Abstract
Insulin resistance is a primary characteristic of type 2 diabetes. Several lines of evidence suggest that accumulation of free fatty acids in skeletal muscle may at least in part contribute to insulin resistance and may be linked to mitochondrial dysfunction, leading to apoptosis. Palmitate treatment of several cell lines in vitro results in apoptosis and inhibits protein kinase B (Akt) activity in response to insulin. However, the role of Bax and Bcl-2 in regulating palmitate-induced apoptosis has not been well studied. Therefore, the purpose of this study was to determine whether palmitate-induced apoptosis in C2C12 myotubes is dependent on Bax to Bcl-2 binding. An additional purpose of this study was to determine whether the changes in Bax to Bcl-2 binding corresponded to decreases in Akt signaling in palmitate-treated myoblasts. Apoptotic signaling proteins were examined in C2C12 myotubes treated overnight with palmitate. Bax to Bcl-2 binding was determined through a coimmunoprecipitation assay that was performed in myotubes after 2 h of serum starvation, followed by 10 min of serum reintroduction. This experiment evaluated whether temporal Akt activity coincided with Bax to Bcl-2 binding. Last, the contribution of Bax to palmitate-induced apoptosis was determined by treatment with Bax siRNA. Palmitate treatment increased apoptosis in C2C12 myotubes as shown by a twofold increase in DNA fragmentation, an approximately fivefold increase in caspase-3 activity, and a 2.5-fold increase in caspase-9 activity. Palmitate treatment significantly reduced Akt protein expression and Akt activity. In addition, there was a fourfold reduction in Bax to Bcl-2 binding with palmitate treatment, which mirrored the reduction in AktSer473 phosphorylation. Furthermore, treatment of the C2C12 myotubes with Bax siRNA attenuated the apoptotic effects of palmitate treatment. These data show that palmitate induces Bax-mediated apoptosis in C2C12 myotubes and that this effect corresponds to reductions in AktSer473 phosphorylation.
Keywords: diabetes mellitus, skeletal muscle cells, free fatty acids, mitochondrial DNA
obesity increases the risk for heart disease, hypertension, and type 2 diabetes mellitus. Increased accumulation of intramuscular lipids without corresponding increases in β-oxidation can lead to insulin resistance, dyslipidemia, and lipotoxicity. Excess lipid accumulation is thought to lead to increased oxidative stress and dysfunction in cardiac muscle (14, 16), although the role in skeletal muscle has not been well studied.
Palmitate is the most abundant systemic saturated fatty acid in the circulation, and therefore, it has been used frequently to examine the effects of saturated fatty acids in dyslipidemia on various tissues (7, 10, 20, 28, 29). Treatment of different cell types with palmitate in vitro has resulted in apoptosis (9, 20, 24) and has been found to inhibit protein kinase B (Akt) activity in response to insulin (6, 7, 25).
Although not previously examined in conjunction with palmitate treatment, one of the primary ways in which Akt inhibits apoptosis is through control of the β-cell leukemia/lymphoma-2 (Bcl-2) family. Akt signaling acts directly and indirectly to promote the binding of the antiapoptotic protein Bcl-2 to the proapoptotic protein Bcl-2-associated X protein (Bax) (13, 15, 30). Once Bax is released from Bcl-2, there is a Bax-Bax oligomerization that inserts into the outer mitochondrial membrane and causes mitochondrial permeabilization (30). Once the mitochondrial membrane is permeabilized there is a release of proapoptotic factors, including apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/Diablo), and cytochrome c (4, 17). When free of the mitochondria, AIF translocates to the nucleus and causes DNA fragmentation independently of caspases. Similarly, when cytochrome c is released from the mitochondria, it activates caspase-9 and subsequently caspase-3, which leads to DNA fragmentation and cell death (4, 18). Last, when released from the mitochondria, Smac/Diablo attenuates the apoptotic inhibitory proteins such as X-linked mammalian inhibitor of apoptosis protein.
Palmitate treatment has been shown to induce apoptosis in L6 myotubes (23), and Bcl-2 overexpression has been demonstrated to inhibit palmitate-induced apoptosis in a nonmuscle cell line (9). However, whether palmitate treatment can attenuate the ability of Bcl-2 to bind Bax has not been examined. Therefore, the purposes of this study were to determine whether palmitate treatment 1) induces apoptotic signaling in mouse C2C12 myotubes, 2) results in the release of mitochondrial apoptotic factors cytochrome c, AIF, and Smac/Diablo, 3) attenuates Bax-to-Bcl-2 binding, and 4) induces apoptosis that is dependent on Bax signaling.
MATERIALS AND METHODS
Cell culture.
C2C12 myoblasts were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in 100-mm polystyrene culture dishes in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% FBS and 1% antibiotic antimycotic mixture and passaged in 0.25% trypsin. The cells were maintained in a humidified incubator under an atmosphere of 5% CO2 at 37°C. For differentiation into myotubes, the myoblasts were grown to confluence and transferred to DMEM containing ITS liquid medium (Sigma-Aldrich, St. Louis, MO). Myotubes were used for experiments following 4 days of differentiation. All experiments were performed in triplicate, with n = 3 for all treatments for each independent replicate.
Palmitate (Sigma Chemical, St. Louis, MO) was administered to cells as described by Chavez and colleagues (6, 7), with dodecanoic acid (laurate) used as a free fatty acid (FFA) control. An additional vehicle-only control group was also included in all experiments. Briefly, palmitate or laurate was dissolved in ethanol and diluted in DMEM containing 2% BSA. C2C12 myotubes were incubated with the respective FFA (0.75 mM final concentration) in 1% FBS-DMEM for 16 h, washed with Hanks' balanced salt solution, and then incubated with the FFA in serum-free DMEM for 2 h. Control cells were treated in the same fashion but without the FFA. One percent FBS was added to the medium 10 min before collection, except where indicated. In addition, a commercially available Akt inhibitor (124005; Calbiochem) was also used as a control for Akt experiments.
siRNA transfection.
The myotubes were transfected with scrambled, nonspecific siRNA, without mammalian homology, or Bax siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) using Lipofectamine 2000 (Invitrogen). Individual siRNAs were transfected using Lipofectamine (80 pmol/transfection) in serum-free DMEM for 6 h. An equal volume of ×2 concentration (2% FBS, 4% BSA, and 1.50 mM FFA) of medium was then added to each well, and the samples were incubated for an additional 16 h, washed with DHANKS, and then incubated with the FFA in serum-free DMEM for 2 h. One percent FBS was reintroduced to the medium 10 min prior to collection, except where indicated.
Cytochrome c assay.
The extent of cytochrome c that was released from the mitochondria was determined in a mitochondrial-free cytoplasmic protein fraction, as described previously (26). C2C12 myotubes were collected after treatment in ice-cold extraction buffer in the presence of a protease inhibitor cocktail (Sigma-Aldrich). Briefly, cells were lysed in ice-cold Mito buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 250 mM sucrose, and 0.1 mM phenylmethylsulfonyl fluoride) by homogenization in a small glass homogenizer with Teflon pestle. The homogenates were centrifuged at 800 g to remove nuclei and cell debris and then at 16,000 g for 20 min at 4°C to pellet the mitochondria. The supernatant contained the mitochondrial-free cytosolic protein fraction, and this was used for determining the release of cytochrome c and Smac/Diablo from the mitochondria.
A cytochrome c ELISA kit (no. 5265; Medical and Biological Laboratories) was used to assess the protein content of cytochrome c in the mitochondria-free cytosolic fraction to evaluate the release of the mitochondrial cytochrome c into the cytosol. The change in color was monitored at a wavelength of 450 nm using a Dynex MRX plate reader. Measurements were performed in duplicate, with all comparisons performed with the same assay. The cytochrome c content was analyzed at OD450 (optical density at 450 nm) and reported normalized to protein concentration.
Nuclear and cytoplasmic protein extracts.
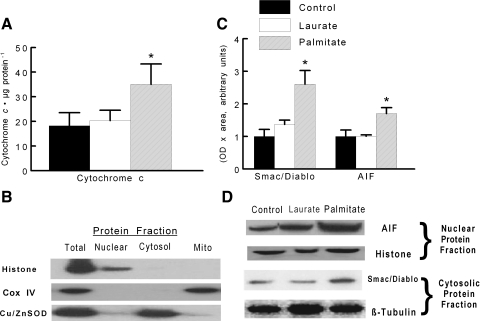
The nuclear and the cytoplasmic protein extracts were obtained using the method described by Rothermel et al. (24) as modified and performed routinely in our laboratory (26). Briefly, the cells were washed three times in phosphate-buffered saline (PBS) and then incubated in lysis buffer (10 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, pH 7.4, 20% glycerol, 0.1% Triton X-100, and 1 mM DTT) for 5 min at 4°C. The cells were gently homogenized in a small glass homogenizer with Teflon pestle. The homogenates were centrifuged at 1,000 g for 1 min at 4°C. The supernatants containing the cytoplasmic protein fraction were collected for subsequent analyses. The remaining nuclear pellet was washed twice in lysis buffer and then resuspended in lysis buffer containing 0.6 M NaCl. The mixture was incubated for 1 h at 4°C and centrifuged at 14,000 g for 15 min at 4°C. The supernatants contained the nuclear protein fraction. The total protein contents of the cytoplasmic extracts were quantified in duplicate by using bicinchoninic acid reagents (Pierce, Rockford, IL) and BSA standards. The purity of the nuclear and cytosolic protein fractions was confirmed through examination of protein expression of histone (nuclear fraction), cytochrome c oxidase IV (mitochondrial fraction), and copper-zinc superoxide dismutase (Cu/ZnSOD; cytosolic fraction), as shown in Fig. 3B.
Fig. 3.
C2C12 myotubes were treated under control, palmitate, or laurate conditions as described in Fig. 1. A: accumulation of cytochrome c in the cytosolic protein fraction as an indicator of cytochrome c release from the mitochondria to the cytosol. B: representative bands demonstrating the purity of the total, mitochondrial, nuclear, and mitochondrial free cytosolic protein fractions. C: quantification of cytosolic second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/Diablo) and nuclear apoptosis-inducing factor (AIF) protein expression. D: representative blots demonstrating approximate equal loading in each of the isolated fractions. The data are displayed as means ± SE. *Experimental samples were different from control- and laurate-treated samples (P < 0.05). COX IV, cytochrome c oxidase IV; Cu/ZnSOD, copper-zinc superoxide dismutase.
A portion of the cytosolic extract (without addition of protease inhibitors) was stored and used for fluorometric caspase activity assays, whereas protease inhibitor cocktail (Sigma-Aldrich) was added to the nuclear and remaining cytosolic portion for further analyses.
Signaling protein levels were determined by immunoblotting. Myotubes were washed three times in PBS, and then 1× SDS sample buffer (50 mM Tris·HCl, pH 6.8, 2% SDS, 6% glycerol, 1% β-mercaptolethanol, 0.02% bromophenol blue) was added directly to the myotubes; 2× SDS lysis buffer was added to an equal volume of total cell lysate, mitochondrial-free cytosolic protein fraction, or nuclear protein fraction, respectively. Samples were lysed with a 25-gauge needle and boiled for 5 min at 100°C. Samples were separated on a 4–12% gradient polyacrylamide gel (Invitrogen). The proteins were blotted to nitrocellulose membranes (Bio-Rad, Hercules, CA) and stained with Ponceau red (Sigma Chemical) to confirm equal loading and transfer among the lanes on the gels.
The membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.05% Tween-20 and then probed with primary antibodies against Akt, Akt1, Akt2, and AktSer473 phosphorylation (Cell Signaling Technology), Bad, Bax, or Bcl-2 (Santa Cruz Biotechnology), and Smac/Diablo and AIF (BD Biosciences). β-Tubulin, Cu/ZnSOD, histone, and cytochrome c oxidase IV (Abcam, Cambridge, MA) were used as loading controls for total lysate, cytosolic, nuclear, and mitochondrial protein fractions, respectively. Secondary antibodies were conjugated to horseradish peroxidase (Chemicon), and the corresponding signals were developed by chemiluminescence (enhanced chemiluminescence advance; Amersham Biosciences) and visualized by exposing the membranes to X-ray films (BioMax MS-1; Eastman Kodak, Rochester, NY). Digital records of the films were captured with a Kodak 290 camera. The resulting bands were quantified as OD × band area by a one-dimensional image analysis system (Eastman Kodak) and expressed normalized to the respective loading control (e.g., β-tubulin, histone, or cytochrome c oxidase IV). The sizes of the proteins were verified by using standard molecular weight markers (Invitrogen).
Fluorometric caspase activity assay.
The activities of caspase-9, -3, and -8 were analyzed by a commercial caspase assay kit (APO-54A-019-KI01; Apotech) according to the manufacturer's procedure and as performed previously in our laboratory (26). Caspase-3 and caspase-9 were measured as indexes of mitochondrial-induced apoptosis, whereas caspase-8 was used as an indicator of nonmitochondrial-dependent caspase signaling.
Briefly, the cytosolic extract without protease inhibitor was incubated in an equal volume of assay buffer (50 mM PIPES, 0.1 mM EDTA, 10% glycerol, 10 mM DTT, pH 7.2), with 100 μM of the fluorogenic 7-amino-4-trifluoromethyl coumarin-conjugated substrate (Alexis, San Diego, CA) at 37°C for 2 h. The change in fluorescence was measured on a spectrofluorometer with an excitation wavelength of 390/20 nm and an emission wavelength of 530/25 nm (CytoFluor; Applied Biosystems, Foster City, CA) before and after the 2-h incubation. Caspase activity was estimated as the change in mean fluorescence intensity expressed as arbitrary units normalized to protein concentration. Measurements were performed in triplicate.
DNA fragmentation and apoptosis.
A cell death detection ELISA kit (Roche Applied Science, Indianapolis, IN) was used to quantitatively determine the apoptotic DNA fragmentation by measuring the cytosolic histone-associated mono- and oligonucleosomes. The change in OD was measured at a wavelength of 405 nm by using a Dynex MRX plate reader controlled through PC software (Revelation; Dynatech Laboratories). Measurements were performed in duplicate, with samples from control and treated samples analyzed at the same time. The OD450 reading was then normalized to control samples. Measurements were performed in triplicate.
As a second approach to confirm the presence of DNA cleavage, which characteristically occurs in apoptotic cells, we identified apoptotic nuclei in myotubes in the presence or absence of an Akt inhibitor. Apoptotic nuclei were examined by using terminal deoxynucleotidyl (TdT)-mediated dUTP nick-end labeling (TUNEL) assay (Roche Applied Science). Myotubes were grown on glass cover slips, fixed in 4% paraformaldehyde in PBS (pH 7.4), and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate. The cells were incubated with TdT and fluorescein-dUTP at 37°C for 1 h. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vectashield mounting medium; Vector Laboratories, Burlingame, CA). Myotubes were counterstained with myosin heavy chain (Developmental Studies Hybridoma Bank, Iowa City, IA). The cells were visualized with an Eclipse E800 fluorescence microscope (Nikon Instruments, Melville, NY).
Bax and Bcl-2 coimmunoprecipitation.
To determine whether Bax-to-Bcl-2 binding corresponded to AktSer473 phosphorylation, myotubes were serum starved for 2 h with or without FFA or the addition of a commercially available Akt inhibitor (124005; Calbiochem); 1% FBS was reintroduced to the medium 10 min before collection of the myotubes. Bax-to-Bcl-2 binding was determined by coimmunoprecipitation of Bax and Bcl-2. Briefly, after treatment, myotubes were harvested in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer (40 mM HEPES, pH 7.5, 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM β-glycerolphosphate, 40 mM NaF, 1.5 mM sodium vanadate, 0.3% CHAPS, 0.1 mM PMSF, 1 mM benzamidine, and 1 mM DTT), rocked for 20 min at 4°C, and then centrifuged at 1,000 g for 10 min at 4°C. An aliquot from the resulting supernatant was measured for total protein. The remaining supernatant was incubated with anti-Bcl-2 (0.2 μg/100 μl; Santa Cruz Biotechnology) overnight at 4°C. Prior to analysis, the immune complexes were collected for 1 h at 4°C with goat anti-mouse BioMag IgG (310004; Qiagen) beads and blocked with 0.1% nonfat dry milk in CHAPS buffer. The beads were collected using a magnetic stand and washed with 200 mM CHAPS and 60 mM HEPES. The precipitates were eluted in 5× SDS sample buffer (250 mM Tris·HCl, pH 6.8, 10% SDS, 30% glycerol, 5% β-mercaptoethanol, and 0.02% bromophenol blue) and then boiled for 5 min. The supernatant was collected by centifugation, and the proteins were separated by SDS-PAGE.
Statistical analyses.
Statistical analyses were performed using the SPSS 10.0 software package. A one-way analysis of variance was performed on the difference in all measured variables. Statistical significance was accepted at P < 0.05. All data are given as means ± SE.
RESULTS
Palmitate attenuated Akt signaling.
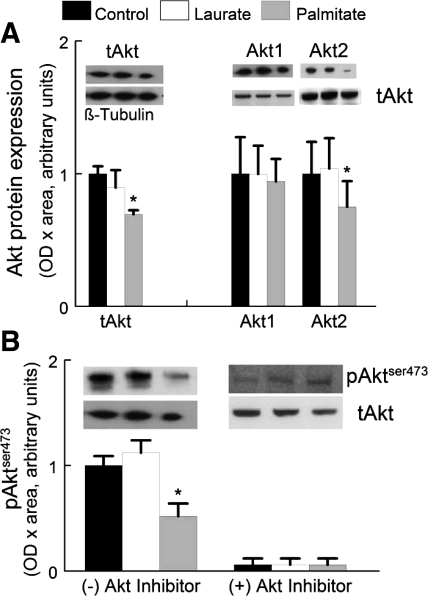
To determine the ability of palmitate pretreatment to inhibit Akt phosphorylation, serum was removed from the medium for 2 h, and then FBS was reintroduced into the medium (final concentration was 1% FBS) for 10 min. Representative blots for total Akt, Akt1, Akt2, and AktSer473 phosphorylation protein expression levels are shown in Fig. 1 for control, laurate, and palmitate treatments. Total Akt protein expression was reduced by ∼30% in palmitate-treated myotubes compared with control-treated myotubes. There was no change in the level of Akt1 protein expression when normalized to total Akt, whereas Akt2 normalized to total Akt was significantly reduced (∼25%, P < 0.05) with a palmitate treatment compared with control- or laurate-treated myotubes. There was a similar ∼41% decrease in AktSer473 phosphorylation in palmitate-treated myotubes (Fig. 1B). Laurate did not result in a decrease in AktSer473 phosphorylation.
Fig. 1.
Palmitate or laurate was dissolved in ethanol and diluted in DMEM containing 2% BSA. Control conditions were identical but without the free fatty acids (FFA). C2C12 myotubes were incubated with or without FFA (0.75 mM final concentration) in 1% FBS-DMEM for 16 h, washed with Hanks' balanced salt solution, and then incubated with the FFA in serum-free DMEM for 2 h; 1% FBS was added to the medium 10 min before collection. The data are presented as optical density (OD) × area expressed in arbitrary units. The graph shows the data expressed as means ± SE. *P < 0.05 vs. control samples. A: total Akt protein expression (tAkt) in C2C12 myotubes was normalized to β-tubulin. Akt1 and Akt2 isoform protein expression were normalized to tAkt protein expression. B: Akt activity as indicated by the protein expression of phosphorylated AktSer473 is shown in the presence or absence of the Akt inhibitor. The data are normalized to tAkt protein expression. The data are presented as OD × area and are expressed in arbitrary units normalized to control values. The graph shows the data expressed as means ± SE. *The experiment data were different from the control data (P < 0.05).
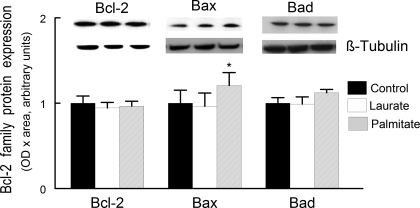
Palmitate increased Bax protein concentration.
Immunoblot analysis was used to determine whether palmitate treatment affected the Bcl-2 family protein expression levels. There was no change in content of the proapoptotic protein Bad, nor was there any change in the concentration in the content of the antiapoptotic protein Bcl-2. However, there was a small increase, ∼20%, in the protein content of the proapoptotic protein Bax (Fig. 2). These data support the hypothesis that palmitate-induced apoptotic signaling may be due to increased Bax-mediated apoptotic signaling.
Fig. 2.
Bcl-2, Bcl-2-associated X protein (Bax), and Bad protein expression were obtained in whole cell lysates prepared from C2C12 myotubes. The conditions for control, palmitate, and laurate treatments were identical to that described in Fig. 1. The data are expressed as OD × area (in arbitrary units) normalized to control samples and displayed as means ± SE. *The experiment data were different from the control data (P < 0.05).
Palmitate mediated release of mitochondrial apoptotic proteins.
The primary means for Bax-regulated apoptotic signaling is via increasing outer mitochondrial membrane permeability. This is followed by the release of proapoptotic factors, including cytochrome c, Smac/Diablo, and AIF, from the mitochondria into the cytosol. The release of these proapoptotic signaling proteins invokes downstream activation of caspase-dependent or caspase-independent signaling, which in turn leads to apoptosis. In these experiments, we wanted to determine whether palmitate regulated apoptosis through altering Bax function and mitochondria permeability. The addition of palmitate to the myotubes increased the cytoplasmic content of cytochrome c twofold compared with control treatments, which is consistent with Bax-mediated apoptotic signaling (Fig. 3A). In addition, palmitate treatment increased the release of Smac/Diablo and AIF from the mitochondria (Fig. 3, C and D). These data demonstrate that mitochondrial proteins are exiting the mitochondria to the cytosolic compartment of the cell, indicating that the mitochondrial membrane is becoming increasingly permeabilized with palmitate treatment.
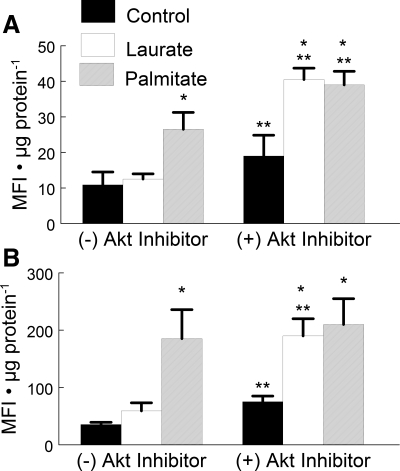
Palmitate increased caspase activity.
Once cytochrome c is released from the mitochondria, it can couple with Apaf-1 to form the apoptosome. The apoptosome cleaves procaspase-9 into its active form, which is followed by subsequent activation of caspase-3 by caspase-9. On the other hand, Akt can stabilize procaspase-9, thus inhibiting cleavage into its active form by the apoptosome (4). Palmitate treatment resulted in a 2.5-fold increase in caspase-9 activity (Fig. 4A) and a fivefold increase in caspase-3 activity (Fig. 4B).
Fig. 4.
The effects of palmitate treatment on caspase-9 and caspase-3 activity. C2C12 myotubes were treated under control, palmitate, or laurate conditions as described in Fig. 1. Concurrent treatment of the samples with 10 μM Akt inhibitor increased the activity of both caspase-9 and caspase-3. The data are expressed as mean fluorescent intensity (MFI) normalized to microgram protein and are displayed as means ± SE. *Experimental vs. control treatment (P < 0.05); **cells treated with the Akt inhibitor were different from cells without Akt inhibitor treatment (P < 0.05).
Akt also phosphorylates and inhibits the cleavage of procaspase-9, thus inhibiting activation by the apoptosome and downstream apoptotic signaling (5). To test whether further inhibition of Akt would exacerbate the palmitate-induced increase in caspase activity, an additional experiment was performed. In this experiment, an Akt inhibitor was added to cells cultured with palmitate. Interestingly, the addition of the Akt inhibitor combined with laurate treatment significantly increased caspase-3 and -9 activities compared with the control treatment to a level that was similar to that of the palmitate-treated myotubes. These data indicate that there may be a combined effect of the FFA treatment and the inhibition of Akt on apoptotic signaling.
Palmitate induced DNA fragmentation.
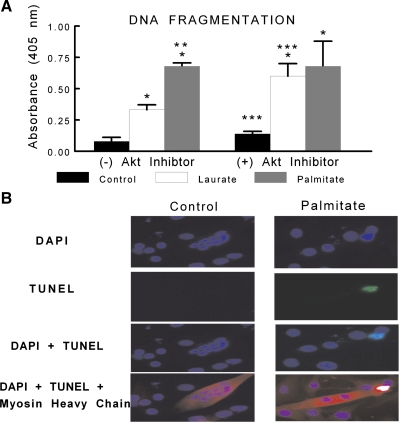
Because caspase-3 is the effector caspase that when activated cleaves DNA, we measured the amount of fragmented DNA in the cytosol of C2C12 myotubes through detection of cytosolic mono- and oligonucleosomes indicating apoptotic cell death. Palmitate treatment induced a twofold increase in DNA fragmentation compared with laurate treatment and a sevenfold increase in DNA fragmentation compared with control (Fig. 5A). However, there was no difference in DNA fragmentation between palmitate- and laurate-treated myotubes that were coincubated with an Akt inhibitor. In addition, to confirm that the myotubes were undergoing apoptosis and not necrosis, TUNEL was performed. The TUNEL data show evidence of apoptotic nuclei in myotubes treated with palmitate (Fig. 5B).
Fig. 5.
C2C12 myotubes were treated under control, palmitate, or laurate conditions as described in Fig. 1. The DNA fragmentation ELISA measures cytosolic mono- and oligonucleosomes and was used as an indicator of apoptotic cell death. A: palmitate treatment significantly increased the content of cytosolic fragmented DNA. In addition, there was a combined effect of the FFA treatment and Akt inhibition, as treatment with laurate and 10 μM Akt inhibitor induced DNA fragmentation similar to that observed in the palmitate-treated myotubes. The data are expressed as OD × area (in arbitrary units) and are displayed as means ± SE. *Experimental vs. control treatment (P < 0.05); **palmitate vs. laurate groups (P < 0.05); ***(+) Akt inhibitor vs. (−) Akt inhibitor (P < 0.05). B: representative terminal deoxynucleotidyl-mediated dUTP nick-end labeling (TUNEL) staining (green) of control- and palmitate-treated myotubes, as described in Fig. 1, colabeled with 4′,6-diamidino-2-phenylindole (DAPI; blue) and myosin heavy chain (red).
Palmitate induced Bax signaling.
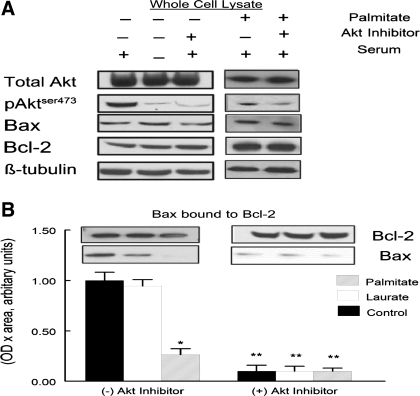
The relationship of Akt activity and Bax-to-Bcl-2 binding was measured to determine whether Bax signaling was a possible mechanism for palmitate-induced apoptosis. C2C12 myotubes were incubated for 10 min with or without 1% FBS serum following 2 h of incubation in serum-free medium. In addition, in some experiments the Akt inhibitor was added to the cells to confirm Akt-specific regulation of Bax-to-Bcl-2 binding (data not shown). In control samples, FBS in the absence of the Akt inhibitor increased the amount of AktSer473 phosphorylation (Fig. 6A). Treatment of samples with palmitate or the Akt inhibitor greatly attenuated the Bax-to-Bcl-2 binding (Fig. 6B).
Fig. 6.
A: protein expression of Akt, Bax, Bcl-2, pAktser473, and β-tubulin in total lysates of C2C12 myotubes. The C2C12 myotubes were cultured in the presence or absence of palmitate, Akt inhibitor, or serum. B: C2C12 myotubes were treated under control, palmitate, or laurate conditions as described in Fig. 1. Palmitate treatment attenuated the expression of Bax protein found in the Bcl-2 coimmunoprecipitate. The data are expressed as OD × area (in arbitrary units) and normalized to control protein bands. The data are shown as means ± SE. *Experimental vs. control treatment (P < 0.05); **(+) Akt inhibitor vs. (−) Akt inhibitor (P < 0.05).
Bax siRNA attenuated palmitate-mediated apoptosis.
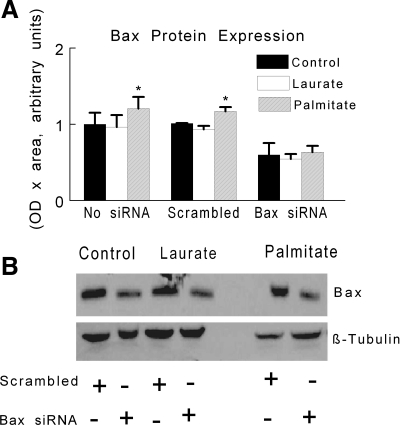
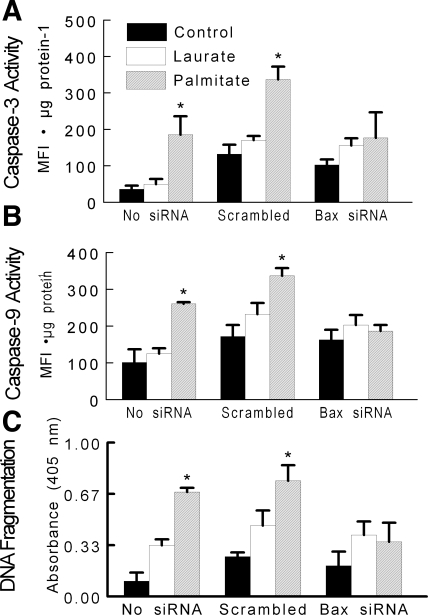
As expected, treatment with Bax siRNA reduced Bax protein content in both control- and palmitate-treated myotubes (Fig. 7A); representative Western blots for these experiments are shown in Fig. 7B. The reduction in Bax protein expression with siRNA attenuated the increase in DNA fragmentation and caspase activity that otherwise occurred after palmitate treatment (Fig. 8, A–C). These data further support the hypothesis that palmitate-mediated apoptotic signaling is dependent on the apoptotic actions of the Bax protein.
Fig. 7.
C2C12 myotubes were treated under control, palmitate, or laurate conditions as described in Fig. 1. A: Bax protein expression was determined after treatment with Bax siRNA or a scrambled siRNA and with or without coincubation of FFA. The data are expressed as OD × area (in arbitrary units) and are displayed as means ± SE. *Experimental vs. control treatment (P < 0.05). B: representative Western blots of Bax (top) and β-tubulin (bottom) are shown with or without siRNA treatment.
Fig. 8.
Apoptotic signaling was assessed in C2C12 myotubes under control conditions or after palmitate or laurate treatments as described in Fig. 1. A: caspase-3 activity. B: caspase-9 activity. C: DNA fragmentation. The data are displayed as means ± SE. *Data representing scrambled siRNA were different from Bax siRNA in the same treatment (P < 0.05).
DISCUSSION
This study confirms two previous separate findings that palmitate treatment can both induce apoptosis and decrease Akt activation in vitro (7, 23). The novel findings of this study are that 1) palmitate treatment inhibits Bax-to-Bcl-2 binding in C2C12 myotubes, 2) knockdown of Bax protein significantly attenuates the ability of palmitate treatment to induce apoptosis in C2C12 myotubes, and 3) there is an additive effect of the free fatty acid treatment and Akt inhibition that induced apoptosis, as indicated by the combined effect of laurate and the Akt inhibitor. These findings are important for understanding the putative effects of free fatty acids on apoptotic signaling in skeletal muscle, because loss of skeletal muscle protein is a serious complication of diabetes and can contribute substantially to patients' morbidity.
Pretreatment of C2C12 myotubes with palmitate has been used to mimic aberrant lipid signaling, which is one particular aspect common with metabolic syndrome and type 2 diabetes (6, 7, 9, 23, 25). Metabolism of palmitate and other long-chain saturated fatty acids results in increased formation of metabolites such as diacylglycerol and ceramide. These two metabolites of aberrant fatty acid metabolism are thought to be responsible for the inhibition of Akt activity (6, 7, 19). We speculate that, in C2C12 myotubes, one or both of these metabolites were elevated after palmitate treatment, which decreased Akt activity and increased apoptotic signaling either directly or indirectly through Bax. This speculation is supported by our control data in cells treated with laurate. Laurate is a shorter-chain fatty acid than palmitate and does not result in the formation of either diacylglycerol or ceramide (7, 8). Furthermore, in our study, laurate-treated cells did not show an inhibition of Akt activity and had only a mild increase in apoptosis (Fig. 5). Together, our data support the hypothesis that palmitate-induced increases in diacylglycerol and/or ceramide mediate inhibition of Akt activity, leading to increased signaling for apoptosis in C2C12 cells.
Because Akt signaling is disrupted with metabolic syndrome and has a major role in inhibiting apoptosis (11, 12, 15), we hypothesized that palmitate would decrease Akt phosphorylation and activate mitochondrial-associated apoptotic signaling in mouse C2C12 myotubes. Specifically, we hypothesized that palmitate would increase mitochondrial-associated apoptosis through attenuation of Bax-to-Bcl-2 binding.
It has been well documented that skeletal muscle apoptosis is an integral part of skeletal muscle loss with aging (1, 27). However, the role of skeletal muscle apoptosis in metabolic syndrome has not been examined. Palmitate treatment has recently been shown to induce mitochondrial DNA damage and apoptosis in L6 myotubes (23). Our study extends the findings of Rachek et al. (23) by showing that mitochondrial-associated Bax signaling was a major component of palmitate-induced apoptosis. Furthermore, our data demonstrate that there may be a combined effect of the saturated free fatty acid and the Akt inhibition, which contributes to increased apoptotic signaling. Treatment of the myotubes with the saturated free fatty acid laurate, which does not inhibit Akt activity, had limited effect on apoptotic signaling. However, when coincubated with an Akt inhibitor there was a significant increase in apoptosis similar to that found in palmitate-only-treated myotubes, as indicated by caspase activation and DNA fragmentation. These data emphasize the need for the use of short-chain fatty acids (i.e., laurate) as well as longer-chain fatty acids (i.e., palmitate) when fatty acid-induced apoptotic signaling is studied.
Mitochondrial-associated apoptosis in C2C12 cells.
When the mitochondrial outer membrane is permeabilized, it releases proapoptotic factors such as AIF, Smac/Diablo, and cytochrome c, each of which can independently increase apoptosis (4, 17). When released from the mitochondria, AIF translocates to the nucleus, where it can directly cleave DNA. Smac/Diablo binds and prevents the actions of a number of apoptosis inhibitory proteins. In contrast, cytochrome c forms a complex with Apaf-1 and procaspase-9 to form the apoptosome. The apoptosome cleaves procaspase-9 into its active form. Caspase-9 then activates caspase-3, an apoptotic effector that leads to DNA fragmentation and cell death (4, 18). The results of the present study verify that palmitate treatment increases nuclear apoptosis in C2C12 cells. This was confirmed through increases in nuclear levels of AIF, increased cytoplasmic levels of cytochrome c and Smac/Diablo, elevated caspase-9 and -3 activity levels, and finally, increased DNA fragmentation (Figs. 3–5). The reason for increased DNA fragmentation with laurate treatment compared with controls is unknown, but it is possible that there was an initial increase in the release of apoptotic signaling proteins from the mitochondria that were mitigated within the time course examined in this study. In addition, it is probable that treatment with any saturated free fatty acid increases the mitochondrial permeability and induces small increases in caspase activity, but the actions of Akt limit this apoptotic effect. However, when the saturated free fatty acid also inhibits Akt activity, there is an unmitigated effect on apoptotic signaling.
Bax-Bcl-2 interactions in palmitate treatment.
Once Bax is released from Bcl-2, there is a Bax-Bax oligomerization. This Bax-Bax protein complex then inserts into the outer mitochondrial membrane, causing mitochondrial permeabilization and release of proapoptotic factors AIF, Smac/Diablo, and cytochrome c (30). The release of these proteins from the mitochondria results in downstream DNA fragmentation and loss of the nucleus (4, 18). It is unlikely that loss of only a few nuclei will result in elimination of the entire myotube, but loss of sufficient numbers of nuclei in a single multinucleated cell would be expected to result in cell death.
The primary novel finding of this study was that palmitate treatment attenuated the ability of growth factor-rich serum (FBS) to increase Bax-to-Bcl-2 binding (Fig. 6B). When growth factors were removed from the culture medium, Bax was dissociated from Bcl-2, but this was quickly reversed (∼10 min posttreatment) when growth factor-rich serum was reintroduced to the culture medium (Fig. 6B). The pattern of Bax and Bcl-2 during palmitate treatment coincides with the amount of Akt phosphorylation. In addition, Bax-to-Bcl-2 binding was prevented when an Akt inhibitor was added to the medium. This supports the idea that Akt acts directly on Bax to increase its binding to Bcl-2 (8). Furthermore, the role of Bax signaling in palmitate-mediated apoptosis was confirmed through the use of siRNA, as there was a significant attenuation of the palmitate-mediated apoptosis with decrease in Bax protein expression.
Metabolic syndrome is the most prevalent metabolic health risk, affecting more than 47 million Americans (21). Skeletal muscle is an important target tissue in the study of metabolic syndrome because it accounts for more than one-half of total body insulin resistance and is also a key storage tissue for excess lipids (2, 3, 22). Therefore, the loss of skeletal muscle can further exacerbate the risks associated with metabolic syndrome. In this study, C2C12 myotubes were used as an in vitro model of skeletal muscle and palmitate was used to mimic one aspect common in metabolic syndrome, dyslipidemia. This study demonstrated that long-chain saturated fatty acids (i.e., palmitate) can reduce Akt signaling and increase Bax-mediated mitochondrial-induced apoptosis. These data suggest that free fatty acid-induced apoptotic signaling may be a critical factor in regulating skeletal muscle atrophy. Therefore, novel strategies that are designed to eliminate or reduce mitochondrial-associated apoptotic signaling and stabilize Bax-Bcl-2 dimers may provide significant therapeutic benefits for the treatment of complications associated with type 2 diabetes and insulin resistance.
GRANTS
This research was supported by National Institute on Aging Grant R01-AG-021530 to S. E. Alway.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol 283: C66–C76, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 49: 272–283, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bjornholm M, Zierath JR. Insulin signal transduction in human skeletal muscle: identifying the defects in Type II diabetes. Biochem Soc Trans 33: 354–357, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51: 1938–1948, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318–1321, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278: 10297–10303, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419: 101–109, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241, 1997. [DOI] [PubMed] [Google Scholar]
- 9.de Pablo MA, Susin SA, Jacotot E, Larochette N, Costantini P, Ravagnan L, Zamzami N, Kroemer G. Palmitate induces apoptosis via a direct effect on mitochondria. Apoptosis 4: 81–87, 1999. [DOI] [PubMed] [Google Scholar]
- 10.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38: 1384–1394, 1997. [PubMed] [Google Scholar]
- 11.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Downward J PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 15: 177–182, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta 1757: 1301–1311, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, Rochette L. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem 283: 147–152, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279: 21085–21095, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Rodrigues B. Cardiac cell death in early diabetes and its modulation by dietary fatty acids. Biochim Biophys Acta 1761: 1148–1162, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol 1–9, 2005. [DOI] [PubMed]
- 18.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Merrill AH Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta 1044: 1–12, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Mishra R, Simonson MS. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol 4: 2, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Bethesda, MD: National Institutes of Health, 2005.
- 22.Olsen DB, Sacchetti M, Dela F, Ploug T, Saltin B. Glucose clearance is higher in arm than leg muscle in type 2 diabetes. J Physiol 565: 555–562, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology 148: 293–299, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem 275: 8719–8725, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565: 309–323, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288: C338–C349, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Staiger K, Staiger H, Weigert C, Haas C, Haring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes 55: 3121–3126, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Welters HJ, Tadayyon M, Scarpello JH, Smith SA, Morgan NG. Mono-unsaturated fatty acids protect against beta-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett 560: 103–108, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 271: 7440–7444, 1996. [DOI] [PubMed] [Google Scholar]