Abstract
Glucose-stimulated insulin secretion (GSIS) is central to normal control of metabolic fuel homeostasis, and its impairment is a key element of β-cell failure in type 2 diabetes. Glucose exerts its effects on insulin secretion via its metabolism in β-cells to generate stimulus/secretion coupling factors, including a rise in the ATP/ADP ratio, which serves to suppress ATP-sensitive K+ (KATP) channels and activate voltage-gated Ca2+ channels, leading to stimulation of insulin granule exocytosis. Whereas this KATP channel-dependent mechanism of GSIS has been broadly accepted for more than 30 years, it has become increasingly apparent that it does not fully describe the effects of glucose on insulin secretion. More recent studies have demonstrated an important role for cyclic pathways of pyruvate metabolism in control of insulin secretion. Three cycles occur in islet β-cells: the pyruvate/malate, pyruvate/citrate, and pyruvate/isocitrate cycles. This review discusses recent work on the role of each of these pathways in control of insulin secretion and builds a case for the particular relevance of byproducts of the pyruvate/isocitrate cycle, NADPH and α-ketoglutarate, in control of GSIS.
metabolic fuel homeostasis is largely controlled by the balance between the anabolic hormone insulin and the catabolic hormone glucagon, both produced by the pancreatic islets of Langerhans. Insulin secretion from islet β-cells is regulated by a number of factors, but the predominant stimulatory signal is the rise in blood glucose that occurs with the ingestion of carbohydrate-containing meals. Glucose not only acts to stimulate insulin secretion from β-cells directly; it enables the potentiating functions of other effectors, including free fatty acids, amino acids, and incretin hormones such as glucagon-like peptide-1, all of which require a threshold level of glucose (generally ≥6 mM) to exert their effects (54). This enabling effect of glucose prevents secretion of insulin under fasting conditions, thereby helping to protect against hypoglycemia. Conversely, robust glucose-stimulated insulin secretion (GSIS) in postprandial periods prevents hyperglycemia. Importantly, impairment of insulin-secretory capacity and loss of normal GSIS underlie the transition from obese and insulin-resistant states to full-blown type 2 diabetes (57).
Surprisingly, despite the central role of insulin secretion in regulation of metabolic fuel homeostasis and the critical impact of loss of normal β-cell function in the development of type 2 diabetes, the fundamental biochemical mechanisms involved in GSIS are still incompletely understood. It has been appreciated for many years that the effect of glucose to stimulate insulin secretion from β-cells is not mediated by a cell surface glucose receptor but rather requires metabolism of the sugar to generate second messengers that trigger exocytosis of secretory granules (22, 51, 54, 55). Nevertheless, the precise metabolic pathways involved, and the attendant second messengers that they generate remain incompletely understood. The purpose of the present chapter is to review progress in this area of central importance in the realm of diabetes and obesity research.
Evidence for KATP Channel-Dependent and -Independent Pathways of GSIS
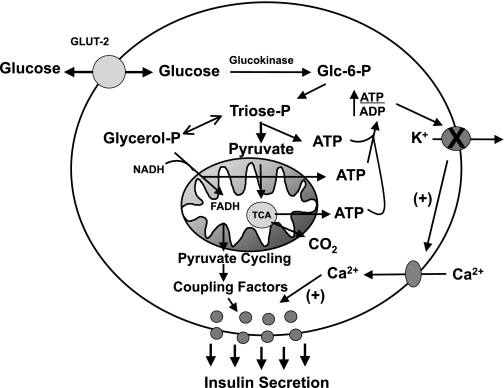
For over 30 years, one particular model of glucose stimulus/secretion coupling has gained wide acceptance. In this construct, the rise in blood glucose induces an increase in β-cell glucose metabolism, resulting in increased production of ATP from several sources: glycolysis, mitochondrial glucose oxidation, and active shuttling of reducing equivalents from the cytosol to the mitochondrial electron transport chain (Fig. 1). The resultant increase in ATP/ADP ratio inhibits ATP-sensitive K+ (KATP) channels, resulting in plasma membrane depolarization, activation of voltage-gated Ca2+ channels, and influx of extracellular Ca2+, which serves to activate granule exocytosis (12, 22, 51, 54, 55, 57). The expression of the molecular components of this signaling network in β-cells and their regulation during glucose stimulation have been extensively documented (reviewed in Ref. 12). However, an expanding body of data also makes it apparent that this KATP channel-dependent mechanism of GSIS does not fully describe the islet glucose response, and signals other than changes in ATP/ADP ratio have been increasingly implicated as important regulators of insulin secretion in recent years, as will be a focus of this article.
Fig. 1.
Overview of ATP-sensitive K+ (KATP) channel-dependent and -independent pathways of glucose-stimulated insulin secretion (GSIS). The canonical model of GSIS holds that an increase in β-cell glucose metabolism leads to production of ATP via glycolysis, pyruvate oxidation, and reducing equivalent shuttles, resulting in an increase in ATP/ADP ratio. This leads to suppression of KATP channels, membrane depolarization, and activation of voltage-gated Ca2+ channels and Ca2+-mediated activation of insulin granule exocytosis. However, multiple lines of evidence reviewed in this article support the idea that pyruvate cycling generates coupling factors in addition to ATP/ADP ratio changes that are essential for the full glucose response, as detailed in the text and in subsequent figures.
The total number of insulin-containing granules present in islet β-cells far exceeds the number secreted during a meal, but only a relatively small proportion (∼10%) of these granules are actually docked at the plasma membrane of β-cells in a “readily releasable” form (72). The KATP channel-dependent mechanism appears to be particularly important in triggering exocytosis of a small number of granules from the readily releasable pool that are responsible for the first, acute phase of insulin release occurring in the first 10 min following glucose stimulation (4, 15, 72). In contrast, in the second and sustained phase of insulin secretion, regulation of KATP channels is required as an initiating event but does not seem to explain the amplification of insulin secretion that occurs beginning at about 15 min after initial glucose stimulation. In fact, amplifying signals have been estimated to contribute as much as 70% of the total GSIS response (22). Second-phase insulin secretion involves both docked, readily releasable granules and newly recruited granules from the intracellular storage pool.
Key pieces of evidence in support of KATP channel-independent pathways of GSIS include studies showing that exposure of islets to the combination of a depolarizing K+ concentration (30 mM) and the KATP channel opener diazoxide, treatments that serve to completely bypass glucose regulation of this channel, still allow very significant increases in insulin secretion in response to increments of glucose in the physiological range (20, 34). Identification of the regulatory (sulfonylurea receptor SUR1) and inward rectifying K+ channel (Kir6.2) subunits of the KATP channel complex led to characterization of mutations in these proteins in humans that influence insulin secretion and glycemic control (1, 74). However, studies of KATP channel knockout mice revealed a surprising retention of regulated insulin secretion and control of glucose homeostasis in animals lacking KATP channel function. For example, SUR1 knockout mice have near-normal control of glucose homeostasis (69). Moreover, another group has reported that, if exposed to a period of culture at low glucose, islets from SUR1 knockout mice exhibit a sixfold increase in insulin secretion in response to 15 mM glucose and also have a sustained and large increase in intracellular Ca2+ levels following a transient drop under these conditions (53, 73). Moreover, mice with homozygous knockout of Kir6.2 have reduced (although not absent) GSIS, whereas heterozygous (Kir6.2+/−) mice actually have increased glucose responsiveness (61). All of the foregoing findings have focused renewed attention on ATP-independent events in control of fuel-regulated insulin secretion.
Evidence for Involvement of Mitochondrial Metabolic Cycles in Control of GSIS
If ATP/ADP ratio and KATP channel regulation are not the major factors controlling the amplification phase of GSIS, what are the alternative signals? This is an important question, since it is reasonable to assume that knowledge of the relevant agents will be helpful for understanding the functional failure of the β-cell in diabetes and development of new drugs for correcting this problem. Recent studies in this area have focused on mitochondrial pathways of glucose metabolism, and the generation of second messengers other than ATP that such pathways might generate. Islet β-cells express both pyruvate carboxylase (PC) and pyruvate dehydrogenase (PDH) in abundance, such that, in the nutritionally replete or fed state, pyruvate flows into mitochondrial metabolic pathways in roughly equal proportions through the anaplerotic (PC) and oxidative (PDH) entry points (32, 37, 38, 68). In hepatocytes, a major purpose of PC is to partner with phosphoenolpyruvate carboxykinase (PEPCK) to initiate gluconeogenesis. In contrast, β-cells lack PEPCK expression (46) and have relatively low levels of fatty acid synthase expression and rates of lipogenesis (11). This suggests that, in β-cells, active PC-mediated anaplerosis could subserve a signaling function rather than a primary role of contributing to gluconeogenesis or lipogenesis.
An important clue to the role of anaplerotic metabolism of pyruvate in β-cells came with the discovery that these cells express enzymes that allow “cycling” of pyruvate via its PC-catalyzed conversion to oxaloacetate, metabolism of oxaloacetate to malate, citrate, or isocitrate in the TCA cycle, and subsequent reconversion of these metabolites to pyruvate and various TCA cycle intermediates via several possible combinations of cytosolic and mitochondrial pathways (37, 40). There are at least three major pathways that can be considered, and each of these will be discussed in more detail in the following sections. Starting from oxaloacetate generated from pyruvate in the PC reaction, a brief description of each cycle is as follows. The first, which we will term the “pyruvate/malate” pathway, involves the conversion of oxaloacetate to malate by mitochondrial malate dehydrogenase, the export of malate to the cytosol by the dicarboxylate (malate) carrier (DIC), and conversion of malate to pyruvate by cytosolic malic enzyme (MEc). The second, termed the “pyvuate/citrate” pathway, involves the conversion of oxaloacetate to citrate, export of citrate to the cytosol via the mitochondrial citrate/isocitrate carrier (CIC), cleavage of citrate to oxaloacetate and acetyl-CoA by ATP-citrate lyase (CL), and recycling of citrate to pyruvate via malate and MEc. The other product of the CL reaction, acetyl-CoA, is converted to malonyl-CoA by acetyl-CoA carboxylase-1 (ACC-1), and malonyl-CoA can be used for lipogenesis via fatty acid synthase (FAS). The last potential cycle is known as the “pyruvate/isocitrate cycle”, and involves export of citrate and isocitrate from the mitochondria to the cytosol via CIC as in the pyruvate/citrate cycle, but now isocitrate (or citrate) is converted to α-ketoglutarate (α-KG) by a cytosolic NADP-dependent isocitrate dehydrogenase (ICDc) and recycled to oxaloacetate or pyruvate by one of several possible cytosolic or mitochondrial pathways. The existence of these pathways in β-cells suggests a means for generating second messengers other than a change in ATP/ADP ratio that could contribute to regulation of insulin secretion (Fig. 1).
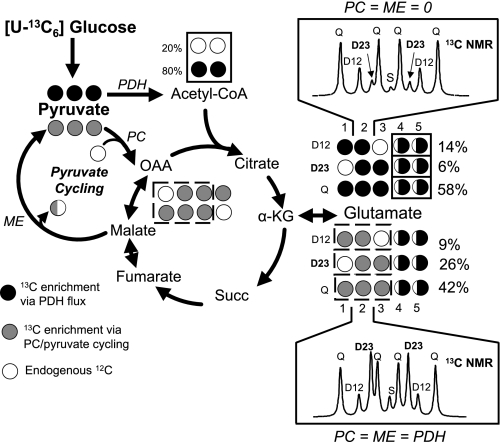
Evidence linking pyruvate cycling to control of GSIS has accumulated in recent years (8, 14, 29, 37). Initial studies were facilitated by creation of a set of clonal cell lines derived from rat insulinoma INS-1 cells (3, 23). These INS-1-derived subclones were discovered to have a range of capacities for GSIS, with responses ranging from less than twofold to more than 10-fold, including two highly glucose-responsive and stable clones, 832/13 and 832/3, which will be referred to throughout the rest of this article (23). Metabolic fates of pyruvate were analyzed in these lines via 13C-NMR-based mass isotopomer analysis (37). This method relies on stimulation of insulin-secreting cells with [U-13C]glucose for periods sufficient to allow isotopic equilibration (generally 2–3 h), followed by extraction of cells and visualization of the 13C distribution in cellular glutamate by NMR. The spectra of each of the glutamate carbons exhibit unique features (13C spin coupling multiplets) that reflect the distribution of 13C in the glutamate molecule (Fig. 2). Since glutamate is derived from α-KG in the TCA cycle, the 13C distribution is determined by the relative flux of glucose into the TCA cycle via oxidative, PDH-catalyzed metabolism of [U-13C]pyruvate vs. PC-catalyzed anaplerotic metabolism of pyruvate. To the extent that PC-catalyzed anaplerotic metabolism of pyruvate is active, this will result in carboxylation of labeled pyruvate with natural abundance 12C, resulting in a 13C labeling pattern of glutamate distinct from that obtained via PDH-catalyzed pyruvate oxidation alone. The glutamate spectra obtained from β-cells stimulated with [U-13C]glucose are used to calculate relative rates of flux through PDH and PC, thereby serving as a measure of pyruvate cycling activity (8, 28, 37).
Fig. 2.
Schematic summary of 13C-NMR technique for measurement of pyruvate cycling. The technique involves incubation of β-cell lines with [U-13C6]glucose, extraction of glutamate and analysis of 13C isotopomers by 13C-NMR spectroscopy. Shown for example are spectra of carbon-2 of glutamate simulated using tcaSim (8, 28, 37) under two idealized situations. Top: the spectrum obtained if pyruvate dehydrogenase (PDH) is the sole pathway for pyruvate to enrich α-ketoglutarate (α-KG) and glutamate (20% unlabeled endogenous substrate is also assumed). Bottom: the spectrum obtained when PDH and pyruvate carboxylase (PC) flux contribute equally to pyruvate entry into the TCA cycle. Several changes in the glutamate isotopomer populations occur as result of PC activity. For example, notice that carboxylation of pyruvate by PC leads to the loss of enrichment in the C1 position and a dramatic increase in the isotopomer population detected as a D23 multiplet. In experimental data, the isotopomer populations determined by the 13C-NMR multiplets of all glutamate carbons are used to calculate relative flux through the oxidative (PDH) and anaplerotic (PC) entry points into the TCA cycle. It is assumed that pyruvate cycling is equivalent to PC flux. See text and refs. 8, 28, and 37 for more details.
Using these methods, we found no differences in PDH-mediated entry of glucose-derived pyruvate into the TCA cycle in our INS-1-derived cell lines with varying capacities for GSIS. In contrast, the varying capacities for GSIS in the different INS-1-derived lines was found to be tightly correlated with PC-catalyzed anaplerotic influx into the TCA cycle and pyruvate cycling (37). Consistent with these findings, pharmacologic inhibition of PC with phenylacetic acid (PAA) caused impairment of GSIS in cell lines and rodent islets (18, 19, 37) and suppressed pyruvate cycling activity as measured by 13C-NMR (37), whereas addition of a membrane-permeant analog of malate, dimethylmalate (DMM), caused an increase in pyruvate cycling activity and a proportional enhancement of GSIS (37). Thus, three key pieces of evidence link pyruvate cycling to GSIS: 1) the strong correlation of GSIS with PC-catalyzed pyruvate cycling, but not PDH-catalyzed glucose oxidation, in variously glucose responsive INS-1-derived cell lines; 2) the inhibition of GSIS in proportion to inhibition of pyruvate cycling by the PC inhibitor PAA; and 3) The proportional stimulation of GSIS and pyruvate cycling activity by the pyruvate cycling intermediate DMM.
On the basis of these findings, we were surprised to find subsequently that suppression of PC in 832/13 cells or rat islets by a molecular approach [small interfering RNA (siRNA)], rather than the pharmacological agent PAA, did not impair GSIS (29). However, suppression of PC protein levels by 60% in 832/13 cells with an adenovirus containing a PC-specific siRNA (Ad-siPC) resulted in only a 20% decrease in PC flux, suggesting an increase in PC specific activity. Acetylcarnitine, a surrogate for acetyl-CoA, an allosteric activator of PC, was clearly increased in Ad-siPC-treated cells, suggesting a mechanism by which PC enzymatic activity is maintained despite suppressed PC protein levels. In addition, the NADPH/NADP ratio, a proposed coupling factor for GSIS (see Identification of Coupling Factors Generated by Pyruvate Cycling and Their Potential Molecular Targets), was unaffected in Ad-siPC-treated cells. We concluded that β-cells activate compensatory mechanisms in response to molecular suppression of PC expression that prevent impairment of anaplerosis, pyruvate cycling, NAPDH production, and GSIS (29). Why, then, does pharmacological suppression of PC impair GSIS while molecular suppression does not? The likely explanation is that the CoA form of PAA impairs the allosteric activation of PC by acetyl-CoA, thereby interfering with a key step in compensation against PC suppression (6, 29). Taken together, all of the foregoing data provide strong support for a key role of PC-catalyzed pyruvate cycling in control of GSIS.
Evidence for Impaired Pyruvate Cycling in Models of β-Cell Dysfunction
There is also evidence to suggest that regulation of pyruvate cycling is perturbed in models of islet cell dysfunction and type 2 diabetes. For example, PC enzymatic activity is increased in islets from insulin-resistant, prediabetic Zucker diabetic fatty (ZDF) rats, suggesting that pyruvate cycling might be increased in concert with the enhanced insulin secretion that offsets insulin resistance in these animals (36). The foregoing studies were based on static measurement of enzyme activities and concentrations of selected metabolic intermediates and therefore did not report on changes in metabolic flux. To address this issue, a recent study employed 13C-NMR to obtain direct measurements of flux through the relevant metabolic pathways in β-cells exposed to elevated fatty acids, a treatment that causes impairment of GSIS (8). Interestingly, this report revealed that exposure of robustly glucose-responsive INS-1-derived 832/13 cells to 1 mM oleate-palmitate (2:1) for 72 h caused an increase in pyruvate cycling activity at basal glucose that paralleled an increase in basal insulin release. Moreover, the increase in basal cycling activity eliminated the normal glucose-induced increment in pyruvate cycling flux, thereby possibly explaining the impairment in insulin secretion observed at stimulatory glucose concentrations in lipid-cultured cells. The mechanism proposed to explain these effects (8) incorporates the fact that chronic lipid culture causes upregulation of β-oxidative enzymes in β-cells (5). This in turn could result in constitutive activation of PC by its known allosteric activator acetyl-CoA, which rises as a consequence of upregulated FA oxidation in lipid-cultured cells (8). This constitutive activation of PC could explain the high rates of pyruvate cycling in lipid-cultured cells at basal glucose, and the failure of stimulatory glucose to elicit a further increase in pyruvate cycling activity.
Further evidence for the potential importance of dysregulated pyruvate cycling in mediating lipid-induced β-cell failure comes from studies with the previously mentioned membrane-permeant ester of malate, DMM. Malate is an intermediate common to all of the potential pyruvate cycling pathways, and addition of DMM to β-cell lines and rat islets potentiates GSIS and increases pyruvate cycling activity (37). Additionally, inclusion of DMM during insulin secretion assays performed on glucose-unresponsive islets from ZDF rats or 832/13 cells with impaired GSIS due to chronic lipid exposure results in a remarkable improvement of GSIS in both cases (8). Overall, recent studies provide strong support for the idea that FA-induced impairment of GSIS is caused, at least in part, by perturbation of normal regulation of pyruvate cycling by glucose.
Studies on the Role of Specific Pyruvate Cycling Pathways in Control of GSIS
More recent studies have focused on identification of the specific pyruvate cycling pathways that may be involved in generation of signals for insulin secretion. There are at least three major pathways that can be considered, and each of these will be discussed in more detail in the following sections. To investigate this important issue, our group has employed an interdisciplinary approach involving metabolic flux analysis by 13C-NMR, targeted metabolic profiling of intermediary metabolites by GC-MS and MS-MS, and modulation of specific metabolic pathways by genetic engineering, using adenovirus vectors to confirm our findings in primary islet preparations. The remainder of this review will summarize and discuss our findings and those of other laboratories in this area.
The Pyruvate-Malate Cycle
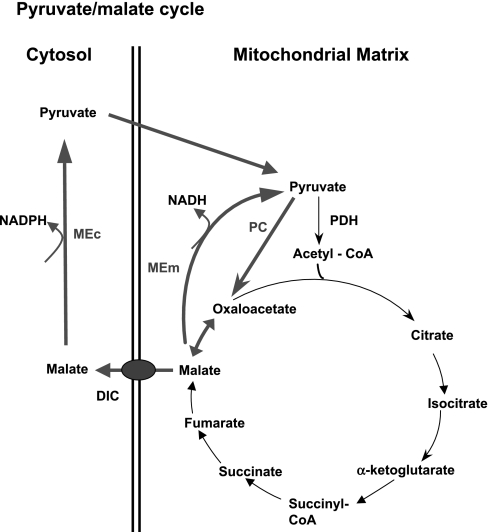
In the pyruvate-malate cycle, oxaloacetate (OAA) is reduced to malate by mitochondrial malate dehydrogenase and then converted back to pyruvate, either by a cytosolic or a mitochondrial form of malic enzyme (Fig. 3). Support for a potential role of malate in insulin secretion comes from studies showing that glucose stimulation of clonal β-cells (18, 29) or isolated rodent islets (39) or pyruvate stimulation of isolated islet mitochondria (38) increases malate levels. Also, increased insulin secretion in response to obesity in Zucker fatty rats (fa/fa) has been shown to correlate with increased activities of PC, malate dehydrogenase, and ME, as well as increases in malate levels in islet cells (36). However, other metabolites such as citrate also increase in response to glucose in β-cells (18, 29). Thus, these earlier studies are consistent with a potential role of malate production in control of GSIS but are correlative and therefore not definitive.
Fig. 3.
Pyruvate/malate cycle. In this cycle, pyruvate enters the TCA cycle via conversion to oxaloacetate by the anaplerotic enzyme PC. To exit the mitochondria, oxaloacetate is converted to malate. Malate can then either be recycled to pyruvate via the mitochondrial, NAD-dependent form of malic enzyme (MEm) or can be transported to the cytosol via the dicarboxylate carrier (DIC). If transported to the cytosol, malate can be reconverted to pyruvate by the cytosolic, NADP-dependent form of ME (MEc).
A recent study showed that short interfering (si)RNA-mediated suppression of the cytosolic, NADP-dependent form of malic enzyme (MEc) in the INS-1-derived 832/13 cell line resulted in ∼90% suppression of MEc mRNA levels, accompanied by an ∼40% reduction of insulin secretion in response to stimulatory glucose or the amino acids leucine and glutamine (58). Similar observations were made in the same cell line in a second, independent study (21). In contract, a modest siRNA-induced reduction in mitochondrial, NAD-dependent molic enzyme (MEm) levels did not affect GSIS but did decrease amino acid-stimulated insulin secretion at basal glucose concentrations (58).
A limitation of both of the foregoing studies involving molecular manipulations of ME expression is that the findings were not confirmed in primary islets, and the biological relevance of the pyruvate-malate cycle in regulation of GSIS therefore remained unclear until recently. Confirming and extending the prior reports, we found that reduction of MEc expression in two independent INS-1-derived cell lines, 832/13 and 832/3, resulted in suppression of GSIS (64). However, strong suppression of either MEc or MEm mRNA levels via adenovirus-mediated siRNA delivery had no effect on GSIS in primary rat islets (64). Moreover, islets from MOD1−/− mice, which lack expression of MEc, exhibit normal GSIS (64). These studies provide strong evidence against the involvement of the pyruvate/malate pathway in regulation of GSIS in normal rodent islets, and focus attention instead on the other two pyruvate cycling pathways.
The Pyruvate-Citrate Cycle
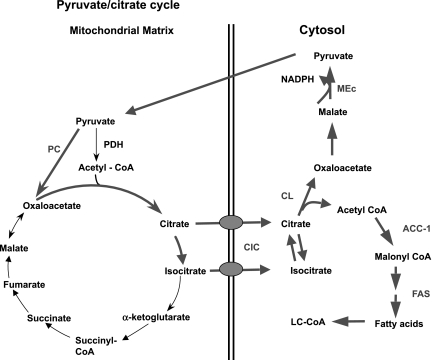
An alternate pyruvate cycling pathway involves generation of citrate from condensation of OAA and acetyl-CoA in the TCA cycle, export of citrate from the mitochondria via the citrate-isocitrate carrier (CIC), cleavage of citrate by ATP-citrate lyase (CL) to OAA and acetyl-CoA, and recycling to pyruvate via a cytsolic malate dehydrogenase and MEc (Fig. 4). This cycle has garnered a great deal of attention over the past fifteen years based on a long-standing “malonyl-CoA/LC-CoA (long-chain acyl-CoA)” hypothesis of GSIS, which holds that PC-mediated anaplerosis is linked to control of insulin secretion via pyruvate-citrate cycling and increases in the levels of byproducts of this pathway, malonyl-CoA and LC-CoA (16, 18, 59). Consistent with this model, treatment of β-cells with glucose causes a rapid rise in malonyl-CoA levels that precedes insulin secretion (16, 59). Glucose stimulation also suppresses FA oxidation, and addition of LC-CoA stimulates insulin granule exocytosis in permeabilized β-cells (17). However, LC-CoA also stimulates KATP channel activity in patch-clamped β-cells (9), an effect seemingly at odds with a role of LC-CoA as a glucose-derived stimulus/secretion coupling factor. Furthermore, prevention of the glucose-induced rise in malonyl-CoA levels by overexpression of malonyl-CoA decarboxylase (MCD) has no impact on GSIS (2, 52, 62). Also consistent with lack of an important role of LC-CoA in GSIS are studies showing that treatment of β-cells with the LC-CoA synthetase inhibitor triacsin C has no effect on GSIS (2, 52, 62). It remains possible, however, that malonyl-CoA and LC-CoA could play a role in FA-induced potentiation of GSIS, since experiments with triacsin C and MCD overexpression diminished this action of FFA in β-cell lines and rat islets in some studies (62), although not in others (52). Adding to the complexity are recent findings suggesting that FAs potentiate GSIS by a receptor-based mechanism involving the G protein-coupled receptor GPR40, although it is also suggested that part of the potentiating effect may be due to a lipid metabolism-generated signal (10, 24, 35, 71).
Fig. 4.
Pyruvate/citrate cycle. In this cycle, pyruvate also enters the TCA cycle via PC. The oxaloacetate formed in the PC reaction then condenses with acetyl-CoA to form citrate and isocitrate, which can exit the mitochondria via the citrate/isocitrate carrier (CIC). Once in the cytosol, citrate is cleaved to oxaloacetate and acetyl-CoA by ATP-citrate lyase (CL), with these two products being metabolized further by two separate pathways. Oxaloacetate is recycled to pyruvate via conversion to malate and engagement with the MEm or MEc to reform pyruvate. Acetyl-CoA goes on to form malonyl-CoA in the acetyl-CoA carboxylase-1 (ACC1) reaction and can then be used for synthesis of long-chain acyl-CoAs (LC-CoA) via fatty acid synthase (FAS).
Evidence against an important role of recycling of citrate to pyruvate via MEc in regulation of GSIS in primary rodent islets has already been presented in a prior section, since suppression of MEc expression in rat islets by adenovirus-mediated delivery of siRNA or ablation of the MEc gene in ME−/− mice had no effect on insulin secretion (64). Recent studies have also investigated the effects of pharmacological or molecular suppression of CL and suppression or knockout of FAS (13, 21, 31, 47). An inhibitor of CL, hydroxycitrate (HC), has been reported to inhibit insulin secretion in some laboratories but not in others. We have recently shown that high concentrations of NaCl created during preparation of HC by standard methods explain the inhibition of GSIS and that removal of the excess NaCl prevents the effect (31).
In addition, suppression of CL mRNA levels by 92% and CL protein levels by 75% by adenovirus-mediated siRNA delivery did not affect GSIS in 832/13 cells compared with cells treated with a control adenovirus. Cells with suppressed CL expression exhibited a strong decrease in cytosolic oxaloacetate and malonyl-CoA levels and inhibition of [U-14C]glucose incorporation into lipids, all expected metabolic outcomes of CL suppression (31). CL knockdown also had no effect on pyruvate cycling activity (29). Consistent with these findings, a study from another laboratory reported no impairment of GSIS in response to an 88% suppression of CL enzyme activity (47), whereas another independent study claimed a modest decrease in insulin secretion at intermediate but not high glucose concentrations in 832/13 cells with suppressed CL expression (21). However, neither of those groups examined the effect of CL suppression in primary islet preparations. We used recombinant adenovirus technology to suppress CL expression by 65% in rat islets and showed that this manipulation had no impact on GSIS (31). Moreover, treatment of 832/13 cells with a recombinant adenovirus containing a siRNA specific to a more distal enzyme in the de novo lipogenic pathway, fatty acid synthase (Ad-siFAS) reduced FAS mRNA levels by 81%, resulting in a strong decrease in [U-14C]glucose incorporation into lipid, but with no effect on GSIS. Finally, treatment of primary rat islets with Ad-siFAS reduced FAS mRNA levels by 52%, but had no effect on GSIS relative to Ad-siControl-treated islets (31). These findings were corroborated recently in studies of FAS knockout mice, in which GSIS was shown to be completely normal (13).
Seemingly inconsistent with the foregoing results, we have also studied the effects of knockdown of acetyl-CoA carboxylase-1 (ACC1), the step immediately preceding FAS in de novo lipogenesis and found that chronic, but not acute, suppression of this enzyme by pharmacological or siRNA methods results in impaired GSIS both in 832/13 cells and in primary rat islets (65). Unexpectedly, treatment of 832/13 cells with an ACC1-specific siRNA duplex (siACC1) also caused a substantial decrease in glucose oxidation and ATP/ADP ratio accompanied by clear decreases in pyruvate cycling activity and TCA cycle intermediates. Exposure of siACC1-treated cells to the pyruvate cycling substrate DMM restored GSIS to normal without recovery of the depressed ATP/ADP ratio, demonstrating the important role that pyruvate cycling can play in control of GSIS. In siACC1-treated cells, glucokinase (GK) protein levels were decreased, correlating with decreases in glycogen and glycolytic flux. Furthermore, acute addition of the ACC inhibitor 5-(tetradecyloxy)-2-furoic acid (TOFA) to β-cells suppressed [14C]glucose incorporation into lipids but had no effect on GSIS, whereas chronic TOFA administration suppressed GSIS and glucose metabolism. In sum, our study found that chronic, but not acute suppression of ACC1 activity impairs GSIS via inhibition of glucose rather than lipid metabolism. In contrast to the effects of ACC1 inhibition on glucose usage and oxidation, such effects were not observed in response to CL suppression, and neither CL nor FAS suppression impairs GSIS (31). Thus, we believe that the findings with ACC1 suppression are a result of a particular linkage of that enzyme to control of glucose metabolism, possibly via modulation of CoA or acetyl-CoA levels. Given the lack of effect of acute ACC1 suppression on GSIS and the clear suppression of lipogenesis under these conditions, our findings do not support a role for lipogenesis in regulation of GSIS. It should be noted that MacDonald et al. (41) have also reported that inhibitors of ACC cause impairment of GSIS in 832/13 cells and rat islets, but in their studies, these effects were seen with both acute and chronic administration of inhibitors. However, their laboratory also reported that CL knockdown has no effect on GSIS (47). The precise mechanism of the effects of ACC1 suppression on glucose metabolism remains to be elucidated, but the findings may be relevant to recent interest in use of ACC inhibitors as therapeutics for obesity and diabetes. ACC inhibitors are predicted to decrease de novo lipogenesis and possibly help remedy the systemic and tissue hyperlipidemia associated with these disorders. Inhibition of insulin secretion via suppression of glucose signaling in β-cells would obviously be an undesired side effect of such inhibitors, and these effects should be carefully monitored.
The findings with ACC1 suppression notwithstanding, the weight of current evidence argues against a direct role of CL, malonyl-CoA, pyruvate/citrate cycling, or FAS-catalyzed de novo lipogenesis in regulation of GSIS when studied in the absence of other potentiators. We do acknowledge the caveat that CL and FAS were not completely suppressed by the siRNA methods applied in the cell line or rat islet studies, and that the residual lipogenic flux could have enabled the glucose response in some way. However, the results obtained with knockdown of MEc, CL, and FAS in primary rat islets certainly establish that there is no linear relationship between pyruvate/citrate cycling or lipogenic flux and GSIS (31, 64). Moreover, complete knockout of FAS in mouse islets also has no effect on GSIS (13). The potential role of these pathways in control of lipid potentiation of GSIS will require more investigation, especially in light of the recent emergence of lipid-regulated G-protein coupled receptors such as GPR40 and GPR119 as potential mediators of these effects.
The Pyruvate/Isocitrate Cycle
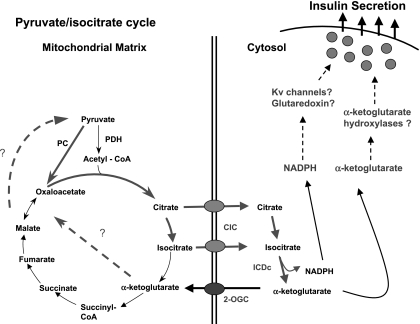
The last of the possible pyruvate cycling pathways is also the least studied but has come to the forefront due to recent findings. In this pathway, citrate and isocitrate exit the mitochondria via the citrate/isocitrate carrier (CIC). In the cytosol, citrate can be converted to isocitrate by a cytosolic aconitase, which interestingly uses an iron-sulfur complex in its catalytic mechanism and also participates in iron homeostasis by binding in its apoprotein form to ferritin mRNA (66). Isocitrate is then converted to α-KG by a cytosolic NAPD-dependent isocitrate dehydrogenase (ICDc) and can cycle back to pyruvate by several combinations of cytosolic and mitochondrial pathways (Fig. 5).
Fig. 5.
Pyruvate/isocitrate cycle. This cycle is again initiated by anaplerotic conversion of pyruvate to oxaloacetate by PC. As in the pyruvate/citrate cycle, citrate and isocitrate leave the mitochondria via CIC. Citrate is then converted to isocitrate by cytosolic aconitase, and isocitrate can then be converted to α-KG by cytosolic, NADP-dependent isocitrate dehydrogenase (ICDc). α-KG can then serve either as a direct signal for insulin secretion, for example by serving as a substrate for α-ketoglutarate hydroxylases, or can be recycled to pyruvate by one of several mitochondrial or cytosolic pathways that remain to be defined (shown as dashed lines). Another byproduct of the pyruvate/isocitrate cycle with potential as an insulin secretagogue is cytosolic NAPDH, possibly acting through Kv channels or the glutathione/glutaredoxin system, as discussed in the text.
The impact of the pyruvate/isocitrate cycle in control of GSIS has been highlighted by two recent studies. In the first, the importance of maintaining active shuttling of citrate and isocitrate from the mitochondria to the cytosol was demonstrated by studies in which the substrate analog 1,2,3-benzenetricarboxylate (BTC) was used to inhibit CIC activity in INS-1-derived 832/13 cells or primary rat islets, resulting in potent inhibition of GSIS (30). This study also included molecular suppression of CIC expression via a recombinant adenovirus containing a CIC-specific siRNA (Ad-siCIC). Ad-siCIC treatment reduced CIC expression in a dose-dependent fashion to a maximum of 77% in 832/13 cells and caused parallel inhibitory effects on citrate accumulation in the cytosol. Ad-siCIC treatment did not affect glucose utilization, glucose oxidation, or ATP/ADP ratio but did inhibit glucose incorporation into fatty acids and glucose-induced increases in NADPH/NADP+ ratio relative to cells treated with a control siRNA virus (Ad-siControl). Ad-siCIC also inhibited GSIS in 832/13 cells, whereas overexpression of CIC enhanced GSIS and raised cytosolic citrate levels. In normal rat islets, Ad-siCIC treatment also suppressed CIC mRNA levels and inhibited GSIS (30). These findings clearly establish that efficient export of citrate and/or isocitrate from the mitochondria to the cytosol is an important step in control of GSIS. This finding could of course implicate either a pyruvate/citrate or pyruvate/isocitrate cycle in GSIS, but results summarized in the previous section present the argument that the former is not the key controlling pathway.
After export of citrate and/or isocitrate from the mitochondria, the next step in the pyruvate/isocitrate pathway is conversion of isocitrate to α-KG by ICDc. The importance of this reaction in GSIS was illustrated by our finding that robust suppression of ICDc expression by siRNA duplex transfection or siRNA delivery with recombinant adenoviruses caused marked impairment of GSIS in two independent INS-1-derived cell lines, 832/13 and 832/3, and in primary rat islets (63). Suppression of ICDc also attenuated the glucose-induced increments in pyruvate cycling activity and in NADPH levels, a predicted byproduct of pyruvate cycling pathways.
From all of the foregoing findings, we conclude that the pyruvate/isocitrate cycle plays the most important role in control of GSIS among the three possible pyruvate cycling pathways. This conclusion is based primarily on results of siRNA-mediated suppression of key enzymes in each of the three pathways carried out in primary rat islets. Key findings that contribute to this conclusions are 1) knockdown of MEc or MEm has no effect on GSIS in rat islets, a result corroborated by findings of a lack of effect of MEc knockout in the ME−/− mouse (64); 2) knockdown of CL or FAS in rat islets has no effect on GSIS, despite a clear impact on metabolic pathways in which those enzymes are engaged (31, 47), which result has also been corroborated by the lack of an insulin secretion phenotype in FAS knockout mice (13); 3) knockdown of CIC or ICDc, key elements of the pyruvate/isocitrate cycle, causes strong impairment of GSIS in rat islets (30, 63). The findings with CIC, ICDc, ME, ACC1, and FAS suppression are consistent in rat islets and the INS-1-derived cell lines 832/13 and 832/3 in our hands, whereas knockdown of MEc impairs GSIS only in the cell lines and not in primary islets, suggesting that pyruvate/malate cycling is a less active pathway in primary rodent islets compared with the INS-1-derived cell lines. We believe that the findings from primary islet and knockout mice are the most physiologically relevant data and that they provide the strongest support for our conclusion that pyruvate/isocitrate cycling plays a key role in control of GSIS.
Identification of Coupling Factors Generated by Pyruvate Cycling and Their Potential Molecular Targets
The striking correlation between pyruvate cycling and GSIS has led to renewed investigation of potential coupling factors generated by these pathways. Candidate factors that have been proposed include malonyl-CoA/LC-CoA (16, 59), glutamate (50), NADPH (25, 43, 63), α-KG (60), short-chain acyl-CoAs (SC-CoA) (44, 45), and GTP produced by the GTP-generating isoform of succinyl-CoA synthetase (SCS-GTP) in the TCA cycle (33). The metabolic pathways that complete the pyruvate/isocitrate cycle remain to be elucidated. Generation of the key signaling intermediate may not require complete recycling of carbon to pyruvate, since α-KG, a product of the ICDc reaction, has itself been directly implicated as a β-cell secretagogue (60). Incubation of mouse islets with α-KG stimulates insulin secretion. The idea that α-KG itself could be a secretion coupling factor is based on the fact that α-KG is metabolized to succinate, but succinate itself does not stimulate insulin secretion in mouse islets. Alternatively, β-cells express a mitochondrial α-KG transporter, also known as the 2-oxoglutarate carrier, that could serve to transport α-KG generated in the ICDc reaction back into the mitochondria. Once returned to the mitochondria, α-KG can be further metabolized in the TCA cycle or could possibly engage in transamination reactions, eventually cycling back to pyruvate via either the cytosolic or mitochondrial forms of ME. The expression of both isoforms of ME in β-cells could explain why molecular suppression of MEc or MEm individually has no impact on GSIS in rodent islets. Further studies will be required to fully characterize the later steps of the pyruvate/isocitrate cycle.
We have already discussed the evidence for and against involvement of malonyl-CoA/LC-CoA in control of GSIS and our reasons for concluding that this is not an important signal. The role of glutamate in GSIS has also been debated. It was originally suggested that glucose stimulation of β-cells stimulates conversion of α-KG to glutamate by glutamate dehydrogenase, resulting in uptake of glutamate into insulin-containing granules, and triggering of granule release (50). This hypothesis was supported by a study in which cytosolic glutamate levels were lowered by overexpression of glutamate decarboxylase, resulting in impaired GSIS (67). However, a number of subsequent reports by other groups failed to establish any clear relationship between changes in glutamate concentration and insulin release (7, 37, 42, 75). Also, a familial form of hyperinsulinism in humans has been identified that is due to an activating mutation of glutamate dehydrogenase (70), but these mutations enhance glutamate oxidation rather than accumulation (42). Thus, the weight of current evidence suggests that glutamate is not a primary signal for insulin secretion.
The idea that SC-CoAs may serve as stimulus/secretion coupling factors has emerged recently (44, 45, 47). This idea is based in part on studies showing that several metabolic fuels, including glucose, pyruvate, leucine, β-hydroxybutyrate, and ketoisocaproate (KIC) cause increases in the levels of several SC-CoAs, including acetyl-CoA, acetoacetyl-CoA, succinyl-CoA, hydroxymethylglutaryl-CoA, and malonyl-CoA in 832/13 cells (44, 45). Suppression of CL, which curtails synthesis of cytosolic acetyl-CoA, impairs insulin secretion in response to the nonmetabolizable analog of leucine, 2-aminobicyclo(2,2,1)heptane- 2-carboxylate (BCH), and this effect could be rescued by addition of β-hydroxybutyrate or acetoacetate, which can be directly converted to SC-CoA. However, this same group has also shown that suppression of CL has no effect on GSIS (47), in agreement with our own findings (31). Also, the potential molecular targets of SC-CoA in control of insulin secretion are not defined. Further studies will be required to understand the role of SC-CoA in GSIS or in mediating the potentiating effects of other metabolic fuels.
NADPH, α-KG, or GTP are attractive candidates because all can be generated as byproducts of the pyruvate/isocitrate cycle, which, based on arguments advanced earlier, appears to be a key pyruvate cycling pathway in control of GSIS. Mitochondrial GTP is produced in the TCA cycle via substrate level synthesis. Evidence in support of a role of GTP production in control of GSIS comes from studies demonstrating that siRNA-mediated suppression of the GTP-generating form of succinyl-CoA synthetase (SCS-GTP) in 832/13 cells and rat islets results in impaired GSIS and decreased GTP levels, whereas suppression of the ATP-generating form of this enzyme (SCS-ATP) enhances insulin secretion at stimulatory glucose levels (33). In these studies, siRNA duplexes were introduced into 832/13 cells and rat islets by transient transfection, resulting in a relatively modest suppression of SCS-GTP (50%) in the cultured islet studies. In islets, the major effect of this suppression appeared to be an increase in insulin secretion at basal glucose (3 mM), accompanied by a small decrease in insulin secretion at 15 mM glucose. Thus, these studies should be confirmed by the more effective strategy of adenovirus-mediated siRNA delivery in rodent islets or via β-cell-specific knockout of SCS-GTP in a mouse model. If a role for GTP in GSIS is confirmed, more study will also be needed to define the mechanism of its effects. Suppression of SCS-GTP impairs glucose-stimulated increases in intracellular Ca2+, whereas suppression of SCS-ATP increases intracellular Ca2+. How changes in mitochondrial GTP levels might bring about changes in cytosolic Ca2+ has not been determined, although knockdown of SCS-GTP appears to cause accumulation of Ca2+ within mitochondria (33).
Among coupling factors that can be generated by pyruvate cycling pathways, perhaps the strongest evidence has accumulated in support of an important role for NADPH. Key findings include the following. 1) The NADPH/NADP ratio increases in direct proportion to media glucose concentration and GSIS in rodent islets and several β-cell lines, whereas this relationship does not exist for NADH/NAD ratio and GSIS (25, 63). 2) Addition of NAPDH to patch-clamped β-cells stimulates exocytosis as measured by increases in cell capacitance, whereas NADH has no effect (25). Analogous to the importance of ATP/ADP ratio in regulation of the KATP channel, NADPH/NADP ratio may be the relevant signal, since addition of a threefold molar excess of NADP reversed the stimulatory effect obtained with NADPH alone (25). 3) As detailed earlier, molecular manipulations that impair GSIS such as suppression of CIC, ICDc, or MEc (in 832/13 cells) are accompanied by suppression of glucose-induced increases in NADPH/NADP ratio (30, 63, 64), whereas maneuvers that fail to impair GSIS, such as molecular suppression of PC, have no effect on NADPH/NADP levels (29).
Although still unfolding, these recent studies that implicate specific reactions and intermediates of glucose metabolism other than ATP in control of insulin secretion hold promise for identification of targets that might be used for enhancing β-cell function in diabetes. Possible molecular targets of two key products of the ICDc reaction, α-KG and NADPH, are worthy of further discussion (Fig. 5). α-KG is a substrate for various α-ketoglutarate hydroxylases that modify proteins on prolyl, lysyl, and aspartyl residues. Perhaps the best-recognized targets of these enzymes are the hypoxia-inducible transcription factors (HIF). HIF-modifying hydroxylases promote the degradation and inactivation of HIFs, leading to enhanced oxidative metabolism through suppression of specific HIF-regulated genes (56). Interestingly, other TCA cycle intermediates such as succinate and fumarate are competitive inhibitors of the hydroxylase enzymes with respect to α-KG. Key questions for further investigation in this area include the following. 1) Does α-ketoglutarate hydroxylase activity change in response to α-KG or other organic acids during glucose stimulation of the β-cell? 2) Does α-ketoglutarate hydroxylase-mediated regulation of HIF or other targets contribute to β-cell functional changes on an acute time scale consistent with GSIS?
Two intriguing targets have also been proposed for NADPH. One very important role of NADPH in a variety of mammalian cells is to control the redox state of glutathione via the activity of NADPH-dependent glutathione reductase. Reduced glutathione, in turn, can be used to create the reduced form of glutaredoxin (GRX), which participates in redox-regulated posttranslational modification of proteins, including exocytosis-regulating t-SNARE proteins (43). Coadministration of GRX and NADPH to the interior of patch-clamped β-cells results in potentiation of NADPH-induced exocytotic activity (25). Further evidence of a role of a glucose-activated NADPH/ glutathione/GRX-mediated pathway of insulin secretion is now needed in intact, non-patch-clamped islet cells. A second interesting potential target of glucose-induced changes in NADPH/NADP ratio might be the voltage-dependent K+ (Kv) channels (48). Kv channels are thought to repolarize glucose-stimulated action potentials and inhibit Ca2+ entry through voltage-gated Ca2+ channels; therefore, Kv channels serve as negative regulators of insulin secretion, and Kv channel antagonists are insulinotropic in a glucose-dependent manner. Kv channels comprise the pore-forming α-subunits (Kv2.1 is thought to be the predominant isoform in islet β-cells) and regulatory β-subunits, analogous to the pore-forming and regulatory subunits of the KATP channel complex. Kv channel β-subunits are proposed to act as intracellular redox sensors, and an increase in cytosolic NADPH/NADP ratio in patch-clamped β-cells was shown to be associated with an increased rate of inactivation of the Kv channel (49). Inhibition of Kv channels by NADPH derived from pyruvate cycling could serve as a logical complementary mechanism to ATP regulation of KATP channel activity, since suppression of Kv channels would slow membrane repolarization, allowing the effects of KATP channel inhibition to be sustained through a second phase of insulin secretion. Although attractive, this model will require more definitive and detailed investigation. It should also be mentioned that glucose-induced activation of group VIA phospholipase A2 (iPLA2β), leading to hydrolysis of membrane phospholipids and accumulation of arachidonic acid, has been invoked as a possible mechanism for control of Kv channels during glucose stimulation of β-cells (26, 27). In fact, it has been reported that glucose and carbachol do not significantly inactivate Kv2.1 channels in islets from iPLA2β knockout mice (27). Whether NADPH, α-KG, or other factors derived from pyruvate/isocitrate cycling could mediate their effects on insulin secretion via modulation of membrane phospholipid turnover remains to be investigated.
Summary and Future Studies
Anaplerotic metabolism of pyruvate in β-cell mitochondria has emerged as an important mechanism for generation of signals for GSIS. Recent studies have refined our knowledge in this area by implicating a specific pyruvate cycling pathway, the pyruvate/isocitrate cycle, as a primary mediator of glucose signaling, especially in primary rodent islet preparations. Interest in this cycle is further enhanced by its ability to generate several byproducts, most notably α-KG and NADPH, for which testable mechanisms of action and defined molecular targets can be proposed. Important questions that remain to be answered in this area include, but are not limited to the following. 1) How is the pyruvate/isocitrate cycle completed (e.g., what are the specific enzymatic steps that mediate recycling of α-KG to pyruvate?)? 2) If NADPH is a key coupling factor generated by pyruvate/isocitrate cycling, what are its specific molecular targets? Kv channels and glutathione/glutaredoxin redox regulation are two plausible mechanisms, but others may still be possible. 3) Similarly, if α-KG has a direct signaling role, is this mediated by its effect to activate protein hydroxylating enzymes? 4) Are pyruvate cycling and the pyruvate/isocitrate pathway as important in regulation of GSIS in human islets as they appear to be in rodent islets? If so, do human islets exhibit impaired regulation of pyruvate cycling activity in response to metabolic stress in a manner similar to that of rodent islets? These questions will undoubtedly be pursued vigorously in the near future.
GRANTS
Work reviewed in this article performed in the authors' laboratories was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-42583 (to C. B. Newgard) and DK-58398 (to C. B. Newgard, A. D. S, and S. C. B).
REFERENCES
- 1.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt Boyd 3rd AE, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268: 423–426, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273: 16146–16154, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130: 167–178, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312: 446–448, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Assimacopoulos-Jeannet F, Thumelin S, Roche E, Esser V, McGarry JD, Prentki M. Fatty acids rapidly induce the carnitine palmitoyltransferase I gene in the pancreatic beta-cell line INS-1. J Biol Chem 272: 1659–1664, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bahl JJ, Matsuda M, DeFronzo RA, Bressler R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem Pharmacol 53: 67–74, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand G, Ishiyama N, Nenquin M, Ravier MA, Henquin JC. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J Biol Chem 277: 32883–32891, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 279: 27263–27271, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Branstrom R, Aspinwall CA, Valimaki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, Larsson O. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in Type 2 diabetes. Diabetologia 47: 277–283, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Brun T, Roche E, Assimacopoulos-Jeannet F, Corkey BE, Kim KH, Prentki M. Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic beta-cell nutrient signaling. Diabetes 45: 190–198, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des 11: 2699–2716, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest 117: 2539–2552, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279: 44370–44375, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311: 271–273, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem 264: 21608–21612, 1989. [PubMed] [Google Scholar]
- 17.Deeney JT, Gromada J, Hoy M, Olsen HL, Rhodes CJ, Prentki M, Berggren PO, Corkey BE. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells). J Biol Chem 275: 9363–9368, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49: 718–726, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Fransson U, Rosengren AH, Schuit FC, Renstrom E, Mulder H. Anaplerosis via pyruvate carboxylase is required for the fuel-induced rise in the ATP:ADP ratio in rat pancreatic islets. Diabetologia 49: 1578–1586, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 89: 1288–1295, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282: 35657–35665, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest 33: 742–750, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49: 424–430, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in 't Veld P, Renstrom E, Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson DA, Philipson LH. Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes Obes Metab 9, Suppl 2: 89–98, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem 282: 7442–7449, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffrey FM, Storey CJ, Sherry AD, Malloy CR. 13C isotopomer model for estimation of anaplerotic substrate oxidation via acetyl-CoA. Am J Physiol Endocrinol Metab 271: E788–E799, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, Newgard CB. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 281: 22342–22351, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281: 35624–35632, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282: 31592–31600, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Khan A, Ling ZC, Landau BR. Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem 271: 2539–2542, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 5: 253–264, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu M, Yajima H, Yamada S, Kaneko T, Sato Y, Yamauchi K, Hashizume K, Aizawa T. Augmentation of Ca2+-stimulated insulin release by glucose and long-chain fatty acids in rat pancreatic islets: free fatty acids mimic ATP-sensitive K+ channel-independent insulinotropic action of glucose. Diabetes 48: 1543–1549, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56: 1087–1094, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YQ, Jetton TL, Leahy JL. Beta-cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem 277: 39163–39168, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci USA 99: 2708–2713, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald MJ Estimates of glycolysis, pyruvate (de)carboxylation, pentose phosphate pathway, and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys 305: 205–214, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald MJ Export of metabolites from pancreatic islet mitochondria as a means to study anaplerosis in insulin secretion. Metabolism 52: 993–998, 2003. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald MJ Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270: 20051–20058, 1995. [PubMed] [Google Scholar]
- 41.MacDonald MJ, Dobrzyn A, Ntambi J, Stoker SW. The role of rapid lipogenesis in insulin secretion: insulin secretagogues acutely alter lipid composition of INS-1 832/13 cells. Arch Biochem Biophys 470: 153–162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald MJ, Fahien LA. Glutamate is not a messenger in insulin secretion. J Biol Chem 275: 34025–34027, 2000. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288: E1–E15, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Macdonald MJ, Hasan NM, Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta 1780: 966–972, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDonald MJ, Longacre MJ, Stoker SW, Brown LJ, Hasan NM, Kendrick MA. Acetoacetate and β-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am J Physiol Cell Physiol 294: C442–C450, 2008. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald MJ, McKenzie DI, Walker TM, Kaysen JH. Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Horm Metab Res 24: 158–160, 1992. [DOI] [PubMed] [Google Scholar]
- 47.MacDonald MJ, Smith AD, 3rd Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 282: 30596–30606, 2007. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360: 2211–2225, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald PE, Salapatek AM, Wheeler MB. Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells. J Physiol 546: 647–653, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402: 685–689, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev 2: 163–214, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Mulder H, Lu D, Finley Jt An J, Cohen J, Antinozzi PA, McGarry JD, Newgard CB. Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) beta-cells. J Biol Chem 276: 6479–6484, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Nenquin M, Szollosi A, Aguilar-Bryan L, Bryan J, Henquin JC. Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic beta-cells. J Biol Chem 279: 32316–32324, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Newgard CB, Matschinsky FM. Substrate control of insulin release. In: Handbook of Physiology, edited by J Jefferson AC. Oxford, UK: Oxford Univ. Press, 2001, p. 125–152.
- 55.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 64: 689–719, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Ozer A, Bruick RK. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat Chem Biol 3: 144–153, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Pfeifer MA, Halter JB, Porte D Jr. Insulin secretion in diabetes mellitus. Am J Med 70: 579–588, 1981. [DOI] [PubMed] [Google Scholar]
- 58.Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282: 200–207, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992. [PubMed] [Google Scholar]
- 60.Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD. α-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 289: E218–E224, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Remedi MS, Rocheleau JV, Tong A, Patton BL, McDaniel ML, Piston DW, Koster JC, Nichols CG. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia 49: 2368–2378, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Roduit R, Nolan C, Alarcon C, Moore P, Barbeau A, Delghingaro-Augusto V, Przybykowski E, Morin J, Masse F, Massie B, Ruderman N, Rhodes C, Poitout V, Prentki M. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 53: 1007–1019, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281: 30593–30602, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, MacNeil D, Howard A, Thornberry N, Ilkayeva O, Lu D, Sherry AD, Newgard CB. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem 283: 28909–28917, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronnebaum SM, Joseph JW, Ilkayeva O, Burgess SC, Lu D, Becker TC, Sherry AD, Newgard CB. Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem 283: 14248–14256, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouault TA The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2: 406–414, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Rubi B, Ishihara H, Hegardt FG, Wollheim CB, Maechler P. GAD65-mediated glutamate decarboxylation reduces glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem 276: 36391–36396, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272: 18572–18579, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem 277: 37176–37183, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 338: 1352–1357, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1: 245–258, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev 18: 451–463, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Szollosi A, Nenquin M, Aguilar-Bryan L, Bryan J, Henquin JC. Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old beta-cells lacking ATP-sensitive K+ channels. J Biol Chem 282: 1747–1756, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, Aguilar-Bryan L, Gagel RF, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 268: 426–429, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Yamada S, Komatsu M, Sato Y, Yamauchi K, Aizawa T, Hashizume K. Glutamate is not a major conveyer of ATP-sensitive K+ channel-independent glucose action in pancreatic islet beta cell. Endocr J 48: 391–395, 2001. [DOI] [PubMed] [Google Scholar]