Abstract
Intestinal mucosal restitution occurs as a consequence of epithelial cell migration and reseals superficial wounds after injury. This rapid reepithelialization is mediated in part by a phospholipase C-γ1 (PLC-γ1)-induced Ca2+ signaling, but the exact mechanism underlying such signaling and its regulation remains elusive. The small GTP-binding protein Rac1 functions as a pivotal regulator of several signaling networks and plays an important role in regulating cell motility. The current study tests the hypothesis that Rac1 modulates intestinal epithelial cell migration after wounding by altering PLC-γ1-induced Ca2+ signaling. Inhibition of Rac1 activity by treatment with its inhibitor NSC-23766 or Rac1 silencing with small interfering RNA decreased store depletion-induced Ca2+ influx and suppressed cell migration during restitution, whereas ectopic overexpression of Rac1 increased Ca2+ influx and promoted cell migration. Rac1 physically interacted with PLC-γ1 and formed Rac1/PLC-γ1 complex in intestinal epithelial cells. PLC-γ1 silencing in cells overexpressing Rac1 prevented stimulation of store depletion-induced Ca2+ influx and cell migration after wounding. Polyamine depletion inhibited expression of both Rac1 and PLC-γ1, decreased Rac1/PLC-γ1 complex levels, reduced Ca2+ influx, and repressed cell migration. Overexpression of Rac1 alone failed to rescue Ca2+ influx after store depletion and cell migration in polyamine-deficient cells, because it did not alter PLC-γ1 levels. These results indicate that Rac1 promotes intestinal epithelial cell migration after wounding by increasing Ca2+ influx as a result of its interaction with PLC-γ1.
Keywords: mucosal injury, early rapid mucosal repair, polyamines, cell migration, capacitative Ca2+ entry, cdx2 gene, small guanosine 5′-triphosphate-binding proteins
early mucosal restitution is an important and primary repair modality in the gastrointestinal tract, and its defective regulation underlies various critical pathological states such as mucosal bleeding and ulcers, disruption of epithelial integrity, and barrier dysfunction (9, 21, 42). Epithelial restitution occurs as a consequence of intestinal epithelial cell (IEC) migration to reseal superficial wounds, a process independent of cell proliferation (6, 21, 50). This rapid reepithelialization is a complex process that is highly regulated by numerous extracellular and intracellular factors, but its exact mechanism at cellular and molecular levels remains unclear. A significant body of evidence indicates that cytosolic free Ca2+ ([Ca2+]cyt) plays a critical role in regulating IEC migration after injury and that increasing [Ca2+]cyt enhances epithelial restitution (27, 28, 33). Ca2+ entry due to store depletion is referred to as capacitative Ca2+ entry (CCE), and it is mediated by Ca2+-permeable channels termed store-operated Ca2+ channels (SOCs) (22, 23). Although the molecular identity of SOCs mediating CCE is not defined yet, the canonical transient receptor potential-1 (TRPC1) protein is highly expressed in IECs and appears to be an excellent candidate for SOCs that regulates [Ca2+]cyt homeostasis after store depletion (31, 55). Recently, it has also been found that phospholipase C-γ1 (PLC-γ1) regulates [Ca2+]cyt by interacting with TRPC channels and that PLC-γ1-induced Ca2+ signaling plays an important role in regulating a variety of cellular functions including cell motility (33, 46, 47).
Rac1 is a member of the Rho family of GTPases that function as molecular switches and control signaling pathways regulating distinct cellular behaviors such as cytoskeleton organization, gene expression, cell cycle progression, apoptosis, and cell motility (2, 7, 17, 38). Similar to all other members of the Rho GTPase family, Rac1 and its homolog Rac2 bind to GTP and cycle between an inactive GDP-bound form and an active GTP-bound form under tight regulation in physiological conditions (20, 37). Several proteins have been identified that can regulate nucleotide exchange or stimulate their intrinsic GTPase activity in vitro (12). The Rac1 gene is expressed in various tissues and cell lines, whereas Rac2 expression is restricted to cells of hematopoietic lineages (19, 41). Accumulating evidence has implicated Rac1 in many aspects of cytoskeleton reorganization and adhesion including lamellipodia formation, RhoA-regulated actin stress fiber and focal adhesion complex formation, and membrane ruffling (20, 37). It has been shown that Rac1 regulates lamellipodia formation and membrane ruffling through p21-activated kinase-dependent and -independent pathways (14, 40), whereas another Rho family GTPase, RhoA, modulates the actin stress fiber and focal adhesion plaque assembly by altering Rho kinase activity (10, 56). Rac1 also acts upstream of RhoA during actin cytoskeleton reorganization and plays a major role in regulating adhesion contacts of cells to integrin (5). Using undifferentiated IECs (IEC-6 line), Ray et al. (35) have reported that all Rac1, RhoA, and Cdc42 are required for optimal epithelial restitution and that Rac1 activation is not only essential but also sufficient for cell migration after wounding. However, the exact mechanism by which Rac1 activation promotes cell migration during epithelial restitution remains elusive.
The PLC family of enzymes catalyzes the production of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol that is implicated in the regulation of [Ca2+]cyt by modulating Ca2+ store mobilization and Ca2+ influx (36). To date, three isoforms of PLC have been identified in mammalian cells: β, γ, and δ; but their expression is cell type-dependent in various tissues. Although all of these PLC isozymes are regulated by multiple mechanisms through different conserved domains, several lines of evidence have suggested that Rac1 and other Rho family GTPases are the upstream regulators of PLC-γ1 in various types of cells (1, 4, 49). For example, Rac1 activation in keratinocytes promotes PLC-γ1-induced Ca2+ signaling and enhances cortactin-cytoskeleton function leading to cell adhesion and differentiation (1). Leukotriene D4-induced RhoA in IECs also interacts with PLC-γ1 and is implicated in regulating the PLC-γ1-mediated Ca2+ mobilization (44). In addition, the crystal structure of Rac1 is shown to bind to its effector PLC-β2 (13). We have recently demonstrated that PLC-γ1-induced Ca2+ signaling is crucial for stimulation of IEC migration after injury and that decreased levels of PLC-γ1 repress epithelial restitution through reduction of Ca2+ influx (33). Here, we sought to further investigate whether Rac1 regulates IEC migration by altering PLC-γ1-induced Ca2+ signaling. Our results indicate that inhibition of Rac1 decreased Ca2+ influx and suppressed cell migration, whereas ectopic overexpression of Rac1 increased Ca2+ influx and promoted cell migration. The data presented here also show that Rac1 physically interacted with PLC-γ1 and that formation of the Rac1/PLC-γ1 complex is necessary for PLC-γ1-mediated Ca2+ influx during restitution.
MATERIALS AND METHODS
Chemicals and supplies.
Tissue culture media, isopropyl-β-d-thiogalactopyranoside (IPTG), LipofectAMINE 2000, and dialyzed fetal bovine serum (dFBS) were obtained from Invitrogen (Carlsbad, CA), and biochemicals were from Sigma (St. Louis, MO). The primary antibody, an affinity-purified mouse monoclonal antibody against Rac1 or PLC-γ1, was purchased from BD biosciences (San Jose, CA); the antibody against RhoA was from Santa Cruz Biotechnology (Santa Cruz, CA). NSC-23766 was purchased from Calbiochem (San Diego, CA), while DFMO was purchased from Genzyme (Cambridge, MA).
Cell culture.
Stable Cdx2-transfected IEC-6 cells were developed and characterized by Suh and Traber (43) and were a kind gift from Dr. Peter G. Traber (Baylor College of Medicine, Houston, TX). The expression vector, the LacSwitch System (Stratagene, La Jolla, CA), was used for directing the conditional expression of the Cdx2 gene, and IPTG served as the inducer for the gene expression (45). IEC-6 cells, derived from normal rat intestinal crypts, were transfected with pOPRSVCdx2 by electroporation technique, and clones resistant to selection medium containing 0.6 mg G418/ml and 0.3 mg/ml hygromycin B were isolated and screened for Cdx2 expression by Northern blot, RNase protection assays, and electrophoretic mobility shift assay. Stock-stable Cdx2-transfected IEC-6 (IEC-Cdx2L1) cells were grown in DMEM supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Before experiments, IEC-Cdx2L1 cells were grown in DMEM containing 4 mM IPTG for 16 days to induce cell differentiation as described in our earlier publications (26, 28, 33).
The Caco-2 cells (a human colon carcinoma cell line) were obtained from American Type Culture Collection at passage 16. They were maintained similarly to the IEC-Cdx2L1 cells except that they were maintained in an atmosphere of 95% air and 5% CO2. The medium used was Eagle's minimum essential medium with 10% heat-inactivated FBS, and passages 18–23 were used for the experiments as described in our previous studies (15).
RNA interference.
The small interfering (si)RNAs that were designed to specifically target the coding regions of Rac1 (siRac1) or PLC-γ1 (siPLC-γ1) mRNAs were synthesized and purchased from Dharmacon (Lafayette, CO). Scrambled control siRNA (C-siRNA), which had no sequence homology to any known genes, was used as the control. The siRac1, siPLC-γ1, and C-siRNA were transfected into cells as described previously (33). Briefly, for each 60-mm cell culture dish, 20 μl of the 5 μM stock siRac1 or siPLC-γ1, or C-siRNA was mixed with 500 μl Opti-MEM medium (Invitrogen). This mixture was added to a solution containing LipofectAMINE 2000 in 500 μl Opti-MEM. The solution was incubated for 20 min at room temperature and gently overlaid onto monolayers of cells in 3 ml medium, and cells were harvested for various assays after 48 and 72 h incubation.
Plasmid construction and transfection.
The transfection grade eukaryotic expression vector pUSEamp(+) containing the full-length cDNA of wild-type human Rac1 gene under the control of cytomegalovirus promoter and its control vector lacking Rac1 cDNA were purchased from Upstate Biotechnology (Lake Placid, NY). The Rac1 cDNA was inserted as an EcoR1/Xho1 fragment into the EcoR1/Xho1 site of pUSEamp(+) multiple cloning site. Caco-2 cells were transfected with either the Rac1 expression vector or control pUSEamp(+) vector using a LipofectAMINE kit, and transfection was performed as recommended by the manufacturer's instructions (Invitrogen).
Reverse transcription and PCR.
Total RNA was isolated by using RNeasy Mini Kit (Qiagen, Valencia, CA). Equal amounts of total RNA (5 μg) were transcribed to synthesize single-strand cDNA with an RT kit (Invitrogen). The specific sense and antisense primers for Rac1 included 5′-GTAAAACCTGCCTGCTCATC-3′ and 5′-GCTTCATCAAACACTGTCTTG-3′, and the expected size of Rac1 fragments was 472 bp. Reverse transcription and PCR were performed as described in our earlier publications (28, 31, 33, 57). To quantify the PCR products (the amounts of mRNA) of Rac1, an invariant mRNA of β-actin was used as an internal control. The optical density (OD) values for each band on the gel were measured by a gel documentation system (UVP, Upland, CA), and their signals were normalized to the OD values in the β-actin signals.
Immunoprecipitation and immunoblotting analysis.
Cell samples, dissolved in ice-cold RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM DTT, 0.5 mM EDTA, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM phenylmethyl-sulfonyl fluoride, 20 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 mM sodium orthovanadate), were sonicated and centrifuged at 4°C, and the supernatants were collected for immunoprecipitation. Equal amounts of proteins (500 μg) for each sample were incubated with the specific antibody against Rac1 or PLC-γ1 (4 μg) at 4°C for 3 h, and protein A/G-PLUS-agarose was added and incubated overnight at 4°C. The precipitates were washed five times with ice-cold Dulbecco's PBS (D-PBS), and the beads were resuspended in SDS sample buffer for subsequent Western blotting analysis. For immunoblotting, samples were subjected to electrophoresis on PAGE gels described previously (31, 33). Briefly, after the transfer of protein onto nitrocellulose membranes, the membranes were incubated for 1 h in 5% nonfat dry milk in 1× TBS-T buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween 20). Immunologic evaluation was then performed overnight at 4°C in 5% nonfat dry milk/TBS-T buffer containing specific antibodies against Rac1, RhoA, and PLC-γ1 proteins. The membranes were subsequently washed with 1× TBS-T and incubated with the secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The immunocomplexes on the membranes were reacted for 1 min with chemiluminescence reagent (NEL-100 DuPont NEN).
Measurement of [Ca2+]cyt.
Details of the digital imaging methods used for measuring [Ca2+]cyt were described in our previous publications (27, 31–33). Briefly, cells were plated on 25-mm coverslips and incubated in culture medium containing 3.3 μM fura-2 AM for 30–40 min at room temperature (22–24°C) under an atmosphere of 10% CO2 in air. The fura-2 AM-loaded cells were then superfused with standard bath solution for 20–30 min at 22–24°C to wash away extracellular dye and permit intracellular esterases to cleave cytosolic fura-2 AM into active fura-2. Fura-2 fluorescence from the cells and background fluorescence were imaged using a Nikon Diaphot microscope equipped for epifluorescence. Fluorescent images were obtained using a microchannel plate image intensifier (Amperex XX1381; Opelco, Washington, DC) coupled by fiber optics to a Pulnix charge-coupled device video camera (Stanford Photonics, Stanford, CA). Image acquisition and analysis were performed with a Metamorph Imaging System (Universal Imaging). The ratio imaging of [Ca2+]cyt was obtained from fura-2 fluorescent emission excited at 380 and 340 nm (16, 33).
Measurement of cell migration.
Migration assays were carried out as described in our earlier publications (26–31). Cells were plated at 6.25 × 104/cm2 in DMEM containing dFBS on 60-mm dishes thinly coated with Matrigel according to the manufacturer's instructions and were incubated as described for stock cultures. Cells were fed on day 2, and cell migration was assayed on day 4. To initiate migration, the cell layer was scratched with a single edge razor blade cut to ∼27 mm in length. The scratch was made over the diameter of the dish and extended over an area 7–10 mm wide. The migrating cells in six contiguous 0.1-mm squares were counted at ×100 magnification beginning at the scratch line and extending as far out as the cells had migrated. All experiments were carried out in triplicate, and the results were reported as number of migrating cells per millimeter of scratch.
Rac1 activation assay.
The Rac1 activation was analyzed using Rac1/Cdc42 Activation Assay Kit (Upstate Biotechnology, Temecula, CA) and performed as described by the manufacturer. In brief, cells were harvested following appropriate treatments in 1.5 ml magnesium-containing lysis buffer [25 mM HEPES (pH 7.5), 150 mM NaCl, 1% Igpal, 10 mM MgCl2, 1 mM EDTA, and 10% glycerol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM sodium fluoride, and 1 mM sodium orthovanadate]. Cell lysates (500 μg) were incubated with 15 μg GST-Pak1 PBD beads for 1 h at 4°C with gentle rocking. Bound proteins were resolved on a 15% SDS-PAGE and transferred to nitrocellulose membranes. Active GTP-bound Rac1 was determined by Western blot analysis using anti-Rac1 antibody.
Polyamine analysis.
The cellular polyamines content was analyzed by high-performance liquid chromatographic (HPLC) analysis as previously described (18, 51). Briefly, after the cells were washed three times with ice-cold D-PBS, 0.5 M perchloric acid was added, and the cells were frozen at −80°C until ready for extraction, dansylation, and HPLC analysis. The standard curve encompassed 0.31–10 μM. Values that fell >25% below the curve were considered undetectable. The results are expressed as nanomoles of polyamines per milligram protein.
Statistical analysis.
All data are expressed as means ± SE from six dishes. PCR and immunoblotting results were repeated three times. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using the Duncan's multiple-range test (11).
RESULTS
Inhibition of Rac1 activity reduces Ca2+ influx and inhibits cell migration.
To determine the role of Rac1 in the regulation of IEC migration after wounding, the following two studies were carried out using differentiated IEC-Cdx2L1 cells. This line of cells represents normal differentiated IECs and provides a unique in vitro model for intestinal epithelial restitution (26, 32, 33). As reported in our previous studies (26, 30), induced expression of the Cdx2 gene by treatment of stable IEC-Cdx2L1 cells with 4 mM IPTG for 16 days resulted in a differentiated phenotype. These differentiated IEC-Cdx2L1 cells exhibited multiple morphological and molecular characteristics of intestinal epithelial differentiation and also migrated over the wounded edge much faster than undifferentiated parental IEC-6 cells after injury.
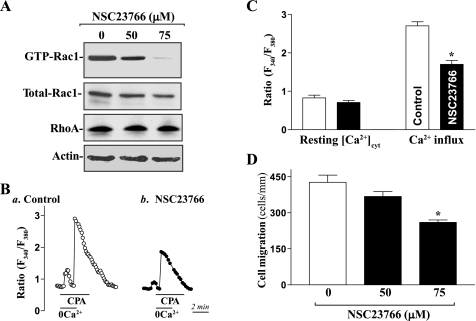
In the first study, we examined the effect of Rac1 inhibition by treatment with its chemical inhibitor, NSC-23766, on store depletion-induced Ca2+ influx and cell migration. As shown in Fig. 1A, exposure of differentiated IEC-Cdx2L1 cells to NSC-23766 significantly repressed Rac1 activity as indicated by a decrease in the levels of active Rac1. NSC-23766 at the concentration of 75 μM repressed Rac1 activity by ∼95%, but it just slightly decreased the levels of total Rac1 protein and failed to alter RhoA content. The reduction in Rac1 activation by NSC-23766 also inhibited store depletion-induced Ca2+ influx induced by cyclopiazonic acid (CPA) (Fig. 1, B and C). In control cells, exposure to CPA, an inhibitor of Ca2+-Mg2+ ATPase in the endoplasmic reticulum and sarcoplasmic reticulum, resulted in an initial transient increase in [Ca2+]cyt in the absence of extracellular Ca2+, which was apparently due to Ca2+ mobilization from intracellular Ca2+ stores. Addition of extracellular Ca2+ to the cell superfusate, when the CPA-induced transient rise in [Ca2+]cyt returned to basal level (i.e., when intracellular stores were depleted), caused a sustained increase in [Ca2+]cyt because of the CCE. In NSC-23766-treated cells, Ca2+ influx due to CCE was decreased by ∼43%, although there were no statistically significant changes in the level of resting [Ca2+]cyt. Treatment with NSC-23766 also markedly inhibited cell migration after wounding (Fig. 1D). The numbers of cells migrating over the wounded edge in NSC-23766-treated cells were decreased by ∼15 at 50 μM and ∼35% at 75 μM, respectively.
Fig. 1.
Effect of inhibition of Rac1 activity by treatment with NSC-23766 on levels of cytosolic free Ca2+ ([Ca2+]cyt) and cell migration in differentiated intestinal epithelial cells (IEC-Cdx2L1 line). Before experiments, stable IEC-Cdx2L1 cells were grown in DMEM containing 4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 16 days to induce cell differentiation. These differentiated IEC-Cdx2L1 cells were exposed to NSC-23766 at different concentrations for 6 h. A: representative immunoblots of Western blot analysis for active Rac1, total Rac1, and RhoA proteins. Levels of active Rac1 (GTP-Rac1) were measured by pulldown assays (see materials and methods). Equal amounts of protein were applied to each lane and subjected to electrophoresis by 15% SDS-PAGE. Immunoblots were hybridized with the antibody specific for Rac1 (∼21 kDa) or RhoA (∼21 kDa). After the blot was stripped, β-actin (∼42 kDa) immunoblotting was performed as an internal control for equal loading. Three separate experiments were performed that showed similar results. B: representative records showing time course of [Ca2+]cyt changes after exposure to cyclopiazonic acid (CPA) in the absence (0Ca2+) or presence of extracellular Ca2+: a, control; b, cells exposed to NSC-23766 (75 μM) for 6 h. C: summarized data showing resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. D: changes in cell migration after wounding in cells described in A. Cell migration was assayed 6 h after part of the monolayer was removed, as described in materials and methods. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with control cells.
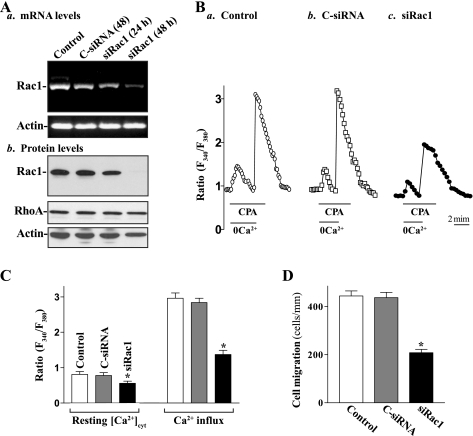
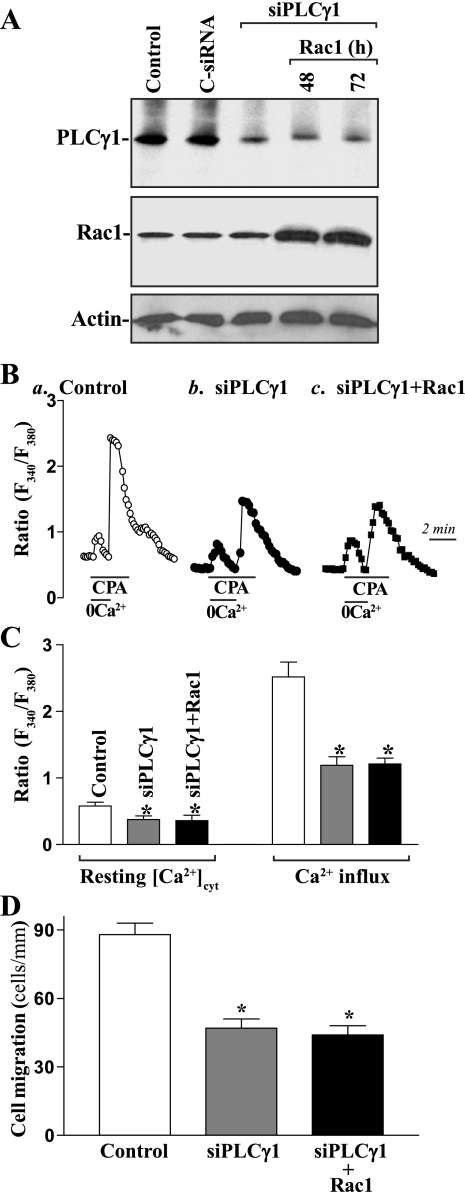
In the second study, we examined changes in levels of [Ca2+]cyt and cell migration after inhibition of Rac1 expression by siRNA specifically targeting the coding region of Rac1 mRNA (siRac1). These specific siRac1 nucleotides were designed to cleave rat Rac1 mRNA by activating endogenous RNase H and to have a unique combination of specificity, efficacy, and reduced toxicity (31, 33). Initially, we determined the transfection efficiency of the siRNA nucleotides in differentiated IEC-Cdx2L1 cells and demonstrated that >95% of cells were positive when they were transfected with a fluorescent FITC-conjugated siRac1 for 24 h (data not shown). As shown in Fig. 2A, transfection of differentiated IEC-Cdx2L1 cells with the siRac1 significantly inhibited expression of Rac1 as indicated by a decrease in levels of Rac1 mRNA and protein. Levels of Rac1 protein were decreased by ∼50% at 24 h and >90% at 48 h after the transfection. To determine the specificity of siRac1 used in this study, we reprobed the membrane with anti-RhoA antibody and showed that levels of RhoA protein were not affected when cells were transfected with siRac1 (Fig. 2Ab). Decreased levels of Rac1 expression by siRac1 also reduced resting [Ca2+]cyt and inhibited store depletion-induced Ca2+ influx (Fig. 2, B and C). The levels of resting [Ca2+]cyt were decreased by ∼25%, while store depletion-induced Ca2+ influx was decreased by ∼55% in cells transfected with siRac1 for 48 h. Furthermore, inhibition of Rac1 expression and subsequent decrease in [Ca2+]cyt by siRac1 suppressed cell migration after wounding (Fig. 2D). The rate of cell migration was decreased by ∼54% in Rac1-silencing cells. Transfection with C-siRNA at the same concentration showed no inhibitory effects on Rac1 expression, Ca2+ influx, and cell migration. In addition, neither siRac1 nor C-siRNA affected cell viability as measured by Trypan blue staining (data not shown). These findings indicate that inhibition of Rac1 activity not only reduces Ca2+ influx but also represses epithelial cell migration as well.
Fig. 2.
Effect of treatment with small interfering (si)RNA targeting the Rac1 mRNA coding region (siRac1) on levels of Rac1 expression, [Ca2+]cyt, and cell migration in differentiated IEC-Cdx2L1 cells. Cells were transfected with either control siRNA (C-siRNA) or siRac1 at a concentration of 0.5 μg/ml by LipofectAMINE technique. Total RNA and whole cell lysates were harvested 24 and 48 h after transfection. A: changes in levels of Rac1 mRNA and protein. a: levels of Rac1 mRNA as measured by RT-PCR analysis. The first-strand cDNAs, synthesized from total cellular RNA, were amplified with the specific sense and antisense primers, and PCR-amplified products displayed in agarose gel for Rac1 (∼472 bp) and β-actin (∼244 bp). b: representative immunoblots of Western analysis for Rac1 and RhoA proteins. Actin immunoblotting was performed as an internal control for equal loading. Three separate experiments were performed that showed similar results. B: representative records showing the time course of [Ca2+]cyt changes induced by exposure to CPA in the absence (0Ca2+) or presence of extracellular Ca2+: a, control cells; b, cells transfected with C-siRNA for 48 h; c, cells transfected with siRac1 for 48 h. C: summarized data showing resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with control cells and cells transfected with C-siRNA. D: summarized data showing cell migration 6 h after wounding by removal of part of the monolayer in cells as described in B. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with controls and cells transfected with C-siRNA.
Overexpression of the Rac1 gene increases Ca2+ influx and promotes cell migration.
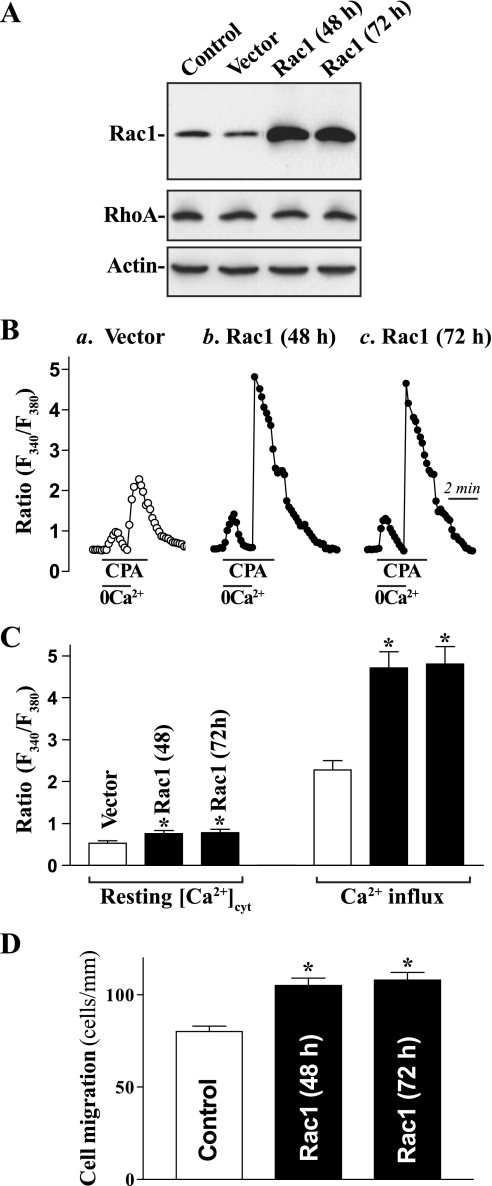
To further define the role of Rac1 activity in the regulation of Ca2+ influx and epithelial restitution, we examined the effect of overexpression of the wild-type Rac1 gene on levels of [Ca2+]cyt and cell migration in Caco-2 cells. This cell line was chosen for this study because it represents human epithelial cells and provides an excellent model for transient transfection. When Caco-2 cells were transfected with the expression vector containing the corresponding Rac1 cDNA under the control of pCMV promoter, levels of Rac1 protein were increased by approximately eightfold at 48 and 72 h thereafter (Fig. 3A). The vector that lacked exogenous Rac1 cDNA was used as a negative control in this experiment and did not alter Rac1 level. In addition, transfection with the Rac1 expression vector had no effect on levels of RhoA protein. As shown in Fig. 3, B and C, forced expression of the Rac1 gene significantly increased the resting [Ca2+]cyt and markedly enhanced store depletion-induced Ca2+ influx induced by CPA. Levels of resting [Ca2+]cyt were increased by ∼20% at 48 and 72 h after the transfection, while levels of store depletion-induced Ca2+ influx were increased by ∼50%. Furthermore, ectopic expression of the Rac1 gene also enhanced cell migration after wounding (Fig. 4D). The numbers of cells migrating over the wounded edge were increased by ∼25% in cells overexpressing Rac1. These results clearly show that increased Rac1 activity by ectopic expression of the Rac1 gene induces Ca2+ influx due to CCE and promotes epithelial cell migration after wounding.
Fig. 3.
Effect of ectopic expression of the Rac1 gene on [Ca2+]cyt and cell migration. A: representative immunoblots of Western blot analysis for Rac1 and RhoA proteins. Caco-2 cells were grown in minimum essential medium containing 10% dialyzed FBS and transfected using either the expression vector containing human wild-type Rac1 cDNA (Rac1) or control vector (vector) lacking Rac1 cDNA by lipofectAMINE technique. Rac1 and RhoA protein levels were measured 48 and 72 h after the transfection; equal loading was monitored by actin immunoblotting. B: representative records showing the time course of [Ca2+]cyt changes after exposure to CPA in the absence (0Ca2+) or presence of extracellular Ca2+: a, cells transfected with the empty vector for 48 h; b, cells transfected with the Rac1 for 48 h; c, cells transfected with the Rac1 for 72 h. C: summarized data showing resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with cells transfected with the vector. D: summarized data showing cell migration 6 h after wounding. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with cells transfected with the vector.
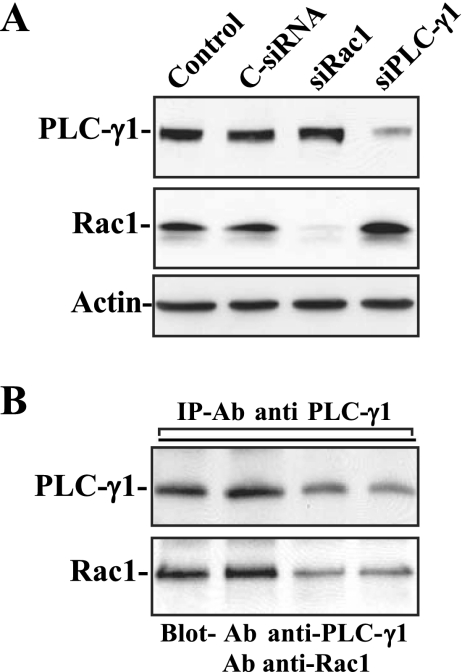
Fig. 4.
Physical interaction between Rac1 and phospholipase C-γ1 (PLC-γ1) proteins in differentiated IEC-Cdx2L1 cells. A: changes in total levels of PLC-γ1 and Rac1 proteins in control cells and Rac1- or PLC-γ1-silencing cells. Cells were transfected with either C-siRNA, siRNA targeting the Rac1 mRNA coding region (siRac1), or siRNA targeting the PLC-γ1 mRNA coding region (siPLC-γ1), and whole cell lysates were harvested 48 h after transfection. Levels of PLC-r1 and Rac1 proteins were examined by Western immunoblotting. B: levels of PLC-γ1 and Rac1 proteins in the complex immunoprecipitated (IP) by the anti-PLC-γ1 antibody. After cell lysates (500 μg) were immunoprecipitated by the specific antibody (Ab) against PLC-γ1, precipitates were subjected to electrophoresis by 10% SDS-PAGE. Levels of PLC-γ1 and Rac1 proteins were measured using Western blot analysis with the antibody against PLC-γ1 or Rac1.
PLC-γ1 interacts with Rac1 and is necessary for Rac1-induced Ca2+ influx and cell migration.
Our previous study (33) has demonstrated that PLC-γ1 is a biological regulator of Ca2+ influx after store depletion in IECs and plays a major role in regulating restitution after wounding. To test the possibility that PLC-γ1 is required for the stimulatory effect of Rac1 on Ca2+ influx and cell migration, we examined the direct interaction of PLC-γ1 with Rac1. Whole cell lysates were harvested and immunoprecipitated with the specific anti-PLC-γ1 antibody, and these precipitates were then examined by Western blot analysis using the antibody against Rac1 or PLC-γ1. As shown in Fig. 4, Rac1 was able to form a complex with PLC-γ1, and this Rac1/PLC-γ1 complex was decreased by silencing either Rac1 or PLC-γ1 through transfection with their specific siRNAs. We also examined the cellular distribution of PLC-γ1 and Rac1 using immunofluorescence staining and demonstrated that both PLC-γ1 and Rac1 were predominantly located in the cytoplasm (data not shown). These results indicate that Rac1 physically interacts with PLC-γ1 in IECs.
To determine whether formation of the Rac1/PLC-γ1 complex plays a role in regulating [Ca2+]cyt and epithelial restitution, we examined the influence of PLC-γ1 silencing on Ca2+ influx and cell migration in cells overexpressing Rac1. Transfection with siPLC-γ1 for 48 or 72 h significantly decreased the protein level of PLC-γ1, which was not altered by ectopic expression of the Rac1 gene (Fig. 5A). Levels of PLC-γ1 protein were decreased by ∼80% regardless with or without Rac1 overexpression. Consistent with our previous observations (33), PLC-γ1 silencing by transfection with siPLC-γ1 not only reduced resting [Ca2+]cyt but also inhibited store depletion-induced Ca2+ influx. The levels of resting [Ca2+]cyt were decreased by ∼25%, whereas CPA-induced Ca2+ influx was diminished by ∼50% in siPLC-γ1-transfected cells (Fig. 5, B and C). Importantly, Rac1 overexpression failed to overcome the reductions of both resting [Ca2+]cyt and store depletion-induced Ca2+ influx. Furthermore, PLC-γ1 silencing also inhibited cell migration after wounding and prevented the stimulation of cell migration by Rac1 overexpression (Fig. 5D). These findings strongly suggest that Rac1 promotes cell migration during restitution by increasing Ca2+ influx through its interaction with PLC-γ1.
Fig. 5.
Effect of PLC-γ1 silencing on [Ca2+]cyt and cell migration in cells overexpressing Rac1. A: representative immunoblots of Western blot analysis for PLC-γ1 and Rac1 proteins. Caco-2 cells were transfected with siPLC-γ1 alone or cotransfected with siPLC-γ1 and the Rac1 expression vector. Levels of PLC-γ1 and Rac1 proteins were measured 48 and 72 h after the transfection. Actin immunoblotting was performed as an internal control for equal loading. Three experiments were performed that showed similar results. B: representative records showing the time course of [Ca2+]cyt changes after exposure to CPA in the absence (0Ca2+) or presence of extracellular Ca2+: a, cells transfected with C-siRNA for 48 h; b, cells transfected with siPLC-γ1 for 48 h; c, cells cotransfected with siPLC-γ1 and Rac1 for 48 h. C: summarized data showing resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with cells transfected with C-siRNA. D: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with cells transfected with C-siRNA.
Decreased levels of Rac1/PLC-γ1 complex by polyamine depletion reduce Ca2+ influx and repress cell migration.
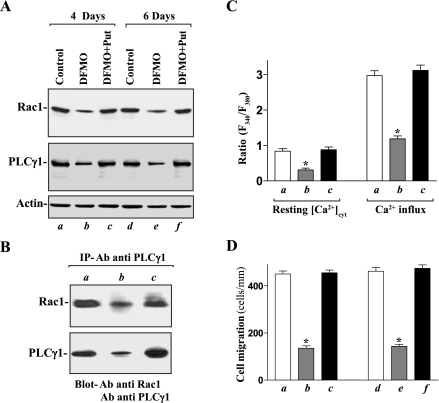
Polyamines are organic cations found in all eukaryotic cells and shown to stimulate early mucosal repair of gastric and duodenal injury in vivo (50, 53) and enhance epithelial restitution in vitro (26, 30, 33, 54). Our previous studies (27–29, 31) demonstrated that cellular polyamines promoted epithelial restitution primarily by controlling [Ca2+]cyt. To determine the role of cellular polyamines in the regulation of formation of Rac1/PLC-γ1 complex, differentiated IEC-Cdx2L1 cells were cultured in the DMEM containing 5 mM d,l-α-difluoromethylornithine (DFMO), a specific inhibitor of polyamine biosynthesis, for 4 and 6 days. Exposure to DFMO completely depleted putrescine within 48 h, but it took 4 days to totally deplete spermidine and substantially decrease spermine (data not shown) as reported in our previous studies (27, 54). Depletion of cellular polyamines by DFMO inhibited expression of Rac1 and PLC-γ1; the levels of Rac1 and PLC-γ1 proteins in the cells treated with DFMO for 4 and 6 days were decreased by ∼70% (Fig. 6A). Consistently, levels of Rac1/PLC-γ1 complex were also decreased by polyamine depletion (Fig. 6B). In the presence of DFMO, addition of exogenous polyamine putrescine (10 μM) to the cultures not only prevented the decreased levels of total Rac1 and PLC-γ1 proteins but also restored content of the Rac1/PLC-γ1 complex to near normal level. Polyamine depletion-induced inhibition of Rac1/PLC-γ1 complex formation was also associated with decreases in [Ca2+]cyt and cell migration. As shown in Fig. 6C, reduced levels of Rac1/PLC-γ1 complex following polyamine depletion were associated with decreases in resting [Ca2+]cyt and store depletion-induced Ca2+ influx. The level of resting [Ca2+]cyt and the amplitude of store depletion-induced Ca2+ influx in polyamine-deficient cells were decreased by ∼60%. The decreased resting [Ca2+]cyt and inhibited Ca2+ influx were also accompanied by an inhibition of cell migration after wounding (Fig. 6D). The number of cells migrating over the wounded edge was decreased by ∼70% in DFMO-treated cells. Restoration of levels of Rac1/PLC-γ1 complex by exogenous putrescine given together with DFMO not only returned resting [Ca2+]cyt and store depletion-induced Ca2+ influx to near normal levels but also abolished the inhibition of cell migration in polyamine-deficient cells.
Fig. 6.
Changes in levels of Rac1 and PLC-γ1 proteins, Rac1/PLC-γ1 complex, [Ca2+]cyt, and cell migration in the presence or absence of cellular polyamines. A: representative immunoblots of Western analysis for Rac1 and PLC-γ1 proteins. Differentiated IEC-Cdx2L1 cells were grown in the DMEM media containing 5 mM α-difluoromethylornithine (DFMO) alone or DFMO plus 10 μM putrescine (Put) for 4 and 6 days. Levels of Rac1 and PLC-γ1 proteins were measured by Western blot analysis, and equal loading was monitored by actin immunoblotting. Three separate experiments were performed that showed similar results. B: levels of Rac1 and PLC-γ1 proteins in the complex immunoprecipitated by the anti-PLC-γ1 antibody from cells exposed to DFMO or to DFMO plus Put for 4 days. C: summarized data showing resting [Ca2+]cyt concentrations (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with control cells and cells exposed to DFMO plus Put. D: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with control cells or cells treated with DFMO plus Put.
Rac1 overexpression fails to rescue [Ca2+]cyt and cell migration in the absence of PLC-γ1 following polyamine depletion.
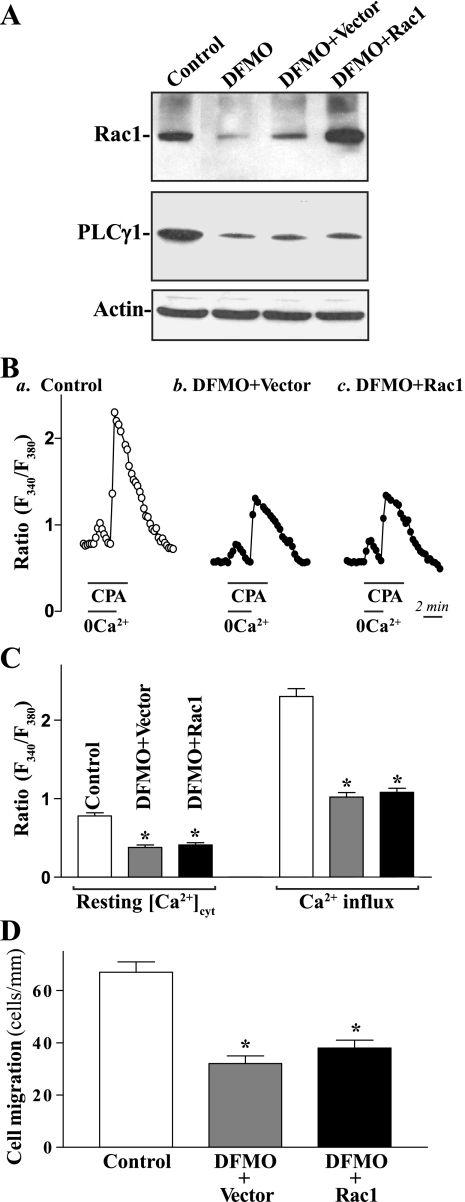
To further define the role of PLC-γ1 in regulation of Rac1/PLC-γ1 complex and epithelial restitution, we examined the effect of Rac1 overexpression on [Ca2+]cyt and cell migration in polyamine-deficient cells. In this study, cells were exposed to DFMO for 2 days and then transfected with the Rac1 expression vector for 48 h in the presence of DFMO. Results presented in Fig. 7 show that ectopic expression of the Rac1 gene in polyamine-deficient cells did not restore the resting [Ca2+]cyt and store depletion-induced Ca2+ influx to normal, because it did not alter levels of PLC-γ1 protein. There were no significant differences in levels of resting [Ca2+]cyt and Ca2+ influx due to CCE between DFMO-treated cells transfected with the Rac1 expression vector and DFMO-treated cells transfected with the control vector. Furthermore, Rac1 overexpression also failed to overcome the inhibition of cell migration after wounding in polyamine-deficient cells. These results indicate that both Rac1 and PLC-γ1 are important for stimulation of Ca2+ influx and epithelial restitution and that polyamine depletion-mediated inhibition of cell migration results from the decrease in [Ca2+]cyt as a result of reduction of Rac1/PLC-γ1 complex formation.
Fig. 7.
Effect of Rac1 overexpression on [Ca2+]cyt and cell migration in polyamine-deficient cells. A: representative immunoblots of Western blot analysis for Rac1 and PLC-γ1 proteins. Caco-2 cells were exposed to 5 mM DFMO for 2 days and then transfected with the Rac1 expression vector (Rac1) or control vector lacking Rac1 cDNA for 48 h in the presence of DFMO. Levels of Rac1 and PLC-γ1 protein levels were measured, and equal loading was monitored by actin immunoblotting. Three experiments were performed that showed similar results. B: representative records showing the time course of [Ca2+]cyt changes after exposure to CPA in the absence (0Ca2+) or presence of extracellular Ca2+: a, control; b, DFMO-treated cells transfected with control vector; c, DFMO-treated cells transfected with Rac1. C: summarized data showing resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) from cells described in B. Values are means ± SE; n = 20. *P < 0.05 compared with controls. D: summarized data showing cell migration 6 h after wounding in cells described in B. Values are means ± SE of data from 6 dishes. *P < 0.05 compared with controls.
DISCUSSION
Restitution in the gastrointestinal mucosa occurs by sloughing the damaged epithelial cells and migration of remaining viable cells from areas adjacent to, or just beneath, the injured surface to cover the wounded area in vivo (42, 51). Although precise regulation of epithelial restitution to reseal superficial wounds is crucial for the maintenance of mucosal integrity under physiological and pathological conditions, the exact mechanism underlying this primary repair modality remains poorly understood. Our previous studies have demonstrated that TRPC1 functions as a store-operated Ca2+ channel in IECs and regulates cell migration after injury (31). PLC-γ1 is implicated in the signaling pathway of control of intracellular Ca2+ homeostasis by altering TRPC1 activity, and inhibition of PLC-γ1 expression reduces levels of IP3, decreases [Ca2+]cyt due to reduction of CCE, and represses IEC migration (33). Here, we provide new evidence showing that Rac1 is involved in regulating IEC migration after wounding by altering PLC-γ1-induced Ca2+ signaling, thereby advancing our understanding of the mechanism of early mucosal restitution. Among the salient findings in this study is the discovery that Rac1 directly interacts with PLC-γ1 in IECs and that induced Rac1/PLC-γ1 complex after wounding is necessary for stimulation of store depletion-induced Ca2+ influx and cell migration during epithelial restitution.
The results reported herein clearly indicate that Rac1 activation promotes epithelial cell migration after wounding in differentiated IECs. Inhibition of Rac1 activity by NSC-23766 (Fig. 1) or Rac1 silencing with siRac1 transfection (Fig. 2) suppressed cell migration in differentiated IEC-Cdx2L1 cells, whereas ectopic overexpression of Rac1 increased cell migration after wounding (Fig. 3). Consistent with our current observations, Rac1 activation is also shown to sufficiently stimulate cell migration after wounding in undifferentiated IEC-6 cells (35). Since the rapid mucosal restitution of superficial wounds in vivo is the function of differentiated IECs from the surface of the mucosa rather than from undifferentiated IECs within the crypts (42, 51, 53), the results reported here from the differentiated IECs are novel observations about our understanding of the mechanism underlying cell migration after mucosal injury and are of important biological significance. In support of our findings, Rac1 directly regulates the polymerization of actin to produce lamellipodia in different types of cells including fibroblasts (10) and IECs (35). In addition, Rac1 also functions as an upstream regulator of RhoA and Cdc42, two other members of Rho family of GTPases, and activates their activities after exposure to stress (37–39). RhoA and Cdc42 are shown to be essential for stimulation of cell migration by regulating stress fiber formation and filopodia, respectively (20).
The data from the current study further demonstrate that Rac1 stimulates cell migration after wounding by activating PLC-γ1-induced Ca2+ signaling. As shown in Fig. 4, Rac1 directly interacted with PLC-γ1 and formed Rac1/PLC-γ1 complex. Overexpression of Rac1 increased [Ca2+]cyt and enhanced epithelial restitution, which were completely prevented by PLC-γ1 silencing (Fig. 5). These results are consistent with our previous studies (32, 33) and others (4, 44) showing that elevated [Ca2+]cyt is a major mediator for the stimulation of IEC migration and that PLC-γ1 plays a critical role in regulating Ca2+ homeostasis during intestinal epithelial restitution. Induction of PLC-γ1 increases [Ca2+]cyt at least in part through IP3-sensitive signaling pathway in IECs, because PLC-γ1 inhibition decreases the level of IP3 and reduces [Ca2+]cyt due to the reduction of CCE, while stimulation of PLC-γ1 increases IP3 and promotes Ca2+ influx (24, 33). Furthermore, IP3 is shown to trigger the release of Ca2+ from intracellular Ca2+ store through binding to IP3 receptors, thus resulting in activation of Ca2+ influx via SOC channels (46, 48). On the other hand, it also has been reported that PLC-γ1 augments Ca2+ entry induced by either a G protein-coupled receptor agonist or Ca2+ store depletion through its direct interaction with other signaling molecules such as TRPC channels (16, 22). Several studies have shown that the interaction of PLC-γ1 with TRPC3 requires the partial pleckstrin homology (PH) domain and that the partial PH domain of PLC-γ1 interacts with a complementary partial PH-like domain in TRPC3 to elicit lipid binding and cell surface expression of TRPC3 (47). PLC-γ1 is also shown to bind to and regulate TRPC1, but not to TRPC4 channels, in human keratinocytes (46). Since IECs highly express TRPC1 that participates in forming SOC channels (31), it is likely that PLC-γ1 activates SOC channels and promotes store depletion-induced Ca2+ entry in IECs by interacting with TRPC1.
The present study also indicates that polyamines are required for expression of Rac1 protein in differentiated IECs and are implicated in modulating the interaction between Rac1 and PLC-γ1 after wounding. Depletion of cellular polyamines decreased the formation of Rac1/PLC-γ1 complex, attenuated store depletion-induced Ca2+ influx, and repressed cell migration during restitution (Fig. 6). The effect of cellular polyamines on Rac1 levels is cell type-dependent, because there are no significant changes in Rac1 content in polyamine depleted IEC-6 cells (34). Overexpression of Rac1 alone failed to rescue Ca2+ influx after store depletion and cell migration in polyamine-deficient cells, because cellular PLC-γ1 was absent (Fig. 7). Polyamines, including spermidine, spermine, and their precursor putrescine, are intimately implicated in a wide variety of distinct biological functions (3, 8) and are shown to enhance early mucosal restitution (18, 26, 33, 50, 53). Increasing body of evidence also indicates that cellular polyamines stimulate epithelial cell migration during restitution primarily by controlling [Ca2+]cyt (27, 28, 55), but the exact process by which polyamines regulate [Ca2+]cyt remains unclear. As reported in our previous studies (28, 55), polyamines modulate [Ca2+]cyt partially by governing membrane potential through control of voltage-gated K+ (Kv) channel expression in IECs. Depletion of cellular polyamines inhibits Kv channel activity (as indicated by a decrease in whole cell Kv currents), causes membrane depolarization, and attenuates agonist-mediated increase in [Ca2+]cyt as a result of reduced driving force for Ca2+ influx. It has also been reported that PLC-γ1 expression requires polyamines and that polyamine-induced PLC-γ1 is involved in the control of [Ca2+]cyt during epithelial restitution (33). The current study provides further evidence that the formation of Rac1/PLC-γ1 complex is necessary for the stimulatory effect of polyamines on Ca2+ influx after wounding.
In summary, these results indicate that induced Rac1 promotes intestinal epithelial restitution after wounding by activating PLC-γ1-induced Ca2+ signaling. Inactivation of Rac1 by treatment with NSC-23766 or Rac1 silencing decreases store depletion-induced Ca2+ influx and suppresses cell migration, whereas ectopic expression of the Rac1 gene increases Ca2+ influx and enhances cell migration. Our study further demonstrates that Rac1 directly interacts with PLC-γ1 protein and that the formation of Rac1/PLC-γ1 complex is critical for activation of PLC-γ1-induced Ca2+ signaling after wounding. The observations from this study also help to further define the mechanism by which polyamines modulate [Ca2+]cyt during intestinal epithelial restitution by providing evidence showing that polyamines regulate the interaction of Rac1 with PLC-γ. These findings suggest that Rac1 functions as an upstream regulator of PLC-γ1-induced Ca2+ signaling and that induced interaction between Rac1 and PLC-γ1 enhances cell migration after wounding by increasing Ca2+ influx. Given the fact that Rac1/PLC-γ1-induced Ca2+ signaling is highly regulated by cellular polyamines and that levels of tissue polyamines in the damaged intestinal mucosa are dramatically increased (51, 52), activation of this signaling pathway is crucial for polyamine-dependent IEC migration after injury and contributes to the maintenance of intestinal epithelial integrity under biological conditions.
GRANTS
This work was supported by a Merit Review Grant from the Department of Veterans Affairs and by National Institutes of Health Grants DK-57819, DK-61972, and DK-68491 (to J.-Y. Wang) and HL-64945 and HL-66012 (to J. X.-J. Yuan).
Acknowledgments
J.-Y. Wang is a Research Career Scientist, Medical Research Service, U.S. Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bourguignon LY, Singleton PA, Diedrich F. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-γ promotes phospholipase Cγ1 activation, Ca2+ signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem 279: 29654–29669, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6: 373–390, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Yang YR, Lee SK, Kim IS, Ha SH, Kim EK, Bae YS, Ryu SH, Suh PG. Phospholipase C-γ1 potentiates integrin-dependent cell spreading and migration through Pyk2/paxillin activation. Cell Signal 19: 1784–1796, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Coniglio SJ, Jou TS, Symons M. Rac1 protects epithelial cells against anoikis. J Biol Chem 276: 28113–28120, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology 106: 1254–1262, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Gerner EW, Meyskens FL. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4: 781–792, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, Wang JY. Polyamines regulate β-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am J Physiol Cell Physiol 283: C722–C734, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem 281: 18652–18659, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Harter JL Critical values for Duncan's new multiple range test. Biometrics 16: 671–685, 1960. [Google Scholar]
- 12.Hiraoka K, Kaibuchi K, Ando S, Musha T, Takaishi K, Mizuno T, Asada M, Ménard L, Tomhave E, Didsbury J. Both stimulatory and inhibitory GDP/GTP exchange proteins, Smg GDS and Rho GDI, are active on multiple small GTP-binding proteins. Biochem Biophys Res Commun 182: 921–930, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-β2. Nat Struct Mol Biol 13: 1135–1140, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87: 519–529, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Liu L, Rao JN, Esmaili A, Strauch ED, Bass BL, Wang JY. JunD stabilization results in inhibition of normal intestinal epithelial cell growth through p21 after polyamine depletion. Gastroenterology 123: 764–779, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Ma HT, Venkatachalam K, Rys-Sikora KE, He LP, Zheng F, Gill DL. Modification of phospholipase C-γ-induced Ca2+ signal generation by 2-aminoethoxydiphenyl borate. Biochem J 376: 667–676, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maly K, Hechenberger G, Strese K, Tinhofer I, Wede I, Doppler W, Grunicke HH. Regulation of calcium signaling by the small GTP-binding proteins Ras and Rac1. Adv Enzyme Regul 47: 169–183, 2007. [DOI] [PubMed] [Google Scholar]
- 18.McCormack SA, Wang JY, Johnson LR. Polyamine deficient causes reorganization of F-actin and tropomyosin in IEC-6 cells. Am J Physiol Cell Physiol 267: C715–C722, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Moll J, Sansig G, Fattori E, van der Putten H. The murine rac1 gene: cDNA cloning, tissue distribution and regulated expression of Rac1 mRNA by disassembly of actin microfilaments. Oncogene 6: 863–866, 1991. [PubMed] [Google Scholar]
- 20.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution: characterization of cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Piccolo E, Innominato PF, Mariggio MA, Maffucci T, Iacobelli S, Falasca M. The mechanism involved in the regulation of phospholipase C-γ1 activity in cell migration. Oncogene 21: 6520–6529, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, Collard JG. Calcium signaling regulates translocation and activation of Rac. J Biol Chem 278: 39413–39421, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Rao JN, Li L, Li J, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol Gastrointest Liver Physiol 277: G1149–G1158, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JXJ, Wang JY. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 280: C993–C1007, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA Yuan JXJ, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: C885–C898, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Rao JN, Wang JY. Ca2+ signaling in epithelial restitution. In: Gastrointestinal Mucosal Repair and Experimental Therapeutics, edited by Cho CH and Wang JY. Basel, Switzerland: Karger, 2002, p. 29–42.
- 30.Rao JN, Guo X, Liu L, Zou T, Murthy KS, Yuan JXY, Wang JY. Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol 284: C848–C859, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JXJ, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol 290: G782–G792, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Rao JN, Wang JY. Regulation of Kv channel activity and intracellular junctions by polyamines in intestinal epithelial cells. In: Polyamine Cell Signaling: Physiology, Pharmacology, and Cancer Research, edited by Wang JY and Casero Jr RA, Totowa, NJ: Humana, 2006, p. 363–381.
- 33.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial cell restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, Johnson LR. Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G983–G995, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, Johnson LR. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem 278: 13039–13046, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Rhee SG Regulation of phosphoinositide-specific phospholipase C. Ann Rev Biochem 70: 281–312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridley AJ, Hall A. Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Quant Biol 57: 661–671, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401–410, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Sander E, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 147: 1009–1022, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol 145: 837–849, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirsat NV, Pignolo RJ, Kreider BL, Rovera G. A member of the ras gene superfamily is expressed specifically in T, B and myeloid hemopoietic cells. Oncogene 5: 769–772, 1990. [PubMed] [Google Scholar]
- 42.Silen W, Ito S. Mechanism for rapid-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985. [DOI] [PubMed] [Google Scholar]
- 43.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619–625, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thodeti CK, Massoumi R, Bindslev L, Sjölander A. Leukotriene D4 induces association of active RhoA with phospholipase C-γ1 in intestinal epithelial cells. Biochem J 365: 157–163, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traber PG, Wu GD. Intestinal development and differentiation: In: Gastrointestinal Cancers: Biology, Diagnosis, and Therapy, edited by Rustgi AK. Philadelphia, PA: Lippincott-Raven, 1995, p. 21–43.
- 46.Tu CL, Chang W, Bikle DD. Phospholipase C-γ1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol 124: 187–197, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434: 99–104, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol 4: E263–E272, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Yang C, Leskow FC, Sun J, Canagarajah B, Hurley JH, Kazanietz MG. Phospholipase Cγ/diacylglycerol-dependent activation of β2-chimaerin restricts EGF-induced Rac signaling. EMBO J 25: 2062–2074, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 259: G584–G592, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Wang JY, Johnson LR. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100: 333–343, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Wang JY, Johnson LR. Luminal polyamines substitute for tissue polyamines in duodenal mucosal repair after stress in rats. Gastroenterology 102: 1109–1117, 1992. [PubMed] [Google Scholar]
- 53.Wang JY, Viar MJ, Johnson LR. Transglutaminase in response to hypertonic NaCl-induced gastric mucosal injury in rats. Gastroenterology 104: 65–74, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Wang JY, McCormack SA, Johnson LR. Role of nonmuscle myosin II in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 270: G355–G362, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, Yuan JXJ. Role of K+ channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 278: C303–C314, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signaling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol 7: 255–261, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387–19394, 2006. [DOI] [PubMed] [Google Scholar]