Abstract
Mitochondrial transcription factor A (TFAM) is essential for mitochondrial DNA transcription and replication. TFAM transcriptional activity is decreased in diabetic cardiomyopathy; however, the functional implications are unknown. We hypothesized that a reduced TFAM activity may be responsible for some of the alterations caused by hyperglycemia. Therefore, we investigated the effect of TFAM overexpression on hyperglycemia-induced cytosolic calcium handling and mitochondrial abnormalities. Neonatal rat cardiomyocytes were exposed to high glucose (30 mM) for 48 h, and we examined whether TFAM overexpression, by protecting mitochondrial DNA, could reestablish calcium fluxes and mitochondrial alterations toward normal. Our results shown that TFAM overexpression increased to more than twofold mitochondria copy number in cells treated either with normal (5.5 mM) or high glucose. ATP content was reduced by 30% and mitochondrial calcium decreased by 40% after high glucose. TFAM overexpression returned these parameters to even higher than control values. Calcium transients were prolonged by 70% after high glucose, which was associated with diminished sarco(endo)plasmic reticulum Ca2+-ATPase 2a and cytochrome-c oxidase subunit 1 expression. These parameters were returned to control values after TFAM overexpression. High glucose-induced protein oxidation was reduced by TFAM overexpression, indicating a reduction of the high glucose-induced oxidative stress. In addition, we found that TFAM activity can be modulated by O-linked β-N-acetylglucosamine glycosylation. In conclusion, TFAM overexpression protected cell function against the damage induced by high glucose in cardiomyocytes.
Keywords: mitochondria, free radicals, SERCA2a
diabetic cardiomyopathy is characterized by impaired cardiac contractility and reduced myocardial performance without an attendant vascular or valvular disease and can lead to congestive heart failure. Studies in diabetic human patients and animal models have demonstrated the early development of diastolic dysfunction prior to the alteration of systolic function (10, 39). Eventually, however, nearly all aspects of cardiac contractility appear to become impaired (30, 37). Increasing evidence indicates that abnormalities in cardiac Ca2+ handling may be an important contributor to decreased contractile function in the diabetic heart. Ventricular contraction and relaxation are controlled largely by Ca2+ release from and uptake into the sarco(endo)plasmic reticulum (SR) (6). All aspects of SR function in the diabetic heart are depressed as documented by decreased SR Ca2+ uptake, reduced SR Ca2+ content, and impaired SR Ca2+ release (4, 22, 29). Impaired SR function appears to result from reduced activity and expression of the SR Ca2+ ATPase (SERCA2a) (12, 37), which is responsible for sequestering Ca2+ and inducing diastolic relaxation. The underlying mechanisms leading to abnormal SR function and calcium flux in the diabetic heart are poorly understood. Several factors have been implicated in the development of diabetic cardiomyopathy, including metabolic, biochemical, and ultrastructural changes within the cardiac myocyte (1, 3, 33). In particular, because of the primary role of mitochondria in ATP production, impairment of mitochondrial respiratory function is a key contributing factor to decreased contractile function of the diabetic heart. The mechanisms that contribute to the diabetes-induced decrease in mitochondrial function have only been incompletely explored.
Diabetic hyperglycemia results in a number of pathophysiological changes in the vascular system, but investigations of its role in cardiac myocyte function are limited. Recently, studies exposing cardiac myocytes to elevated extracellular glucose resulted in impaired cardiomyocytes contractility and calcium flux (31) and increased cytosolic Ca2+ (14). In addition, hyperglycemia elicits an increase in reactive oxygen species (ROS) production, because of increased input of reducing equivalents into the mitochondrial electron transport chain (5, 25). Hyperglycemia-induced cell damage is a consequence of increased flux through metabolic pathways such as the polyol pathway flux, advanced glycation end product formation, activation of protein kinase C (PKC) isoforms and increased hexosamine pathway flux (5, 9, 26, 32). Mitochondrial function is regulated by the mitochondrial DNA (mtDNA) as well as factors that regulate mtDNA transcription and/or replication. Mitochondrial function is highly impaired by hyperglycemic conditions in part because of decreased mitochondrial transcription factor A (TFAM) activity and/or expression (7, 19, 27, 28). TFAM is essential for the maintenance of mtDNA (20). Moreover, targeted disruption of TFAM in cardiac myocytes induced deletion of mtDNA and dilated cardiomyopathy (23, 38). Therefore, TFAM can be considered as a therapeutic target for mitochondrial dysfunction. In line with these principles, it has been reported that overexpression of TFAM ameliorates mitochondrial deficiencies in an ischemic model (18).
In this report we sought to investigate whether overexpression of TFAM could ameliorate hyperglycemia-induced calcium handling alterations by improvement of mitochondrial function. In addition, we investigated the mechanism by which hyperglycemia impairs TFAM function. Our findings showed that TFAM overexpression was able to improve cytosolic calcium transients and mitochondrial function of neonatal rat cardiac myocytes (NCM) exposed to hyperglycemic conditions. Furthermore, we found that TFAM activity is modulated by O-linked β-N-acetylglucosamine glycosylation (O-GlcNAcylation).
MATERIALS AND METHODS
All animal protocols were approved by the University of California, San Diego, Animal Subjects Committee and conform to the Guide for the Care and Use of Laboratory Animals as outlined by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Isolation and culture of NCM.
Primary cultures of NCM were prepared as described previously (15). Cells (3 × 106 cells per 10-cm plate) were plated onto gelatin-coated culture dishes. Medium consisted of 4.25:1 Dulbecco's modified Eagle's medium; medium 199, 10% horse serum, 5% fetal bovine serum, 1% penicillin/streptomycin/fungizone, and 5.5 mmol/l d-glucose. Cells were allowed to adhere to the plates for at least 24 h before different treatments. Cells were treated with medium supplemented with glucose at either normal glucose (NG; 5.5 mM plus 25 mM mannitol) or high glucose (30 mM) concentrations. Cells were also treated with adenovirus encoding mouse TFAM (Adv-TFAM), human O-linked β-N-acetyl-d-glucosaminidase (GCA), or vector control (Adv-Ctr) with a multiplicity of infection (MOI) of 20 for both viruses. The culture medium was changed daily for 2 days until the cells were harvested at the times indicated in the figures.
Construction of recombinant adenoviruses.
Mouse TFAM or human GCA [enzyme that removes O-linked β-N-acetyl-d-glucosamine (O-GlcNAc) residues from the protein] cDNAs were subcloned into a replication-deficient adenoviral vector under control of the promoter-enhancer region of the human cytomegalovirus targeting nuclear DNA. The general procedure has been previously described (16). The resulting plasmids were confirmed with the use of restriction enzyme digestion and DNA sequencing. The TFAM and GCA-expressing plasmids were then cotransfected into 293 cells with PJM17 using PolyFect transfection reagent (Qiagen, Valencia, CA). Plaques were isolated, propagated in 293 cells, and purified. The contamination of wild-type virus was ruled out by RT-PCR (40). Viral titers of purified stocks were then determined. An empty adenovirus without transgene (Adv-Ctr) was used as control group.
Calcium transients.
Calcium transients were recorded in single myocytes, with the use of indo-1. The method has been described previously (13, 36). Calcium transients were recorded from at least 20 cells per experiment from 3 separated experiments. Diastolic and systolic intracellular Ca2+ levels were inferred from the basal and maximal indo-1 ratio per cycle, respectively. Diastolic Ca2+ decay time (Tdecay) was calculated from the normalized Ca2+ transient.
ATP production rate and mitochondrial Ca2+ measurement.
ATP production rate from mitochondrial respiratory chain was measured as reported before (35) with some modifications. After the glucose/virus treatments, NCM were washed with phosphate-buffered saline (PBS, 150 mM NaCl, 10 mM potassium phosphate, pH 7.4) for two times, trypsinized, and pelleted by centrifugation for 5 min at 400 g. Cells were resuspended in PBS and incubated at 37°C for 15 min to deplete residual intracellular ATP stores and sources. NCM were added at a final concentration of 0.2 mg protein/ml into respiratory reaction mixtures containing 150 mM KCl, 75 mM Tris·HCl pH 7.4, 2 mM EDTA, 10 mM potassium dihydrogen phosphate, 0.1% (wt/vol) bovine serum albumin, 10 μg/ml digitonin, 10 mM succinate, and 1 mM ADP. The final reaction volume was 50 μl. The reactions were performed at 37°C for 15 min. After incubation, 50 μl of an 8% (wt/vol) trichloroacetic acid and 2 mM EDTA solution were added to terminate the reaction on ice. After vortexing, the tubes were kept at 4°C for 10 min. Thereafter, 500 μl Tris-acetate buffer was added to neutralize the extracts. ATP was measured with ATP assay reagents in bioluminescent somatic cell assay kit (Sigma).
Mitochondrial Ca2+ concentration (m[Ca2+]) was measured in living cells using ratiometric mitochondrial pericam as described before by us (2). Mitochondrial pericam has been constructed and described by Nagai et al. (24). Pericam is a circularly permuted yellow fluorescent family protein that was fused to calmodulin (CaM) and M13, a 26-residue peptide derived from the CaM-binding region of the skeletal muscle myosin. The Ca2+-bound CaM and M13 peptide form a stable and compact complex. We used the ratiometric pericam, which has a bimodal excitation spectrum peaking at 415 and 494 nm. To target pericam specifically to the mitochondrial matrix (mitochondrial pericam), it was fused to a cytochrome oxidase sequence. We cloned the gene coding for mitochondrial pericam into a replication-deficient adenoviral vector under control of the promoter-enhancer region of the human cytomegalovirus (Adv-mito-pericam).
Cells were infected with Adv-mito-pericam using 50 plaque-forming units (pfu)/cell. Cells cultured on chambered coverglass were perfused with HEPES-buffered media at room temperature. Chambers were mounted in a Nikon Diaphot epi-fluorescence microscope equipped with a ×100 fluor objective (oil immersion) interfaced to a dual-excitation lamp system (Solamere Technologies, Salt Lake City, UT) set at 410 nm for E1 and 485 nm for E2 via a set of filters. Fluorescence emission was filtered at 510 nm and directed to a photomultiplier tube. Additionally, an aperture mechanism allowed fluorescence to be collected from a selected portion of the field, which was always positioned over the cytoplasmic region of individual cells. Data were simultaneously collected from each emission channel at a rate of 20 Hz. Fluorescence measurements were performed in buffer containing 2 mM CaCl2. Measurements were typically carried out for 10–20 s on an individual cell, a time period during which there was minimal photobleaching of mitochondrial pericam. This was repeated with additional cells in other fields so that a total of up to 20 cells each group were surveyed. Mitochondrial pericam fluorescence was collected, and the ratio between the intensities of the two excitations (410/485) was calculated providing for relative comparisons of the mitochondrial calcium concentration between experimental treatments.
mtDNA copy number measurement with real-time PCR.
mtDNA copy number per nuclear genome was quantified with a described method (34). Cytochrome b was used as a marker for mtDNA and β-actin for nuclear DNA. DNA was extracted from NCM with DNAzol reagent (Invitrogen, Carlsbad, CA). Total DNA concentration was determined with ND-100 Spectrophotometer (Nanodrop Technology). Sequences for rat β-actin were 5′-TTCTGACCCATACCCACCAT-3′ and 5′-TTCTGACCCATACCCACCAT-3′ Sequences for rat cytochrome b for were 5′-CCGAAAATCTCACCCCCTAT-3′ and 5 ′-GCAGATGTGGGTGACTGATG-3′ Primers were designed with Primer3 software. SYBR Green PCR Master Mix (Applied Biosystems) was mixed with 200 nM primers, H2O, and DNA. 0.2 Nanograms of DNA were used for cytochrome b reaction and 20 ng DNA was used for β-actin reaction. RT-PCR was run in 7300 Real-Time PCR System (Applied Biosystems) at 50°C for 2 min, 95°C for 10 min, and then 95°C for 15 s followed by 60°C for 1 min for 40 cycles. The results were analyzed by 7300 System Sequence Detection Software, version 1.3.1. mtDNA per nuclear genome was calculated as the ratio of cytochrome b DNA level to β-actin DNA level.
Mitochondrial content.
Mitochondrial content was assessed using MitoTracker Green probe (Molecular Probes) as described before (8). MitoTracker Green probe preferentially accumulates in mitochondria regardless of the mitochondrial membrane potential and provides an accurate assessment of mitochondrial mass. Cells were washed with PBS and incubated at 37°C for 30 min with 100 nM MitoTracker Green FM. Cells were harvested using trypsin/EDTA and resuspended in PBS. Fluorescence intensity was measured with excitation and emission wavelengths of 490 and 516 nm, respectively, and values were normalized for total protein (mg/ml).
DNA binding activity of TFAM by electrophoretic mobility shift assay.
The assay was performed as previously described (19) with minor modifications. Briefly, a radioactive probe containing the nucleotide sequence of the heavy-strand promoter was prepared by annealing paired oligonucleotides with the sequences 5′-TTTCCTCCTAACTAAACCCTCTTTAC-3′ and 5′-GTAGGCAAGTAAAGAGGGTTTAGTTA-3′ and was labeled using [α-32P]dATP and DNA polymerase (New England). The reaction was performed at room temperature for 20 min in a volume of 20 μl. The reaction mixture contained 25 μg whole cell lysate, 2 μg poly(dI-dC) (Amersham), 10 mM Tris·HCl (pH 7.5), 50 mM NaCl, 0.5 mM EDTA, 0.5 mM dithiothreitol, 1 mM MgCl2, 4% glycerol, and 100,000 counts/min of labeled oligonucleotides. After the incubation, samples were loaded onto 6% polyacrylamide gels in 0.5× Tris-borate-EDTA buffer and run at 200 V for 2 h. The gels were dried, and the bands were visualized by autoradiography. Same amount proteins for each sample were loaded in 4–12% gradient Bis-Tris gel for TFAM protein analysis by Western blot.
Western blot analysis.
To determine TFAM, SERCA2a, and cytochrome-c oxidase subunit 1 (CytOx1) protein levels, myocytes were rinsed with PBS and then extracted with 0.2 ml lysis buffer (20 mM Tris, pH 7.4, 20 mM NaCl, 0.1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, and 1 mM dithiothreitol). Lysates were incubated on ice for 30 min, and cellular debris was pelleted at 10,000 g for 20 min at 4°C. Protein concentration in the supernatant was determined by Bio-Rad protein reagent. Protein (10–20 μg) diluted in lysis buffer and 4× Laemmli [62.5 mM Tris·HCl (pH 6.8), 2.35% SDS, 100 mM DTT, 10% glycerol, 1 mM EDTA, and 0.001% bromphenol blue] sample buffer was loaded after 5 min boiling onto precast 4–20% Bis-Tris polyacrylamide gel (Invitrogen) and transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked overnight at 4°C using 5% nonfat milk powder in Tris-buffered saline (+0.05% Tween 20) and exposed for 1 h to primary antibodies, washed 15 min, and incubated 1 h with secondary antibodies. Primary antibodies were SERCA2a, a goat polyclonal [SERCA2 (N-19): sc-8095, Santa Cruz Biotechnology]; TFAM antibody (a gift from Dr Samson T. Jacob, Ohio State University, Columbus, OH); and mouse CytOx1 antibody (Mitosciences, Eugene, OR). Secondary antibody were anti-mouse IgG-horseradish peroxidase (HRP) conjugated (Amersham) and anti-goat IgG-HRP conjugated (Pierce). Blots were also probed by a mouse monoclonal α-actin antibody as an internal control to ensure equivalent protein loading and protein integrity. Bands were visualized by reacting with chemiluminescent substrate (PerkinElmer Life Sciences) and exposed to film. Protein bands were quantified from scanned images using ImageJ software (NIH) and are expressed in arbitrary units.
Protein carbonyl assay.
Cell protein extracts were prepared as described for Western blots, and 20 μg protein was assayed for protein carbonyls as per the manufacturer's instructions (OxyBlot, Chemicon). Briefly, proteins were diluted into a final concentration of 6% SDS and reacted with 2,4-dinitrophenylhydrazine for 15 min. After the reaction, samples were neutralized, electrophoresed on NuPage gels (Invitrogen), transferred to nitrocellulose, and Western blotted using an antibody specific to the dinitrophenylhydrazone-derivatized residues on oxidatively damaged proteins. Blots were stripped and subsequently reprobed for actin to confirm equivalent protein loading.
Detection of glycosylated TFAM with wheat germ agglutinin-conjugated beads.
Wheat germ agglutinin-conjugated beads were used to precipitate the O-GlcNAcylated TFAM with the same procedure described previously (11). Cell lysate of NCM from a 10-cm plate was incubated with beads. After incubation, wheat germ agglutinin-conjugated beads were centrifuged at 5,000 rpm for 5 min and the supernatant was collected. The pellet containing the beads was mixed with 2× Laemmli buffer, boiled 5 min, and analyzed by Western blot. Unbound TFAM in the supernatant was analyzed in the same Western blot. The ratio of O-GlcNAc modified and unmodified TFAM was calculated with band densities.
Statistical analysis.
Results are presented as means ± SE from at least three different experiments. Statistical significance between different groups was evaluated using one-way ANOVA with Bonferroni's multiple-comparison posttest. P < 0.05 denotes the presence of a statistically significant difference.
RESULTS
General.
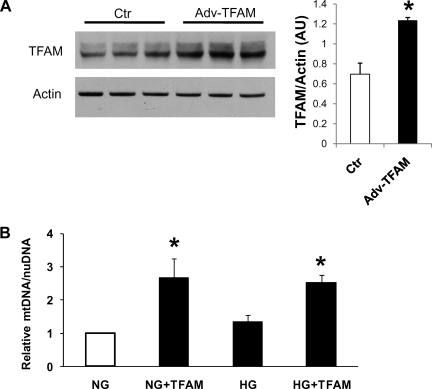
We tested Adv-TFAM in neonatal cardiac myocytes. Figure 1A shows that infection of myocytes for 48 h with Adv-TFAM increased TFAM protein levels by almost 100% as demonstrated by Western blot. There were no signs of cell toxicity by adenoviral TFAM overexpression at the MOI used in this study (20 pfu/cell).
Fig. 1.
A: adenoviral overexpression of mitochondrial transcription factor A (TFAM) in neonatal rat cardiomyocytes (NCM). TFAM adenovirus Adv-TFAM increased TFAM protein levels by 100%. B: TFAM overexpression effectively increases mitochondrial copy number in cardiomyocytes. Cardiomyocytes were treated 48 h with 5.5 mM glucose + 25 mM mannitol [normal glucose (NG)], NG plus Adv-TFAM (NG+TFAM), 30 mM glucose [high glucose (HG)], or HG plus Adv-TFAM (HG+TFAM). Relative mitochondrial DNA (mtDNA) was determined. Adenoviral TFAM overexpression dramatically increased mtDNA in cells treated either with NG (NG+TFAM) or with HG (HG+TFAM). Ctr, control; AU, arbitrary units; nuDNA, nuclear DNA. *P < 0.05 compared with NG from 3 experiments.
TFAM overexpression increases mitochondrial copy number.
Relative mtDNA was quantified in cardiac myocytes exposed to either NG (5.5 mM glucose, 25 mM mannitol) or high glucose (HG; 30 mM glucose) for 48 h treated with adenovirus encoding TFAM (Adv-TFAM) or an empty adenovirus as control. Cells infected with Adv-TFAM presented relative mtDNA increased by 150% independent of glucose concentration (Fig. 1B). HG did not change mtDNA. Mitochondrial mass assessed with MitoTracker Green was not altered by HG or TFAM overexpression (NG: 2.35 ± 0.18; NG+TFAM: 2.12 ± 0.032; HG: 2.23 ± 0.5; HG+TFAM: 2.45 ± 0.32, arbitrary fluorescent units/mg protein).
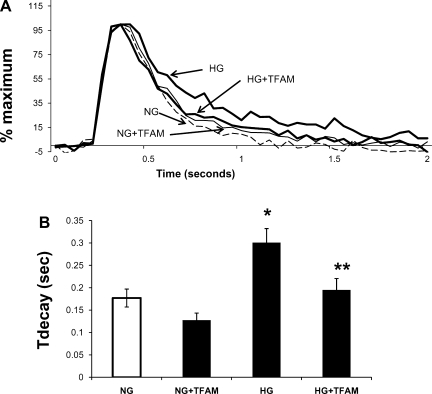
Influence of TFAM overexpression on calcium transients of cardiomyocytes exposed to HG.
Calcium transients in cardiomyocytes exposed to HG for 48 h were prolonged compared with transients of cells cultured in NG (Fig. 2). Time to calcium decay (Tdecay) in HG-treated cells was increased by 70% compared with NG (Fig. 2B). TFAM overexpression in HG-treated cells restored calcium decay to control values. Overexpression of TFAM in cells treated with NG tended to decrease Tdecay but was not statistically significant (Fig. 2B). Cells in NG not exposed to adenovirus presented similar Tdecay values to cells in NG exposed to adenovirus control (0.20 ± 0.023 vs. 0.18 ± 0.020 s, respectively).
Fig. 2.
Effects of TFAM overexpression on calcium transients of hyperglycemic cardiac myocytes. A: superimposed tracings of averaged cytosolic Ca2+ transients in cardiac myocytes treated with NG, NG+TFAM, HG, and HG+TFAM. Cells in NG and HG were treated with equivalent amount of empty adenovirus, which codifies no protein. Myocytes were electrically paced at 0.3 Hz. B: averaged Ca2+ decay time (Tdecay) values. Values are means ± SE of at least 20 myocytes per group from 3 experiments. (*P < 0.05 compared with NG, **P < 0.05 compared with HG).
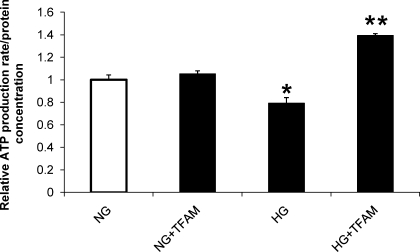
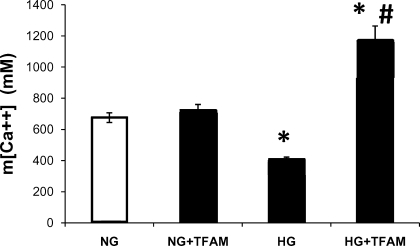
Reduced ATP production rate and m[Ca2+] in hyperglycemia are restored after TFAM overexpression.
ATP production was decreased by 30% in HG (Fig. 3); surprisingly, TFAM overexpression improved ATP production to values even higher than control (Fig. 3). ATP production rate in cells that were not exposed to adenovirus was similar to that found in cells in normal glucose treated with control adenovirus shown in Fig. 3 (0.97 ± 0.015 vs. 1.0 ± 0.04, respectively). m[Ca2+] was markedly reduced by 40% in cardiomyocytes cultured in HG for 48 h compared with cells exposed to NG. TFAM overexpression returned m[Ca2+] above control levels (Fig. 4). TFAM overexpression did not change m[Ca2+] in cells treated with NG (Fig. 4).
Fig. 3.
Cellular ATP content was decreased by HG and increased by expression of TFAM. ATP was measured in myocytes treated as in Fig. 1B. *P < 0.05 compared with NG, **P < 0.05 compared with NG and HG.
Fig. 4.
Mitochondrial Ca2+ concentration (m[Ca2+]) in NCM treated with HG and TFAM. HG decreased mitochondrial Ca2+ levels, and expression of TFAM (HG+TFAM) increased mitochondrial Ca2+ toward control levels (NG). TFAM expression in normal glucose condition (NG+TFAM) had no effect on mitochondrial Ca2+. Cells were maintained in NG or HG for 48 h and were then infected with Adv-mito-pericam and either Adv-Ctr or Adv-TFAM for 48 h. *P < 0.05 vs. NG and NG+TFAM, #P < 0.05 vs. HG. Values are means ± SE; n = 15 cells/group from 3 experiments.
Changes in mtDNA-encoded CytOx1.
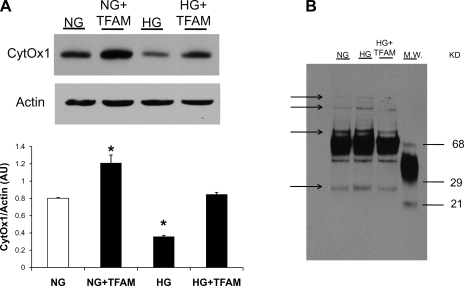
Cardiomyocytes exposed to HG presented reduced CytOx1 protein levels. TFAM overexpression restored CytOx1 to control values (Fig. 5).
Fig. 5.
A: effect of HG and TFAM overexpression on the expression of mtDNA-encoded cytochrome-c oxidase subunit 1 (CytOx1) in cardiac myocytes. Cells were treated with 5.5 mM glucose (NG), 30 mM glucose (HG), and 30 mM glucose plus Adv-TFAM (HG+TFAM). The Western blot shows that CytOx1 levels were decreased in HG and restored by TFAM overexpression. *P < 0.05 compared with NG. B: Western blot analysis of protein oxidation. HG increased protein oxidation (arrows show bands of carboxylated proteins). TFAM overexpression returned protein oxidation to NG levels.
SERCA2a protein is reduced by hyperglycemia and improved by TFAM overexpression.
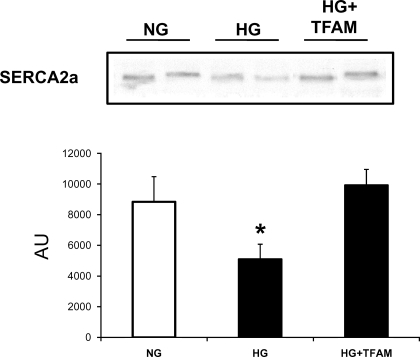
Cardiomyocytes exposed to HG exhibited reduced SERCA2a protein expression by 45% (Fig. 6). In contrast, high glucose-treated cells overexpressing TFAM exhibited SERCA2a protein levels in the control range.
Fig. 6.
Western blot showing the influence of HG and TFAM overexpression on the expression of sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) in cardiac myocytes. Cells were treated with 5.5 mM glucose (NG), 30 mM glucose (HG), and 30 mM glucose plus Adv-TFAM (HG+TFAM). SERCA2a levels were decreased in HG and restored by TFAM overexpression. *P < 0.05 compared with NG.
TFAM is glycosylated.
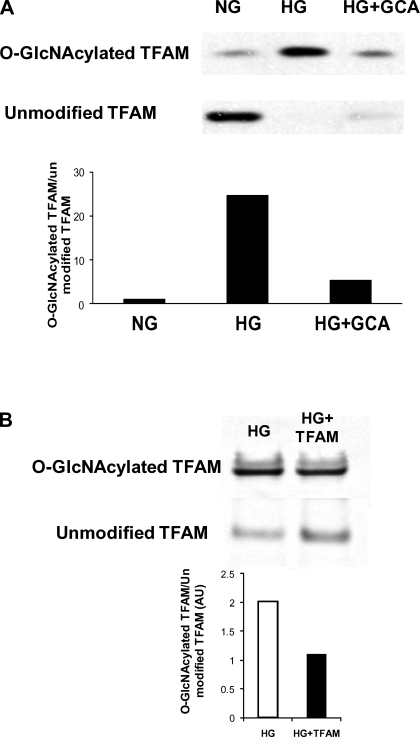
O-glycosylation can modulate protein activity. Therefore, we investigated whether TFAM is susceptible to O-GlcNAcylation. O-GlcNAc-modified proteins were precipitated from cell lysates with wheat germ agglutinin-conjugated beads, separated by electrophoresis, and tested with the anti-TFAM antibody. Results show that TFAM can be O-GlcNAc modified under NG conditions (Fig. 7A). High glucose treatment induced a dramatic increase of the glycosylated TFAM fraction. Specificity for O-GlcNAc modification was proved by the fact that cell lysates from cells overexpressing the enzyme that removes O-GlcNAc residues from the protein, GCA, presented levels of glycosylated TFAM similar to control cells (Fig. 7A). TFAM overexpression in HG-treated cells increased the nonglycosylated fraction of TFAM. The ratio of GlcNAcylated TFAM to unmodified TFAM was dramatically reduced after TFAM overexpression (Fig. 7B).
Fig. 7.
A: Western blot showing O-linked β-N-acetyl-d-glucosamine (O-GlcNAc) modification of TFAM. O-glycosylated proteins were precipitated with agarose wheat germ agglutinin beads and probed with TFAM antibody. Precipitated and nonprecipitated fractions were analyzed. HG resulted in a dramatic increase of O-GlcNAcylated TFAM. O-linked β-N-acetyl-d-glucosaminidase (GCA) overexpression specifically reduced GlcNAcylated TFAM after HG. Bar graph shows that ratio of GlcNAcylated TFAM to unmodified TFAM was decreased after GCA overexpression. B: Western blot showing influence of TFAM overexpression on O-GlcNAc modification of TFAM. O-glycosylated proteins were precipitated with agarose wheat germ agglutinin beads and probed with TFAM antibody. Precipitated and nonprecipitated fractions were analyzed. Unmodified TFAM expression increased by 98% after transfection (HG+TFAM). GlcNAcylated TFAM was not changed. Bar graph indicates that ratio of GlcNAcylated TFAM to unmodified TFAM was decreased after TFAM overexpression.
TFAM DNA O-GlcNAcylation.
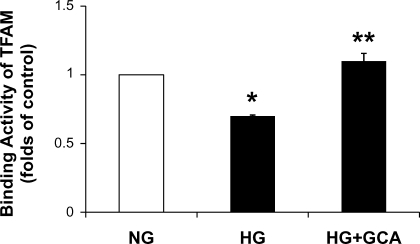
The binding activity of TFAM to the [α-32P]-labeled oligonucleotide containing the heavy-strand promoter sequence within the d-loop was investigated using an electrophoretic gel mobility shift assay. The specificity of the shifted band was confirmed by the finding that the band disappeared in the presence of a 50-fold molar excess of unlabeled oligonucleotide (data not shown). The binding activity of TFAM in NCM treated with HG was reduced by 30% (Fig. 8). Adenoviral GCA expression fully recovered TFAM binding activity in hyperglycemic conditions.
Fig. 8.
Binding activity of TFAM to the d-loop region in NCM treated with NG (5.5 mM glucose + 25 mM mannitol), HG (30 mM glucose), and HG plus Adv-GCA. Binding activity of TFAM was assessed by electrophoretic mobility shift assay in NCM. The binding activity was normalized with TFAM protein contents analyzed in Western blot. *P < 0.05 compared with NG, **P < 0.05 compared with HG (n = 3).
DISCUSSION
Exposure of cardiomyocytes to hyperglycemia impairs cell function, but the contributing mechanisms are only incompletely explored. Impairment of the function of TFAM may be a contributing factor not yet fully explored. We therefore determined whether overexpression of TFAM could restore mitochondrial function and improve cytosolic calcium handling of cardiac myocytes exposed to hyperglycemic conditions. Our results show for the first time that TFAM overexpression restored calcium transients to normal by improving mitochondrial ATP production and reducing ROS-induced protein oxidation. We also demonstrated that TFAM is susceptible of O-GlcNAcylation that was increased by hyperglycemia decreasing its activity.
Mitochondrial dysfunction is a key factor in the development of diabetic complications. Hyperglycemia is known to directly cause mitochondrial damage by increasing ROS production, which in turn will trigger increase of metabolic pathways with deleterious effects. The most studied among these pathways are polyol pathway flux, advanced glycation end product formation, activation of PKC isoforms, and increased hexosamine pathway flux (5, 9, 26, 32). One consequence of these alterations is the reduction of mitochondrial transcription factors, which results in an impaired mitochondrial ATP production. A large number of studies have documented a toxic effect of hyperglycemia in the transcription activity and expression of TFAM (7, 19, 27, 28). In view of the essential role of TFAM in maintaining mtDNA and modulating mitochondrial biogenesis (20), it can be suggested as a therapeutic target to prevent hyperglycemia-induced cell toxicity. In the present study, we found a reduced mitochondrial function as evaluated by the rate of ATP production and mitochondrial calcium concentration. These parameters were decreased after cardiomyocytes were exposed to 30 mM glucose. TFAM overexpression restored these parameters to levels found in cells treated with normal glucose (5.5 mM). ATP was increased in HG + TFAM to higher levels than control values (Fig. 3). The fact that ATP production and mitochondrial Ca2+ concentration increased after TFAM overexpression indicates that the oxidative phosphorylation was improved. ATP production could increase if mitochondrial mass increases; however, we did not find increase in mitochondrial mass. To increase mitochondrial Ca2+ concentration, an increase in mitochondrial membrane potential (ΔΨ) is necessary. Increase in ΔΨ is linked with an improvement in mitochondrial electron transport and coupling. These results open the possibility that increased substrate availability (high glucose) and a more efficient oxidative phosphorylation after TFAM overexpression could lead to increased ATP production compared with normal glucose condition. These beneficial effects of TFAM can be explained by an increase in mitochondrial copy number that we observed as a result of TFAM overexpression. We did not observe increase in mitochondrial mass assessed with a fluorometric assay. These data are consistent with findings in a TFAM cardiac-specific transgenic mouse with elevated TFAM expression but normal mitochondria number and size (18). An additional mechanism could be an increase in mtDNA-encoded proteins in the respiratory chain due to increased transcriptional activity of TFAM. In the present study, we investigated the expression level of the mtDNA-encoded protein CytOx1. Exposure of cardiomyocytes to high glucose leads to reduction of CytOx1 expression. However, TFAM overexpression increased CytOx1 to control levels despite the hyperglycemic conditions, which is consistent with an increase in the transcriptional activity of TFAM. In our study we did not observe a reduction of TFAM protein levels induced by high glucose in the times tested (data not shown), which is in accordance with results obtained in HepG2 cells cultured in the presence of 30 mM glucose for 48 h (28). These results suggest that hyperglycemia impairs the transcriptional activity of TFAM rather than the expression of the protein, which is consistent with results observed in vivo in the diabetic heart (19, 27). However, the mechanism responsible for a reduced TFAM activity in hyperglycemia is unknown. In the present work, alterations in mitochondrial function and a reduced CytOx1 expression suggest a diminished TFAM transcriptional activity in HG, which can be in part due to reduced TFAM DNA binding observed here. Hyperglycemia stimulates the hexosamine pathway flux promoting the O-GlcNAcylation of several proteins in the cell. The O-GlcNAc modification is able to alter the biochemical properties of the proteins in different ways. We explored the possibility that TFAM could be modulated by O-GlcNAc modification. On the basis of results with wheat germ agglutinin-conjugated bead precipitation, we concluded that TFAM is O-GlcNAcylated. This idea was further supported by experiments immunoprecipitating TFAM with the TFAM-specific antibody and identification of the protein with the O-GlcNAc-specific antibody RL-2 (17) (data not shown). In addition, overexpression of the enzyme that removes O-GlcNAc residues from proteins, β-N-acetyl-d-glucosaminidase (GCA), diminished the O-GlcNAc modified TFAM. We found that TFAM DNA binding was reduced in HG and that removal of O-GlcNAc modification by GCA overexpression returned TFAM DNA binding activity towards normal, suggesting that O-glycosylation of TFAM leads to reduced activity of the protein. Furthermore, GCA overexpression is able to improve HG-induced mitochondrial dysfunction in cardiomyocytes and can be an alternative approach to improve mitochondrial function (unpublished observations). TFAM overexpression increased mainly the levels of nonglycosylated TFAM. Therefore, more unmodified (active form) TFAM protein would be available to improve mitochondrial function.
TFAM overexpression was able to improve mitochondrial ATP production in hyperglycemic conditions. This beneficial effect of TFAM in the cellular energy balance may lead to recover cytosolic calcium handling since SERCA2a activity is ATP dependent. Here we observed prolonged calcium transients in cells exposed to HG. In contrast, cells treated with HG and TFAM overexpression presented calcium transients in the normal range, indicating a beneficial effect of TFAM overexpression on the overall cellular function as deduced by the improved calcium transient. SERCA2a expression was reduced by HG and restored by TFAM overexpression. It has been reported that oxidative stress can downregulate SERCA2a gene transcription through modification of Sp1 binding (21). Here we found that hyperglycemia increased protein oxidation and TFAM overexpression was able to return it to normal glucose levels, explaining in part the increase in SERCA2a protein levels under HG conditions and TFAM overexpression (Fig. 5B). These results indicate that TFAM overexpression is able to protect against hyperglycemia-induced oxidative stress damage acting primarily on mitochondrial level but having an impact on overall cell function. Further investigation is needed to evaluate the beneficial effects of TFAM on diabetic organisms that are more complex than isolated cardiomyocytes.
In summary, we demonstrated that TFAM overexpression is able to restore the depressed mitochondrial function provoked by hyperglycemia. Improved ATP production was associated with normalization of calcium flux. The decreased transcriptional activity of TFAM in hyperglycemia can be mediated by an O-GlcNAc modification of the protein.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-66917 (to W. H. Dillmann) and partially supported by Grant P60 MD00220 from the San Diego EXPORT Center, National Center of Minority Health and Health Disparities, National Institutes of Health. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279: E1104–E1113, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol 292: H1755–H1763, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bhimji S, Godin DV, McNeill JH. Myocardial ultrastructural changes in alloxan-induced diabetes in rabbits. Acta Anat (Basel) 125: 195–200, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard RA, Bose D. Influence of experimental diabetes on sarcoplasmic reticulum function in rat ventricular muscle. Am J Physiol Heart Circ Physiol 260: H341–H354, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Carafoli E Intracellular calcium homeostasis. Annu Rev Biochem 56: 395–433, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Choi YS, Kim S, Pak YK. Mitochondrial transcription factor A (mtTFA) and diabetes. Diabetes Res Clin Pract 54, Suppl 2: S3–S9, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4: 75–87, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Fein F, Kornstein L, Strobeck J, Capasso J, Sonnenblick E. Altered myocardial mechanics in diabetic rats. Circ Res 47: 922–933, 1980. [DOI] [PubMed] [Google Scholar]
- 11.Gandy JC, Rountree AE, Bijur GN. Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1. FEBS Lett 580: 3051–3058, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol Endocrinol Metab 244: E528–E535, 1983. [DOI] [PubMed] [Google Scholar]
- 13.Giordano FJ, He H, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation 96: 400–403, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RK, Wittenberg BA. 19F nuclear magnetic resonance studies of free calcium in heart cells. Biophys J 65: 2547–2558, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartong R, Villarreal FJ, Giordano F, Hilal-Dandan R, McDonough PM, Dillmann WH. Phorbol myristate acetate-induced hypertrophy of neonatal rat cardiac myocytes is associated with decreased sarcoplasmic reticulum Ca2+ ATPase (SERCA2) gene expression and calcium reuptake. J Mol Cell Cardiol 28: 2467–2477, 1996. [DOI] [PubMed] [Google Scholar]
- 16.He H, Meyer M, Martin JL, McDonough PM, Ho P, Lou X, Lew WY, Hilal-Dandan R, Dillmann WH. Effects of mutant and antisense RNA of phospholamban on SR Ca2+-ATPase activity and cardiac myocyte contractility. Circulation 100: 974–980, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Holt G, Snow C, Senior A, Haltiwanger R, Gerace L, Hart G. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104: 1157–1164, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, Tsutsui H. Overexpression of mitochondrial transcription factor A ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112: 683–690, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa A, Nishio Y, Kashiwagi A, Inagaki H, Kikkawa R, Horiike K. Reduced activity of mtTFA decreases the transcription in mitochondria isolated from diabetic rat heart. Am J Physiol Endocrinol Metab 282: E778–E785, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7: 39–44, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Koitabashi N, Arai M, Tomaru K, Takizawa T, Watanabe A, Niwano K, Yokoyama T, Wuytack F, Periasamy M, Nagai R, Kurabayashi M. Carvedilol effectively blocks oxidative stress-mediated downregulation of sarcoplasmic reticulum Ca2+-ATPase 2 gene transcription through modification of Sp1 binding. Biochem Biophys Res Commun 328: 116–124, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D. Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol Heart Circ Physiol 270: H1529–H1537, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Wang J, Wilhelmsson H, Hansson A, Thoren P, Duffy J, Rustin P, Larsson NG. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc Natl Acad Sci USA 97: 3467–3472, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci USA 98: 3197–3202, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl 77: S26–S30, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Nishio Y, Kanazawa A, Nagai Y, Inagaki H, Kashiwagi A. Regulation and role of the mitochondrial transcription factor in the diabetic rat heart. Ann NY Acad Sci 1011: 78–85, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol 225: 214–220, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Penpargkul S, Fein F, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13: 303–309, 1981. [DOI] [PubMed] [Google Scholar]
- 30.Penpargkul S, Schaible T, Yipintsoi T, Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res 47: 911–921, 1980. [DOI] [PubMed] [Google Scholar]
- 31.Ren J, Gintant GA, Miller RE, Davidoff AJ. High extracellular glucose impairs cardiac E-C coupling in a glycosylation-dependent manner. Am J Physiol Heart Circ Physiol 273: H2876–H2883, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Robertson RP Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279: 42351–42354, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem 180: 53–57, 1998. [PubMed] [Google Scholar]
- 34.Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM Jr, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287: E896–E905, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Steyn SJ, Pieterse DJ, Mienie LJ, Van der Schyf CJ. Measurement of mitochondrial respiration in permeabilized murine neuroblastoma (N-2alpha) cells, a simple and rapid in situ assay to investigate mitochondrial toxins. J Biochem Biophys Methods 62: 25–40, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Suarez J, Belke DD, Gloss B, Dieterle T, McDonough PM, Kim YK, Brunton LL, Dillmann WH. In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 286: H68–H75, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 51: 1166–1171, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson NG. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet 21: 133–137, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Zarich S, Arbuckle B, Cohen L, Roberts M, Nesto R. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll Cardiol 12: 114–120, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Zhang WW, Koch PE, Roth JA. Detection of wild-type contamination in a recombinant adenoviral preparation by PCR. Biotechniques 18: 444–447, 1995. [PubMed] [Google Scholar]