ADP-ribose) polymerase-1 regulates vimentin expression in lung cancer cells. Am J Physiol Lung Cell Mol Physiol 293: L1127–L1134, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Corsini A, Verri D, Raiteri M, Quarato P, Paoletti R, Fumagalli R. Effects of 26-aminocholesterol, 27-hydroxycholesterol, and 25-hydroxycholesterol on proliferation and cholesterol homeostasis in arterial myocytes. Arterioscler Thromb Vasc Biol 15: 420–428, 1995. [DOI] [PubMed] [Google Scholar]
- 12.DeBose-Boyd RA, Ou J, Goldstein JL, Brown MS. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci USA 98: 1477–1482, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Castillo-Olivares A, Gil G. Role of FXR and FTF in bile acid-mediated suppression of cholesterol 7alpha-hydroxylase transcription. Nucleic Acids Res 28: 3587–3593, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem 277: 34182–34190, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109: 1125–1131, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100: 12027–12032, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci USA 96: 266–271, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Javitt NB 25R,26-Hydroxycholesterol revisited: synthesis, metabolism, and biologic roles. J Lipid Res 43: 665–670, 2002. [PubMed] [Google Scholar]
- 20.Kim DS, Jeong SK, Kim HR, Kim DS, Chae SW, Chae HJ. Effects of triglyceride on ER stress and insulin resistance. Biochem Biophys Res Commun 363: 140–145, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic β-cells. Am J Physiol Endocrinol Metab 294: E540–E550, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Landis MS, Patel HV, Capone JP. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J Biol Chem 277: 4713–4721, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Pandak WM, Erickson SK, Ma Y, Yin L, Hylemon P, Ren S. Biosynthesis of the regulatory oxysterol, 5-cholesten-3beta,25-diol 3-sulfate, in hepatocytes. J Lipid Res 48: 2587–2596, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lu TT, Repa JJ, Mangelsdorf DJ. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J Biol Chem 276: 37735–37738, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Malerod L, Juvet LK, Hanssen-Bauer A, Eskild W, Berg T. Oxysterol-activated LXRalpha/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem Biophys Res Commun 299: 916–923, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Pandak WM, Ren S, Marques D, Hall E, Redford K, Mallonee D, Bohdan P, Heuman D, Gil G, Hylemon P. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem 277: 48158–48164, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev 8: 571–575, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Ren S, Hylemon P, Marques D, Hall E, Redford K, Gil G, Pandak WM. Effect of increasing the expression of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid synthesis. J Lipid Res 45: 2123–2131, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ren S, Hylemon P, Zhang ZP, Rodriguez-Agudo D, Marques D, Li X, Zhou H, Gil G, Pandak WM. Identification of a novel sulfonated oxysterol, 5-cholesten-3beta,25-diol 3-sulfonate, in hepatocyte nuclei and mitochondria. J Lipid Res 47: 1081–1090, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Ren S, Hylemon PB, Marques D, Gurley E, Bodhan P, Hall E, Redford K, Gil G, Pandak WM. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology 40: 910–917, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Ren S, Li X, Rodriguez-Agudo D, Gil G, Hylemon P, Pandak WM. Sulfated oxysterol, 25HC3S, is a potent regulator of lipid metabolism in human hepatocytes. Biochem Biophys Res Commun 360: 802–808, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 14: 2819–2830, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16: 459–481, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Saucier SE, Kandutsch AA, Taylor FR, Spencer TA, Phirwa S, Gayen AK. Identification of regulatory oxysterols, 24(S),25-epoxycholesterol and 25-hydroxycholesterol, in cultured fibroblasts. J Biol Chem 260: 14571–14579, 1985. [PubMed] [Google Scholar]
- 35.Schroepfer GJ Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev 80: 361–554, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow CP, Baffic J, Lam MH, Lund EG, Adams AD, Fu X, Hayes N, Jones AB, Macnaul KL, Ondeyka J, Singh S, Wang J, Zhou G, Moller DE, Wright SD, Menke JG. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J Biol Chem 277: 10021–10027, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, Schulman IG, Glass CK. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol 23: 5780–5789, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res 95: 780–788, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L, Liao H, Liu Y, Lee TS, Zhu M, Wang X, Stemerman MB, Zhu Y, Shyy JY. Sterol-responsive element-binding protein (SREBP) 2 down-regulates ATP-binding cassette transporter A1 in vascular endothelial cells: a novel role of SREBP in regulating cholesterol metabolism. J Biol Chem 279: 48801–48807, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Gurley EC, Jarujaron S, Ding H, Fang Y, Xu Z, Pandak WM Jr, Hylemon PB. HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am J Physiol Gastrointest Liver Physiol 291: G1071–G1080, 2006. [DOI] [PubMed] [Google Scholar]