Abstract
We have previously established a correlation between reduced mitochondrial glycerol-3-phosphate acyltransferase-1 (GPAT-1) activity and decreased proliferation in splenic T-lymphocytes from aged rats. To better understand the immunoregulatory role of GPAT-1, we examined T-lymphocyte function in young GPAT-1 knockout (KO) mice. We show that without GPAT-1, T-lymphocyte proliferation is inhibited and activation induced apoptosis is increased. Th-1 (IL-2 and IFN-γ) cytokine secretion is reduced, and Th-2 (IL-4 and IL-10) cytokine secretion is increased. These changes may be due to alterations in membrane lipid composition since we found changes in the relative content of individual phospholipid species. Furthermore, we show increased arachidonate content and subsequent increased prostaglandin E2 secretion, which may inhibit T-lymphocyte proliferation. Taken together, we show a novel link between GPAT-1 and changes in T-lymphocyte function. These data have broad health implications because GPAT-1 suppression has recently been implicated as a new target for preventing insulin sensitivity and hepatic steatosis and we show that immune function may also be affected. Interestingly, the changes in young GPAT-1 KO splenic T-lymphocytes are similar to defects commonly seen in T-lymphocytes from aged rodents, which further underscores the significance of GPAT-1 in T-lymphocyte function.
Keywords: lipid mediators, cell proliferation, spleen and lymph nodes
glycerol-3-phosphate acyltransferase (GPAT) catalyzes the initial step in de novo glycerophospholipid and triacylglycerol (TAG) biosynthesis. GPAT converts glycerol-3-phosphate and acyl-CoA into lysophosphatidic acid (lysoPA). The lysoPA is then acylated to phosphatidic acid, which is the precursor to TAG and glycerophospholipids (14). Currently, three isoforms of GPAT have been identified. Two isoforms are found on the mitochondria (GPAT-1 and GPAT-2) (18) and one is found on the microsome (GPAT-3) (3). The exact contribution of each GPAT isoform to cellular phospholipid composition is not clear. Recent evidence has shown that GPAT-1 increases palmitate content in the sn-1 position of phospholipids. Furthermore, several reports in vitro and in vivo have shown that GPAT-1 is important in TAG and VLDL production in the liver (11, 17, 19). However, the exact role of GPAT-1 in cellular function remains unclear.
The T-lymphocyte is a key immune cell regulating both the type and extent of an immune response. The T-lymphocyte influences immune function by proliferating in response to antigen stimulation and secreting immunoregulatory molecules like cytokines and eicosanoids. For example, Th-1 cytokines [interleukin-2 (IL-2), interferon-γ (IFN-γ)] promote cell- mediated immunity, whereas Th-2 cytokines (IL-4, IL-10) promote humoral immunity. Reduced T-lymphocyte IL-2 secretion and subsequent proliferation is a hallmark of aging in both humans and rodents. A major mechanism thought to be involved in the reduced proliferation is an increase in the membrane cholesterol-to-phospholipid ratio thereby reducing plasma membrane receptor function. Altered membrane lipid composition is commonly observed in many aged cell types and has led to the “membrane gate theory” to explain aged cellular dysfunction (28). The biochemical and molecular mechanism(s) explaining this observation are relatively unexplored. Attempts have been made to regulate T-lymphocyte function by adding or removing cholesterol by dietary feeding or using cyclodextrin, which binds cholesterol (10, 16). To date, there is no known method or model to alter glycerophospholipid content in T-lymphocytes. Therefore, part of the objective of the present study is to determine whether the GPAT-1 knockout (KO) mouse may be a good model to examine alterations in glycerophospholipid content in T-lymphocyte function.
Our lab has presented evidence to support GPAT-1 regulating T-lymphocyte proliferation. We have previously reported a correlation between reduced GPAT-1 activity and decreased proliferation in aged rat splenic T-lymphocytes (8). We next established a cause/effect relationship by showing that splenic T-lymphocyte proliferation is dramatically reduced in young GPAT-1 KO mice (9). T-lymphocyte proliferation is a general indicator of immune function. T-lymphocyte proliferation can be regulated by multiple mechanisms such as changes in membrane lipid composition, cytokine and/or eicosanoid production, and apoptosis. Therefore we examined these parameters to gain insight into the potential mechanisms by which GPAT-1 regulates T-lymphocyte function in KO mice.
MATERIALS AND METHODS
Materials.
All antibodies were purchased from BD Pharmingen (La Jolla, CA) unless otherwise noted. All chemicals were reagent grade or better from Sigma-Aldrich (St. Louis, MO).
Mice, T-lymphocyte isolation, and stimulation.
C57BL/6 GPAT-1 KO mice were obtained from Dr. Rosalind Coleman (University of North Carolina at Chapel Hill) and bred in our animal facilities. Mice were fed a commercial chow diet (Prolab Rat/Mouse/Hamster 2000) provided by the animal facility. Offspring were numbered to monitor sex differences or differences between litters. Since no differences in sex or litter were observed, all the data reported here include male and female mice from the same and different litters. Mice were housed on a 12:12-h light-dark cycle and had free access to commercial chow food and water. At 6–8 wk of age, splenic T-lymphocytes were isolated from wild-type, heterozygous, and KO mice using negative selection Immulan columns (Biotecx, Houston, TX) as per the manufacturer's instructions, yielding a 90% pure splenic T-lymphocyte population. Isolation by negative selection prevents perturbation of the T-lymphocyte's receptor during the isolation procedure, as occurs with isolation via positive selection. T-lymphocytes were counted using the Cell-Dyn 900 Hematology Analyzer (Sequoia-Turner, Mountain View, CA). T-lymphocytes were stimulated at 37°C for the indicated times in prewarmed complete RPMI 1640 culture media (10% heat-inactivated fetal bovine serum plus 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μM 2-mercaptoethanol, and 100 mM l-glutamine) with either 10 μg/ml plate-bound anti-CD3 and 1 μg/ml anti-CD28 in NaHCO3 or 10 nM phorbol myristate acetate (PMA) and 1 μM ionomycin (9). All animal procedures used were approved by the University of Texas Animal Use and Care Committee.
Flow cytometric receptor and activation-induced apoptosis analysis.
Splenic T-lymphocytes were cultured with either CD3/CD28, PMA/ionomycin, or without stimulus at 2 × 106 cells per well for 20 h. Splenic T-lymphocytes were treated with 200 μl 1× RBC Lysis buffer (eBioscience, San Diego, CA) for 2 min to lyse any remaining red blood cells that might interfere with analysis. Lysis was ceased with the addition of 5 ml PBS and centrifugation at 250 g. Cell pellets were incubated with Fc block for 10 min and then stained with appropriate antibodies to determine markers of splenic activation [CD3, CD4, CD8, CD69, CD28, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and CD25]. Excess antibodies were removed by washing with PBS with 0.1% NaN3, and cells were fixed in 0.5% paraformaldehyde in PBS. Analysis was performed using a BD FACSCalibur flow cytometer and CellQuest Pro software (BD Pharmingen, San Diego, CA). Following 20 h of stimulation with either CD3/CD28 or PMA/ionomycin, splenic T-lymphocytes were analyzed for cell death using the Vybrant Apoptosis Assay (Molecular Probes, Carlsbad, CA) as described by the manufacturer. Cells were stained with propidium iodide and annexin V kit components, diluted with binding buffer, and analyzed with the BD FACSCalibur flow cytometer and CellQuest Pro software (13).
T-lymphocyte proliferation.
Splenic T-lymphocytes were cultured at 37°C with complete media in the presence or absence of 10 μg/ml plate-bound anti-CD3 for 0, 8, 16, 20, or 24 h as described in Mice, T-lymphocyte isolation, and stimulation. T-lymphocyte proliferation was measured using the MTT Cell Proliferation Assay as described by the manufacturer (American Type Cell Culture, Manassas, VA). Plastic 96-well plates were precoated with either anti-CD3 and anti-CD28, PMA and ionomycin, or NaHCO3 alone before the addition of cells. The CD3/CD28 plates were incubated in a humidified incubator containing 5% CO2 at 37°C for 4 h to facilitate binding of the antibody to the wells. Plates were washed with sterile PBS and 1.5 × 106 T-lymphocytes in 100 μl cell culture media were added to their corresponding wells. Cells were incubated for 18 h at 37°C to induce proliferation. Then, 20 μl MTT reagent was added to each well, mixed gently, and incubated for ∼4 h. The cells were permeabilized with 100 μl of detergent and incubated at room temperature for 2 h. The plate was read at an absorbance of 570 nm, and values were corrected for background absorbance with wells containing no cells (9).
Cytokine secretion.
IL-2, IFN-γ, IL-4, and IL-10 secretion into the culture media was measured using commercially available ELISA kits from eBioscience (San Diego, CA). Stimulated and unstimulated cells were prepared as described above for proliferation studies, except that only the 20-h time point was used since this is when maximum proliferation was observed. The cells were pelleted following stimulation, and their supernatant was collected. The ELISA was performed according to the manufacturer's instructions, and cytokines were quantitated using standards supplied by the manufacturer. Absorbencies were read at 450 nm, with a reference filter of 570 nm, using an UltraMarc plate reader (Bio-Rad, Hercules, CA). All samples were run in triplicate, and the average absorbance was used for quantitation (7).
Phospholipid mass analysis.
Frozen T-lymphocytes were thawed, and lipid extraction was initiated by the addition of 1 ml of 2-propanol to the tube containing the cells. This was added to a test tube containing 3 ml hexane, and the original tube containing the cells was rinsed with another aliquot of 2-propanol (12, 25). The single-phase extract was thoroughly mixed, and the residue was pelleted by centrifugation at 1,000 g. The lipid containing organic fraction was removed, concentrated to dryness under nitrogen, and rehydrated with solvent. The pellet was kept for protein quantitation. The lipid samples were quantitatively spotted on a heat-activated Whatman LK6 thin-layer chromatography plate and developed in a solvent system containing chloroform:methanol:acetic acid:water (60:30:3:1 by volume). Individual bands corresponding to commercially prepared standards were scraped into acid-washed tubes, and the phosphorus content was analyzed. The protein pellet was air-dried overnight and then incubated with 0.2 M KOH at 65°C overnight to resolubilize the proteins. Protein concentration was determined using the Bradford method (2).
Cholesterol mass analysis.
Total lipids were extracted by resuspending splenic 5 × 106 T-lymphocytes in a 3:2 solution of hexane:propanol. Cell solution was vortexed and centrifuged at 1,000 g for 5 min. The lipid supernatant fraction was transferred into glass vials, filtered through a 0.2-μm filter, and dried under nitrogen. Lipids were resuspended in 50 μl isopropanol with 10% polyoxyethylene octylphenyl ether for cholesterol measurement. The protein pellet was dried overnight at room temperature, digested in 0.2 M KOH at 65°C for 1 h, and quantitated using the Bradford method. Cholesterol was measured in lipid samples using a Free Cholesterol E Microtiter procedure from Wako Chemical (Richmond, VA). Cholesterol standards were prepared using serial dilutions of the standard provided by Wako to yield standards representing 197 mg/dl (5.05 nmol/μl), 100 mg/dl (2.56 nmol/μl), and 50 mg/dl (1.28 nmol/μl). Samples were assayed undiluted and at 1:5 and 1:10 dilution, each analyzed in triplicate, to ensure accuracy of measurements. The color reagent solution (300 μl/well) was added to each well, and plates were incubated at 37°C for 5 min. Absorbance was measured at 600 nm, and sample concentrations were determined by plotting the absorbance vs. concentration of the standard curve. Cholesterol content was expressed as nmol/mg protein following protein quantitation of samples.
Fatty acid analysis by gas chromatography.
Fatty acid analysis was performed on T-lymphocytes. Lipids were extracted from cells with n-hexane:2-propanol 3:2 (vol:vol) (22). The lipid extracts were centrifuged at 800 g to pellet cellular debris. The lipid-containing supernatants were filtered through a 0.2 μM nylon filter, dried under nitrogen, and redissolved in methanol. The samples were converted to fatty acid methyl esters (FAMEs) using the method of Brockerhoff (96). Briefly, methanolic KOH was added, followed by 0.2 M aqueous H2SO4. The FAMEs were extracted by n-hexane, dried under nitrogen, and cleaned by thin layer chromatography. The FAMEs were again extracted by n-hexane before separation by gas-liquid chromatography. FAMEs were separated using a Varian 3900 gas chromatography instrument equipped with a flame ionization detector and a Varian CPWax 52CB WCOT fused silica 50-m × 0.32-mm column. The injector and detector temperatures were set to 200°C and 270°C, respectively. The split ratio was 1:10, and helium was used as the carrier gas at a flow of 1.5 ml/min. The column temperature was initially set to 50°C, increased to 140°C at a rate of 20°C/min, held for 5 min, increased to 200°C at 4°C/min, increased to 240°C at 2°C/min, and held for 30 min. FAME standards, as well as a 17:0 internal standard, were used to establish relative retention times. Peak area data were collected and analyzed using Varian Star Chromatography Workstation Version 5.52 software (Varian, Palo Alto, CA).
Eicosanoid production.
EIA kits from Cayman Chemical (Ann Arbor, MI) were used to measure prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) in the culture supernatant following 20 h of stimulation as described above for cytokine secretion per the manufacturer's instructions. Absorbance was measured at 415 nm, and the concentration of eicosanoids in samples was inversely proportional to the absorbance value. Concentrations were calculated using analysis software provided by the manufacturer (www.caymanchem.com/analysis).
Statistical analysis.
Statistical significance was determined using one-way analysis of variance. Data were considered significantly different from control values when P < 0.05. Post hoc analysis was conducted using Tukey's multiple-comparison test with GraphPad Prism software (San Diego, CA).
RESULTS
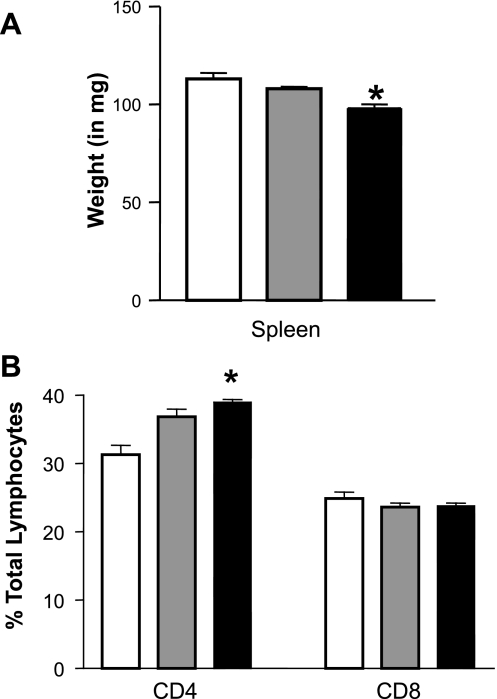
GPAT-1 KO mice are a new model for examining T-lymphocyte function; therefore we first wanted to determine spleen weight and T-lymphocyte subset proportions within the splenic T-lymphocyte population used in these experiments. Shifts in T-lymphocyte CD4+ or CD8+ subset proportions can give insight into the types of immune function that may be impacted in combination with examining cytokine secretion. Figure 1A shows that spleen weight is significantly reduced by 14% in GPAT-1 KO mice. Next we determined the impact of GPAT-1 on the proportions of the two major T-lymphocyte subsets (CD4+ and CD8+) in the spleens. Within the splenic T-lymphocyte population in GPAT-1 KO mice, the CD4+ T-lymphocytes are significantly increased by 20% while the CD8+ population is not affected (Fig. 1B). There was no significant difference in the expression of plasma membrane receptors (CD3, CD4, CD8, CD69, CD28, CTLA-4, and CD25) associated with regulating T-lymphocyte proliferation in the CD4+ or CD8+ subsets (unpublished observations).
Fig. 1.
Splenic T-lymphocyte subsets are altered in glycerol-3-phosphate acyltransferase-1 (GPAT-1) knockout (KO) mice. A: spleens were removed from GPAT-1+/+ (wild type; open bar), GPAT-1+/− (heterozygous; shaded bar), and GPAT-1−/− (homozygous; solid bar) KO mice and weighed. Splenic lymphocytes were then isolated as described in materials and methods. B: an aliquot of splenic lymphocytes was stained with the T-lymphocyte markers (anti-CD3) and anti-CD4 or anti-CD8 to distinguish the two major T-lymphocyte subsets, and 10,000 cells per sample were examined by flow cytometry. Each bar represents the mean ± SE of 4 individual mice. *Significantly different (P < 0.05) from GPAT-1+/+ mice.
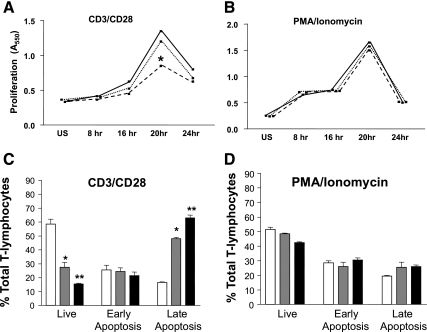
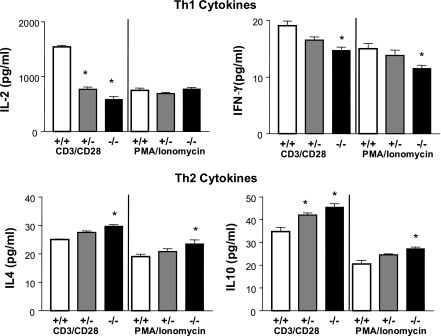
We have previously shown that stimulating splenic T-lymphocyte CD3 and CD28 receptors mimicking an in vivo response resulted in reduced proliferation in GPAT-1 KO mice. We use splenic T-lymphocytes instead of individual T-lymphocyte subsets to more closely mimic an in vivo immune response in which many T-lymphocyte subsets are present and can potentially impact one another's function. Here we determined whether T-lymphocytes activated with PMA and ionomycin, which bypass the plasma membrane and induce intracellular signaling pathways directly, would have similar effects in GPAT-1 KO mice. Figure 2A shows that, similar to our previous observation, CD3/CD28 stimulation is reduced by 40% in GPAT-1 KO mice. In contrast, stimulation with PMA and ionomycin was identical in GPAT-1 KO and wild-type mice (Fig. 2B). We hypothesized that the reduced proliferation in GPAT-1 KO mice may be due to increased activation-induced apoptosis. Figure 2C shows that activation-induced apoptosis was significantly increased in GPAT-1 KO mice in response to CD3/CD28 stimulation, whereas PMA and ionomycin stimulation had no effect in GPAT-1 KO mice (Fig. 2D). If the reduced proliferation in GPAT-1 KO mice modifies T-lymphocyte function, this would be detected by alterations in cytokine production. Figure 3 shows that following activation for 20 h, splenic T-lymphocyte Th-1 (IL-2 and IFN-γ) and Th-2 (IL-4 and IL-10) cytokine secretion is altered in the absence of GPAT-1. Interleukin-2 secretion is reduced by 67% in response to CD3/CD28 stimulation, whereas PMA and ionomycin-induced IL-2 secretion is not affected in GPAT-1 KO mice. IFN-γ secretion was significantly reduced in response to both CD3/CD28 and PMA and ionomycin in GPAT-1 KO mice. In sharp contrast, IL-4 and IL-10 secretion was significantly increased in GPAT-1 KO mice following both CD3/CD28 and PMA and ionomycin stimulation. The changes in proliferation and cytokine production were not due to altered expression of key stimulatory and inhibitory receptors because the densities of CD28, CD69, CD25, and CTLA-4 were not impacted by the KO (unpublished observations).
Fig. 2.
Effect of GPAT-1 KO on T-lymphocyte proliferation. A and B: splenic T-lymphocytes were isolated from GPAT-1+/+, GPAT-1+/−, and GPAT-1−/− KO mice. T-lymphocytes, 1.5 × 106 from each spleen, were left unstimulated (US) or were stimulated for 8, 16, 20, or 24 h with anti-CD3 and anti-CD28 (A) or phorbol myristate acetate (PMA) and ionomycin (B), and proliferation was measured using the MTT assay as described in materials and methods (8). Solid line, GPAT-1+/+; dotted line, GPAT-1+/−; dashed lined, GPAT-1−/−. C and D: T-lymphocytes were stimulated for 20 h with anti-CD3 and anti-CD28 (C) or PMA and ionomycin (D), stained with annexin-V and propidium iodide, and analyzed by flow cytometry (13). Live cells are annexin-V and propidium iodide negative, early apoptotic cells stain annexin-V positive and propidium iodide negative, and late apoptotic/necrotic cells are both annexin-V and propidium iodide positive. Open bar, GPAT-1+/+; shaded bar, GPAT-1+/−; solid bar, GPAT-1−/−. Values represent means ± SE of 4 individual mice. *Significantly different (P < 0.05) from GPAT-1+/+ and GPAT-1−/− mice. **Significantly different (P < 0.05) from GPAT-1+/+ and GPAT-1+/−.
Fig. 3.
Th-1 and Th-2 cytokine secretion is altered in GPAT-1 KO mice. Splenic T-lymphocytes, 2 × 106 from each spleen, from GPAT-1+/+ (open bar), GPAT-1+/− (shaded bar), and GPAT-1−/− (solid bar) mice were stimulated with anti-CD3 and anti-CD28 or PMA plus ionomycin for 20 h. Culture supernatants were collected and interleukin-2 (IL-2), interferon-γ (IFN-γ), interleukin-4 (IL-4), and interleukin-10 (IL-10) were quantitated by ELISA kits as described in materials and methods. Each bar represents the mean ± SE of 4 individual mice. *Significantly different (P < 0.05) from GPAT-1+/+ mice.
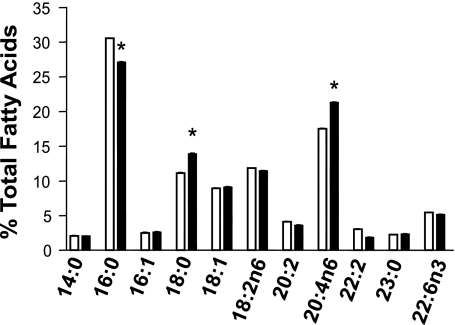
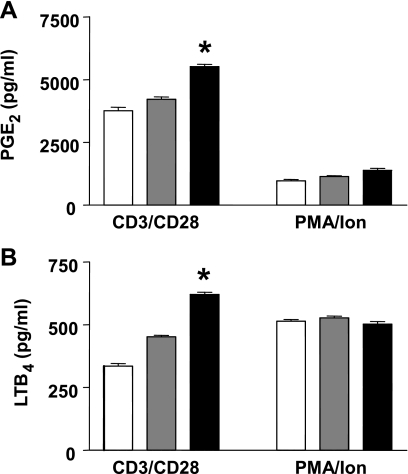
The altered proliferation and cytokine secretion in GPAT-1 KO mice splenic T-lymphocytes may be due to changes in membrane lipid composition. Therefore, we next examined phospholipid and protein mass (Table 1), cholesterol mass, and fatty acid composition (Fig. 4) of T-lymphocyte membranes in GPAT-1 KO mice. Phospholipid mass, protein mass, cholesterol mass, and fatty acid composition were measured in freshly isolated T-lymphocytes. The lipid extracts used for analysis were from whole T-lymphocytes (no subcellular fractionation) without incubation to determine the overall cellular lipid levels present at the time stimulation is initiated. Table 1 shows that the mass of all the major glycerophospholipid species (phosphatidic acid, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidylcholine) is significantly reduced in GPAT-1 KO mouse T-lymphocytes. In contrast, sphingomyelin and cardiolipin content is not changed in GPAT-1 KO mice. Interestingly, when phospholipid species are expressed as mole percent, the relative proportions of each phospholipid is relatively unchanged in GPAT-1 KO with the exception of phosphatidic acid, which is reduced (Table 1). Furthermore, protein mass was significantly reduced in GPAT-1 KO T-lymphocytes. In contrast, cholesterol mass is not affected in splenic T-lymphocytes from GPAT-1 wild-type (22.8 ± 2.4 nmol/mg protein), heterozygous (22.4 ± 3.2 nmol/mg protein), or homozygous (22.0 ± 2.4 nmol/mg protein) KO mice. Figure 4 shows that the splenic T-lymphocyte membrane fatty acid composition is also altered in GPAT-1 KO mice. Palmitate (16:0) is significantly decreased while stearate (18:0) and arachidonate (20:4, n-6) are significantly increased. Arachidonate is the substrate for eicosanoid production. Therefore we determined PGE2 and LTB4 production in GPAT-1 KO mice. Figure 5 shows that both PGE2 and LTB4 are significantly increased, 35% and 85% respectively, in GPAT-1 KO mice in response to CD3/CD28 stimulation. In contrast, PMA and ionomycin-induced PGE2 and LTB4 production was not affected in GPAT-1 KO mice.
Table 1.
Splenic T-lymphocyte phospholipid mass and composition
| Phospholipid |
Mass, nmol/106 T-lymphocytes |
Mole % Composition
|
||||
|---|---|---|---|---|---|---|
| GPAT-1+/+ | GPAT-1+/− | GPAT-1−/− | GPAT-1+/+ | GPAT-1+/− | GPAT-1−/− | |
| Cardiolipin | 0.31±0.14 | 0.25±0.07 | 0.22±0.11 | 4.19±1.83 | 4.19±1.35 | 5.05±3.62 |
| Phosphatidic acid | 0.34±0.09 | 0.21±0.08* | 0.12±0.09* | 4.68±1.21 | 3.49±1.31 | 2.44±1.41* |
| Phosphatidylethanolamine | 1.54±0.16 | 1.21±0.20* | 0.93±0.18* | 20.85±1.86 | 20.24±3.35 | 18.83±3.14 |
| Phosphatidylinositol | 0.63±0.06 | 0.50±0.03* | 0.40±0.04* | 8.64±1.05 | 8.33±0.90 | 8.25±1.74 |
| Phosphatidylserine | 0.51±0.10 | 0.45±0.02 | 0.34±0.02* | 6.97±1.52 | 7.53±0.51 | 7.23±1.90 |
| Phosphatidylcholine | 2.68±0.14 | 2.19±0.25* | 1.81±0.53* | 36.61±2.62 | 36.69±3.70 | 35.34±3.74 |
| Sphingomyelin | 1.31±0.30 | 1.16±0.31 | 1.21±0.58 | 17.87±3.83 | 19.40±4.70 | 22.83±7.29 |
| Total | 7.34±0.28 | 6.00±0.36* | 5.00±1.14* | |||
| GPAT-1+/+ | GPAT-1+/− | GPAT-1 −/− | |
|---|---|---|---|
| Protein, mg protein/106 T-lymphocytes | 0.019±0.001 | 0.017±0.001* | 0.017±0.001* |
Values are means ± SE of 6 individual mice. Splenic T-lymphocytes, 10 × 106 per spleen, were isolated from glycerol-3-phosphate acyltransferase-1 (GPAT-1) wild-type (GPAT-1+/+), heterozygous (GPAT-1+/−), or homozygous knockout (KO; GPAT-1−/−) mice. T-lymphocytes were counted and the lipids were extracted from 10 × 106 cells. Individual phospholipid species were isolated by thin layer chromatography from whole cell lipid extracts, and phospholipid species mass was determined by measurement of phosphorous mass. Protein mass was determined by the Bradford method.
Significantly different (P < 0.05) from GPAT-1+/+.
Fig. 4.
T-lymphocyte fatty acid composition is altered in GPAT-1 KO mice. Splenic T-lymphocytes, 5 × 106 from each spleen, were isolated from GPAT-1+/+ (open bar) and GPAT-1−/− (solid bar) mice. Total lipids were extracted from unfractionated, whole T-lymphocytes without prior incubation or stimulation with a hexane/propanol solution and were converted to fatty acid methyl esters for fatty acid analysis by GC as described in materials and methods. Values represent means ± SE of 4 individual mice. *Significantly different (P < 0.05) from GPAT-1+/+ mice.
Fig. 5.
GPAT-1 KO increases T-lymphocyte eicosanoid production. Splenic T-lymphocytes were isolated from GPAT-1+/+ (open bar), GPAT-1+/− (shaded bar), and GPAT-1−/− (solid bar) mice. T-lymphocytes, 2 × 106 from each spleen, were stimulated for 20 h with anti-CD3 or PMA plus ionomycin (Ion), and prostaglandin E2 (PGE2) (A) and leukotriene B4 (LTB4) (B) were measured in cell culture supernatants as described in materials and methods. Values represent means ± SE of 4 individual mice. *Significantly different (P < 0.05) from GPAT-1+/+ mice.
DISCUSSION
Recently, we established a correlation between reduced GPAT-1 activity and decreased proliferation in T-lymphocytes from aged rats. We then showed that T-lymphocyte proliferation is reduced in GPAT-1 KO mice, establishing a cause/effect relationship. We show here that GPAT-1 KO has a profound effect on T-lymphocyte membrane lipid composition, cytokine production, apoptosis, and eicosanoid production. These data are important for two major reasons. First, we show for the first time that GPAT-1 can regulate T-lymphocyte function. Second, these data in the T-lymphocyte provide critical information to multiple health-related fields, including aging, obesity, diabetes, and nutrition.
Two potential reasons exist to explain the decreased proliferation we found in anti-CD3 and anti-CD28 stimulated T-lymphocytes from GPAT-1 KO mice. First, the T-lymphocytes may be anergic, i.e., alive but not responding. Second, stimulation may cause the T-lymphocytes to undergo activation-induced apoptosis. Two pieces of evidence support the latter possibility. We show that activation-induced apoptosis is increased in anti-CD3 and anti-CD28, but not PMA and ionomycin, stimulated T-lymphocytes from GPAT-1 KO mice. Also, we show that IL-2 secretion is reduced even though the plasma membrane expression of CD25 was not altered (data not shown) following anti-CD3 and anti-CD28, but not PMA and ionomycin, stimulation. This is significant because the secretion of IL-2 and increased expression of CD25, the high-affinity IL-2 receptor α-subunit, is critical to proper T-lymphocyte proliferation. We can rule out changes in the expression of other key receptors associated with proliferation on the T-lymphocytes. We did not find any differences in the expression of CD3 or CD28 receptors on CD4+ or CD8+ T-lymphocytes, which is important because these are the receptors used for stimulation in our system. Furthermore, the expression of receptors associated with promoting proliferation (CD25 and CD69) or the receptor known to inhibit proliferation (CTLA-4) was not altered (data not shown). PMA and ionomycin stimulation may not have been affected because they are pharmacological stimuli that act by bypassing the requirement for plasma membrane receptor stimulation to induce T-lymphocyte proliferation and directly activate PKC and increase intracellular Ca2+ levels. Similar observations have been directly made in T-lymphocytes in aged rodents in which cells responded normally to PMA and ionomycin stimulation (5, 21). These findings may be due to changes in the production of diacylglycerol and ceramide. Diacylglycerol, derived from phosphatidylinositol and phosphatidylcholine, is a proproliferative signal by stimulating PKC. Ceramide, derived from sphingomyelin, is an antiproliferative signal inducing the apoptotic signaling machinery (4). We show that phosphatidylinositol and phosphatidylcholine mass lowers relative to sphingomyelin mass; thus the balance of stimulatory and inhibitory intracellular lipid second messengers may be shifted to favor apoptosis. We also cannot rule out the potential for lysoPA production to be altered since it is known to be a potent mitogen. However, the lysoPA levels in our system were below detectable levels. The mechanism by which relative phospholipid levels and protein mass are altered is unknown but may involve changes in phospholipid synthesis or structural changes to the T-lymphocytes. For example, the number of mitochondria may be altered, overall cell size, or the ability of the T-lymphocyte to form immune synapses, which are the clusterings of key receptors and signaling molecules at the plasma membrane to properly induce proliferation. Interestingly, PMA/ionomycin stimulation did not normalize IFN-γ, IL-4, or IL-10 secretion in GPAT-1 KO mice, suggesting that production of these cytokines involves additional signals downstream of PKC signaling. These data suggest that GPAT-1 is important in T-cell receptor/CD3 and CD28 plasma membrane receptor-dependent T-lymphocyte proliferation, but that T-lymphocytes can still respond to other stimuli such as PMA/ionomycin, perhaps due to compensation by GPAT-3. This is consistent with our observations that T-lymphocyte subset numbers are relatively normal in the spleen, showing that a lack of GPAT-1 is not a catastrophic event to the T-lymphocyte.
Alternatively, the decreased proliferation may be due to increased production of PGE2. Our data show, in agreement with that reported in liver glycerophospholipids, that there was a significant decrease in palmitate and an increase in arachidonate (11). This is not surprising since GPAT-1 increases palmitate incorporation into phospholipids. We hypothesized that the increased availability of arachidonate for phospholipase A cleavage may lead to increased eicosanoid production. Indeed, we show that secretion of both PGE2 and LTB4 is increased in T-lymphocytes from GPAT-1 KO mice, indicating that the elevation of arachidonate alone may have functional significance. This is significant because PGE2 is known to function in an autocrine and paracrine manner to directly inhibit T-lymphocyte proliferation. The decrease in proliferation may be due to the ability of PGE2 to inhibit two important signaling pathways in T-lymphocytes, namely, calcium mobilization (6) and p59fyn protein tyrosine kinase activity (15).
These data provide evidence for a potentially novel mechanism by which aging suppresses T-lymphocyte proliferation because many of the results presented here are similar to those seen in aged T-lymphocytes. First, lack of GPAT-1 impairs T-lymphocyte proliferation and increases activation-induced apoptosis. Reduced T-lymphocyte proliferation is one of the most consistent observations in aging from rodents to humans (20). Second, Th-1 (IL-2 and IFN-γ) cytokine secretion is reduced and Th-2 (IL-4 and IL-10) cytokine secretion is increased. Third, the cholesterol-to-phospholipid ratio is increased. An increased cholesterol-to-phospholipid ratio, as shown in young GPAT-1 KO mice, is a consistent observation made in most all aged tissues and cells examined including T-lymphocytes (26). It is important to point out that previous reports using the GPAT-1 KO mice have not shown a change in relative phospholipid levels (11). This could be due to the differences in cell type studied. Liver tissue or hepatocytes, which produce large amounts of TAG for lipoprotein synthesis, have been the major focus of research on GPAT-1's role in lipid metabolism. In this study, we use T-lymphocytes, which are unique in that they become glucose addicted when proliferating, and spare fatty acids for membrane lipid biosynthesis. Thus, T-lymphocyte membrane phospholipids may be more sensitive to alterations in GPAT-1 activity.
These data also have important implications for obesity and diabetes, especially since there is a strong interaction between the two diseases. GPAT-1 has been a major recent focus of research in improving insulin sensitivity (23), hepatic steatosis (24), and lipoprotein levels (11, 19, 27). The GPAT-1 KO mouse has a decreased susceptibility to developing insulin resistance and hepatic steatosis. These beneficial effects have been linked to decreased liver TAG production, which resulted in an improved lipoprotein profile. Female GPAT-1 KO mice also have approximately a 20% decrease in body weight, which is attributed to decreased fat mass. On the basis of these observations, it has been proposed that GPAT-1 may be a novel target for preventing or treating diabetes and obesity through novel pharmacological agents or dietary regimens. While this is correct, our data provide evidence that caution should be taken when designing these strategies because a complete loss of GPAT-1 KO may be detrimental to the immune system. Future studies will have to be performed to determine how much GPAT-1 activity can be inhibited without adversely impacting the immune system.
Taken together, the importance of the data presented here is twofold. We are the first to report that manipulating lipid metabolic enzymes like GPAT-1 can have a profound effect on the immune system. Second, and perhaps most importantly, because of the similarities seen in T-lymphocytes from aged rodents, GPAT-1 may be a new link between aging, lipid metabolism, and T-lymphocyte function.
GRANTS
This work was supported by National Institutes of Health Grant AG-20651 (to C. A. Jolly).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aspinall R Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J Immunol 158: 3037–3045, 1997. [PubMed] [Google Scholar]
- 2.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Li JL, Li D, Tobin JF, Gimeno RE. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc Natl Acad Sci USA 103: 19695–19700, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapkin RS, McMurray DN, Jolly CA. Dietary N-3 polyunsaturated fatty acids modulate T-lymphocyte activation: clinical relevance in treating diseases of chronic inflammation. In: Nutrition and Immunology: Principles and Practice, edited by Gershwin ME, German BJ, and Keen CL. Totowa, NJ: Humana, 2000, p. 121–134.
- 5.Chopra RK, Holbrook NJ, Powers DC, McCoy MT, Adler WH, Nagel JE. Interleukin 2, interleukin 2 receptor, and interferon-gamma synthesis and mRNA expression in phorbol myristate acetate and calcium ionophore A23187-stimulated T cells from elderly humans. Clin Immunol Immunopathol 53: 297–308, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Choudhry MA, Ahmed Z, Sayeed MM. PGE2-mediated inhibition of T cell p59fyn is independent of cAMP. Am J Physiol Cell Physiol 277: C302–C309, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Collison LW, Collison RE, Murphy EJ, Jolly CA. Dietary n-3 polyunsaturated fatty acids increase T-lymphocyte phospholipid mass and acyl-CoA binding protein expression. Lipids 40: 81–87, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Collison LW, Kannan L, Onorato TM, Knudsen J, Haldar D, Jolly CA. Aging reduces glycerol-3-phosphate acyltransferase activity in activated rat splenic T-lymphocytes. Biochim Biophys Acta 1687: 164–172, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Collison LW, Jolly CA. Phosphorylation regulates mitochondrial glycerol-3-phosphate-1 acyltransferase activity in T-lymphocytes. Biochim Biophys Acta 1761: 129–139, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fulop T, Douziech N, Goulet AC, Desgeorges S, Linteau A, Lacombe G, Dupuis G. Cyclodextrin modulation of T lymphocyte signal transduction with aging. Mech Ageing Dev 122: 1413–1430, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol 22: 8204–8214, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90: 420–426, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Jolly CA, Muthukumar A, Reddy Avula CP, Fernandes G. Maintenance of NF-κB activation in T-lymphocytes and a naive T-cell population in autoimmune-prone (NZB/NZW)F(1) mice by feeding a food-restricted diet enriched with n-3 fatty acids. Cell Immunol 213: 122–133, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Jolly CA, Kananan L. Phosphatidic acid metabolism in mammals. In: Lipids: Glycerolipid Metabolizing Enzymes, edited by Haldar D and Das SK. Kerala, India: Research Signpost, 2002, p. 29–41.
- 15.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol 12: 242–249, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Larbi A, Douziech N, Khalil A, Dupuis G, Gherairi S, Guerard KP, Fulop T Jr. Effects of methyl-beta-cyclodextrin on T lymphocytes lipid rafts with aging. Exp Gerontol 39: 551–558, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lewin TM, Wang S, Nagle CA, Van Horn CG, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the metabolic fate of exogenous fatty acids in hepatocytes. Am J Physiol Endocrinol Metab 288: E835–E844, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Lewin TM, Schwerbrock NM, Lee DP, Coleman RA. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J Biol Chem 279: 13488–13495, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Linden D, William-Olsson L, Ahnmark A, Ekroos K, Hallberg C, Sjogren HP, Becker B, Svensson L, Clapham JC, Oscarsson J, Schreyer S. Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J 20: 434–443, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Miller RA The aging immune system: primer and prospectus. Science 273: 70–74, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Miller RA Immunodeficiency of aging: restorative effects of phorbol ester combined with calcium ionophore. J Immunol 137: 805–808, 1986. [PubMed] [Google Scholar]
- 22.Murphy EJ, Stiles T, Schroeder F. Sterol carrier protein-2 expression alters phospholipid content and fatty acyl composition in L-cell fibroblasts. J Lipid Res 41: 788–796, 2000. [PubMed] [Google Scholar]
- 23.Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem 282: 14807–14815, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2: 55–65, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Rouser G, Siakotos A, Fleischer S. Quantitative analysis of phospholipids by thin layer chromatography and phosphorus analysis of spots. Lipids 143: 85–86, 1969. [DOI] [PubMed] [Google Scholar]
- 26.Shmeeda HR, Golden EB, Barenholz Y. Membrane lipids and aging. In: Biomembranes: Structural and Functional Aspects, edited by Shinitzky M. New York: VCH, 1994, p. 1–79.
- 27.Xu H, Wilcox D, Nguyen P, Voorbach M, Suhar T, Morgan SJ, An WF, Ge L, Green J, Wu Z, Gimeno RE, Reilly R, Jacobson PB, Collins CA, Landschulz K, Surowy T. Hepatic knockdown of mitochondrial GPAT1 in ob/ob mice improves metabolic profile. Biochem Biophys Res Commun 349: 439–448, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Yeo EJ, Park SC. Age-dependent agonist-specific dysregulation of membrane-mediated signal transduction: emergence of the gate theory of aging. Mech Ageing Dev 123: 1563–1578, 2002. [DOI] [PubMed] [Google Scholar]