Abstract
Vasopressin (VP)-induced exocytosis was dissected in native and aquaporin-2 (AQP2)-expressing renal LLC-PK1 cells by a fluorimetric exocytosis assay based on soluble secreted yellow fluorescent protein (ssYFP). YFP was targeted to the secretory pathway by addition of an 18-amino acid signal peptide from hen egg white lysozyme. Immunofluorescence labeling, together with analysis of Alexa 555-dextran internalization, revealed that ssYFP is exclusively located in the secretory pathway. Immunofluorescence and immunogold electron microscopy showed significant colocalization of ssYFP and AQP2. Fluorimetry and Western blot analysis demonstrated similar constitutive ssYFP secretion in native LLC-PK1 and AQP2-expressing cells. In AQP2-expressing cells, a twofold increase in ssYFP secretion was observed within 15 min of VP stimulation. This transient burst of ssYFP secretion was abolished by the PKA inhibitor H-89 and was not observed in native cells. The endocytotic inhibitor methyl-β-cyclodextrin, which also promotes membrane accumulation of AQP2, had no effect on ssYFP secretion. Although cells expressing phosphorylation-deficient AQP2-S256A showed significantly lower baseline levels of constitutive secretion, VP induced a significant increase in exocytosis. Our data indicate that 1) this assay can monitor exocytosis in cultured epithelial cells, 2) VP has an acute stimulatory effect on ssYFP secretion in AQP2-expressing, but not native, cells, and 3) phosphorylation of AQP2 at S256 may be involved in the regulation of constitutive AQP2 exocytosis and play only a minor role in the VP-induced burst. These results support the idea that, in addition to its role in reducing AQP2 endocytosis, VP increases AQP2 exocytosis.
Keywords: recycling, antidiuretic hormone, phosphorylation, epithelial cells, kidney
vasopressin (VP), a cyclic peptide hormone secreted by the posterior pituitary gland, is responsible for regulating body fluid and electrolyte balance. Aquaporin-2 (AQP2) is the major VP-responsive water channel in the kidneys, where it plays a critical role in mediating the effect of VP on urine concentration and body water homeostasis. In humans, congenital and acquired forms of nephrogenic diabetes insipidus, a disease characterized by massive water loss via the kidneys, have been linked to defects in the VP signaling cascade that lead to AQP2 accumulation at the apical plasma membrane of collecting duct principal cells. Excessive water retention in congestive heart failure and pregnancy has also been linked to AQP2 dysregulation (11, 42, 48).
Within renal cells, VP stimulation initiates a signaling cascade that causes an increase in intracellular cAMP and Ca2+ levels, activating PKA, which subsequently phosphorylates AQP2 on S256. The classic view of the short-term regulation of AQP2 trafficking by VP is that, after synthesis, AQP2 is sorted into intracellular storage vesicles. When VP is present, the storage vesicles are mobilized and fuse with the plasma membrane, delivering AQP2 to the cell surface, where it accumulates, increasing the water permeability of the cell. On removal of the stimulus, AQP2 is internalized via endocytosis and resorted into its storage vesicles, ready for the next round of signaling (11, 42).
The underlying assumption of this classic hypothesis is that specific exocytosis of AQP2 leads to its accumulation at the cell surface. The observation that a constitutive cycle for AQP2 exists, however, has called this basic assumption into question, inasmuch as AQP2 can accumulate at the cell surface simply by blocking endocytosis using a dominant-negative GTPase-deficient K44A dynamin (17, 49, 54). Indeed, a mathematical model of the VP response in the kidney devised almost 15 years ago suggested that water channel accumulation at the cell surface was probably due to an increase in the rate of water channel exocytosis, as well as a decrease in its rate of internalization (30). Further studies showed that blockade of endocytosis by the chemical inhibitor methyl-β-cyclodextrin (mβCD) was sufficient to produce a rapid and robust accumulation of AQP2 at the cell surface (35), a phenomenon that was confirmed to occur in vivo (47). Interestingly, the kinetics and extent of accumulation were rapid and sufficient to account for the entire VP effect on AQP2. Subsequently, AQP2 was also found to reside in endocytosis-resistant membrane microdomains, suggesting that specific inhibition of AQP2 internalization is at least partially responsible for VP-induced AQP2 accumulation at the cell surface (7).
Many other studies have also provided evidence to support a much more complex view of the intracellular trafficking of AQP2. For example, it is known that, upon endocytosis, AQP2 is not only recycled, but it is also shuttled to proteasomes and into lysosomes and multivesicular bodies (20) that can fuse with the plasma membrane and shed AQP2 into the urine (44, 58); however, the biological significance of this shedding event is uncertain. AQP2 vesicles in the resting cytoplasm partially colocalize with Rab11 (50), and AQP2 is possibly sorted in recycling endosomes in a Rab11-dependent manner (39, 57). Indeed, a proteomic study supported the idea that, at steady state, most AQP2-bearing vesicles contain endosomal or recycling markers (3). Finally, AQP2, in addition to being sorted in the recycling endosome, also traverses and can rapidly accumulate at the trans-Golgi network (TGN) under some conditions (17); thus the TGN may serve as an initial sorting station for newly synthesized AQP2 and may also be involved in the sorting of recycling AQP2.
The fact that AQP2 traverses so many intracellular compartments implies that regulation of AQP2 can occur at any step or (more likely) at multiple steps of its intracellular itinerary. In this light, the role of regulatory mechanisms such as phosphorylation on S256 (or other residues) would be to increase or decrease the rate of AQP2 exchange from one compartment to another, for example, from the TGN to the plasma membrane (11, 55). Indeed, we recently showed that S256 phosphorylation modulates the interaction of AQP2 with the accessory protein heat shock conjugate/heat shock protein 70 (hsc/hsp70) which is involved in the endocytotic arm of the AQP2 recycling process (36). Other phosphorylation-regulated interactions that regulate AQP2 recycling will undoubtedly emerge in the future.
Although several cell biological tools that employ exogenous endocytotic and recycling markers to follow vesicle trafficking have been developed, few methods utilize independent labeling of the secretory/post-TGN pathway. Furthermore, most methods that monitor AQP2 trafficking measure its cell surface accumulation, rather than bona fide exocytosis, leaving open the possibility that a blockade of AQP2 endocytosis might be largely responsible for the effects. On the basis of these considerations, we developed a fluorescence-based assay that measures exocytosis of post-TGN vesicles, which can be clearly distinguished from endocytic vesicles. The principle behind the assay is simple: cells are transfected with a plasmid coding for a soluble enhanced yellow fluorescent protein (EYFP) preceded by a signal peptide [secreted soluble yellow fluorescent protein (ssYFP)]. Upon expression, ssYFP molecules are translocated into the lumen of the endoplasmic reticulum (ER). From the ER, they transit the Golgi and then passively enter and label secretory vesicles as they are released from the TGN. The EYFP content is secreted upon vesicle exocytosis, and ssYFP fluorescence in the extracellular medium is assayed using a fluorimeter. We found an increase in ssYFP secretion only in AQP2-expressing cells upon VP stimulation, supporting the view that an exocytotic response indeed occurs upon VP stimulation and is related to AQP2 expression within the cell. Moreover, our data suggest that S256 phosphorylation plays only a minor role in this process.
MATERIALS AND METHODS
Antibodies and reagents.
Rabbit anti-YFP (A-6455), mouse anti-golgin-97 (A-21270), Alexa 555-dextran (10,000 kDa), LysoTracker Red DND-99 (L7528), goat anti-rabbit Alexa 488 (A11008), and goat anti-mouse Alexa 555 (A31622) were purchased from Molecular Probes (Carlsbad, CA); goat anti-AQP2 (sc-9882) from Santa Cruz Biotechnology (Santa Cruz, CA); and rabbit anti-Rab11 (ab3612) and rabbit anti-immunoglobulin-binding protein (BiP; ab21685) from Abcam (Cambridge, MA). Horseradish peroxidase (HRP)-, 15-mm gold-, 10-mm gold-, and Cy3-conjugated secondary antibodies were obtained from Jackson Immunoresearch Laboratories (Bar Harbor, ME) and the secondary antibody goat anti-mouse-HRP from Amersham Biosciences (Piscataway, NJ). A previously described mouse anti-c-myc MAb (15) was produced in our laboratory. Cell culture reagents, including Geneticin (G418), DMEM, PBS, FBS, l-glutamine (Glu), and Hanks' balanced salt solution (HBSS, 10×) were purchased from GIBCO-BRL (Carlsbad, CA); lysine-VP, forskolin (FK), methyl-β-cyclodextrin (mβCD), HEPES, lysine, sodium periodate, sucrose, Triton X-100, sodium azide, and d-glucose from Sigma (St. Louis, MO); paraformaldehyde (PFA) from Electron Microscopy Sciences (Hatfield, PA); n-propyl gallate from MP Biomedical (Solon, OH); and H-89 from Biosource (Carlsbad, CA). The construct containing ssYFP (a gift from J. Lippincott-Schwarz, National Institutes of Health) consisted of the hen egg white lysozyme (GenBank accession no. NM_205281) signal peptide RSLLILVLCFLPLAALGK followed by EYFP in the pEYFP-C3 vector (Clonetech, Palo Alto, CA).
Cell culture and generation of stable cell lines.
All cells were cultured at 37°C in a 5% CO2 atmosphere in DMEM + 10% FBS + 2 mg/ml Glu, with the addition of 1 mg/ml G418 where applicable, and passaged using TrypLE (GIBCO-BRL). ssYFP was transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) into the following previously characterized epithelial cell lines: LLC-PK1, LLC-PK1 cells expressing AQP2-c-myc (LLC-AQP2), and LLC-PK1 cells expressing the phosphorylation-deficient mutant AQP2-S256A (27, 28). The cells were cultured in G418 for 1 wk after transfection, and sorted by fluorescence-activated cell sorting (Massachusetts General Hospital FACS Core Facility) into 96-well plates. For each cell line, 10–12 clones were selected, grown on 12-mm-diameter coverslips (no. 1.5), and fixed in 4% PFA in 0.5 M phosphate buffer. Clones displaying medium-to-bright fluorescence in a vesicular pattern were chosen for further study. Cell lines expressing AQP2 were further tested with VP (10−8 M) for 30 min and stained with anti-c-myc or anti-AQP2 to ensure that the expected AQP2 pattern and trafficking were not disturbed. Expression levels of ssYFP were determined by Western blotting (see below), and clones with similar expression levels were chosen as the final set for all other experiments.
Immunofluorescence microscopy.
Cells were fixed in 4% PFA in PBS + 50 g/l sucrose (PFS) for 20 min. Fixed coverslips were permeabilized in 0.1% Triton X-100 and 0.2% sodium azide in PBS for 5 min, washed three times in PBS, and blocked in 1% BSA or Image-IT-Fx (Molecular Probes; for goat anti-mouse Alexa 555 staining). The coverslips were incubated with primary antibodies for 1 h at room temperature. Primary antibodies were applied at the following dilutions in Dako diluent (Carpinteria, CA): anti-AQP2 at 1:250, anti-BiP at 1:100, anti-golgin-97 at 1:60, anti-Rab11 at 1:400, rabbit anti-YFP at 1:400, mouse anti-ssYFP at 1:100, and undiluted anti-c-myc. After three washes in PBS, coverslips were incubated in secondary antibody for 1 h. Secondary antibodies were diluted at 1:500, except for Alexa 555, which was diluted at 1:100. After four final washes, the coverslips were mounted in 90% glycerol-Tris (pH 8.0) + 1% n-propyl gallate. For double staining, primary antibodies were incubated separately, while secondary antibodies were added simultaneously in the case of BiP-YFP and Rab11-YFP, whereas golgin-97-YFP staining was performed by application of anti-YFP and then its cognate secondary antibody followed by the anti-golgin-97 and then its cognate secondary antibody.
Immunogold electron microscopy.
Cells were fixed in 4% PFA containing 75 mM lysine and 10 mM sodium periodate for 4 h, pelleted, and resuspended in 2.0% agarose (Invitrogen) for ease of handling. The blocks were cryoprotected in 2.3 M sucrose at 4°C overnight and then mounted on aluminum supports and frozen in liquid nitrogen. Ultrathin frozen sections were cut on an ultracryomicrotome (model FCS, Leica, Depew, NY) and collected on Formvar-coated 200-mesh grids. The sections were incubated on drops of goat anti-AQP2 (1:100 dilution) and anti-YFP (1:50 dilution) in Dako diluent for 1 h at room temperature. AQP2 was labeled with rabbit anti-goat-15-nm gold, while YFP was labeled with goat anti-rabbit-10-nm gold. The sections were rinsed on drops of distilled water and then stained on drops of a Tylose-uranyl acetate mixture on ice. Sections were examined in a transmission electron microscope (model 1011, JEOL, Tokyo, Japan) at 80 kV, and images were collected using a digital camera (Advanced Microscopy Techniques, Danvers, MA).
Endocytosis assay and LysoTracker staining.
Cells were seeded on glass coverslips (no. 1.5) in a 24-well cell culture-treated plate (BD, Franklin Lakes, NJ) and grown to 80% confluence. The medium was changed to serum-free DMEM 1 h before the experiment. Cells were then incubated in serum-free DMEM containing 1.5 mg/ml dialyzed Alexa 555-dextran or 5 μM LysoTracker for 0, 5, 10, or 15 min (dextran) or 0, 10, or 15 min (LysoTracker). Coverslips were fixed in PFS for 20 min for visualization.
Fluorescence exocytosis assay.
Cells were grown on 24-well cell culture-treated plates until confluence (3 days after seeding). All cells were used between passages 4–30. Although AQP2 is expressed mostly basolaterally in LLC-PK1 cells, at confluence, the cells grown on coverslips are not fully polarized, and molecules in the medium are accessible to and from both poles of the cells. Initial studies were performed on two clones of each cell line (wE8 and wF9 for LLC-AQP2-ssYFP, l5E3 and lE10 for LLC-ssYFP, and 11A and 9A for LLC-AQP2-S256A-ssYFP); when similar results were observed, the clones listed first were chosen for further replicates and for generation of the reported results. Each well was washed twice and incubated in 250 μl of HBSS (supplemented with 20 mM HEPES and 2 g/l glucose; hence referred to simply as HBSS) for 1 h before manipulation. Cells were then incubated in 250 μl of control HBSS, HBSS + 10−8 M VP-10−5 M FK (VP/FK), or HBSS + mβCD (10 mM) for 0, 15, 30, or 60 min at 37°C. These concentrations of VP, FK, and mβCD have been previously established to produce the maximal effect on AQP2 cell surface accumulation and water permeability (28, 35, 53). At the end of the incubation, 150 μl of medium were transferred from each well to a black half-area 96-well plate (Corning, Corning, NY). For H-89 (8 μM) experiments, cells were preincubated for 15 min before measurements were recorded. The ssYFP fluorescence in each well was read immediately after collection using a multimode plate reader (model DTX880, Beckman-Coulter, Fullerton, CA). The following settings were used: fluorescence intensity top method, 0.1 s integration time, 485-nm excitation filter, and 535-nm emission filter. The values represent at least five independent experiments, with results at each time point measured in triplicate. Background fluorescence values were obtained from empty wells, and fluorescence values are reported as a ratio of each background- and zero-subtracted value to the corresponding LLC-AQP2-ssYFP 15-min control value.
cAMP analysis.
The intracellular cAMP levels in control, VP (10−8 M)-stimulated, FK (10−5 M)-stimulated, and VP/FK-stimulated LLC-ssYFP and LLC-AQP2-ssYFP cells grown in 12-well culture plates (Corning) were determined using the ELISA-based cAMP Direct BioTrak enzyme immunoassay (Amersham Biosciences) according to the manufacturer's instructions in the presence and absence of the phosphodiesterase inhibitor IBMX (Sigma), as previously described (5). Since absolute values were similar for VP/FK conditions, although they were less variable in the presence of IBMX, only the IBMX values are reported. Absorbance values were measured using the DTX880 multimode plate reader (Beckman-Coulter) with the following settings: absorbance method, 450-nm excitation filter, and 620-nm reference excitation filter. The results were analyzed as previously described (5).
VP type 2 receptor [3H]VP binding assay.
The average amount of VP type 2 receptor (V2R) per cell was determined for LLC-ssYFP and LLC-AQP2-ssYFP cells grown to confluence on Transwell filters (Corning) using a [3H]VP binding assay performed as previously described (9). Briefly, the culture medium was replaced by ice-cold PBS containing 16 nM [3H]8-AVP (Perkin-Elmer, Waltham, MA), and the cells were incubated for 3 h at 4°C. Nonspecific binding was determined in the presence of excess unlabeled VP (1.6 μM). Cells were rinsed twice with ice-cold PBS; then the filters were excised and solubilized in scintillation vials containing 500 μl of NaOH (0.1 N). After 5 min, 5 ml of Optic-Fluor scintillation fluid (Packard, Groningen, The Netherlands) were added, and bound radioactivity was determined using a liquid scintillation analyzer (Tricarb 2200 CA, Packard). Data from three experiments were analyzed as previously described (9).
Western blotting.
Cells were grown on P6 plates and lysed with a cell scraper in 100 μl of radioimmunoprecipitation (RIPA) buffer (Boston Bioproducts, Boston, MA) supplemented with a Complete-Mini protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The lysates were incubated in a rotor at 4°C for 20 min and centrifuged for 10 min at 14,000 rpm on a centrifuge (model 5414 C, Eppendorf, Westbury, NY). Total protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Samples were then diluted to 0.4 μg/μl in RIPA buffer, NuPage SDS sample buffer, and NuPage sample reducing agent to make up the final loading sample. Ten micrograms of total protein were loaded into each lane of a NuPage 1.5-mm 10-well 4–12% Bis-Tris gel. Ten microliters of SeeBluePlus2 protein ladder were loaded as a size marker (NuPage System, Invitrogen). Samples were electrophoresed for ∼60 min at 100 V and subsequently transferred to a Sequi-Blot (Bio-Rad, Hercules, CA) or an iBlot (Invitrogen) polyvinylidene difluoride membrane. Protein-loaded membranes were rinsed in 0.05% Tris-buffered saline-Tween (for anti-c-myc) or 0.3% PBS-Tween (PBT; for all others), blotted in 5% milk powder-PBT for 1 h at room temperature or overnight at 4°C, and incubated in primary antibody diluted in 3% milk powder-PBT for 1 h or overnight at 4°C. Anti-YFP and anti-AQP2 (for band confirmation) were applied at a dilution of 1:5,000, anti-c-myc at a dilution of 1:6, and anti-actin at a dilution of 1:20,000. The membranes were then washed five times for 10 min each in PBT, incubated in secondary antibody diluted in 3% milk powder-PBT for 1 h at room temperature, and washed again five times for 10 min each in PBT before visualization using a Western Lightning chemiluminescence kit (Perkin-Elmer) and Biomax XAR film (Kodak, Rochester, NY).
Real-time PCR analysis.
Total RNA from each cell line was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was generated from 1 μg of RNA using SuperScript II RNase H reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed and analyzed as described previously (22). Primers used for detection of porcine P0 were 5′-TCCAGGAAGCGAGAATGCA-3′ and 5′-GCAGCATCTACAACCCTGAAGTG-3′; those for AQP2 were 5′-TCAACCCCGCCGTGACT-3′ and 5′-AGGCAGCTCGAAGGAAGGA-3′ as well as 5′-GGAGCGGGCTGGATTCA-3′ and 5′-GGCCACCTCCTTGGGATCT-3′; those for ssYFP were 5′-GTCCGCCCTGAGCAAAGA-3′ and 5′-TCCAGCAGGACCATGTGATC-3′ as well as 5′-GGGCACAAGCTGGAGTACAAC-3′ and 5′-TCTGCTTGTCGGCCATGATA-3′.
Image and data analysis.
Images were collected using a Bio-Rad/Zeiss Radiance 2000 confocal microscope with a Zeiss ×63 1.4 NA Plan Apo objective. z-Slices were captured at 0.15-μm intervals. Three-dimensional reconstruction and colocalization analyses were performed using Volocity (Improvision, Waltham, MA). Western blot images were quantified using ImageJ (NIH, Bethesda MD), and MS Excel and Prism (Graphpad, San Diego, CA) were used for the exocytosis assay statistical analyses. One- and two-way ANOVAs with Bonferroni's post test were used to determine statistical significance, which was set at P < 0.05. Unless otherwise stated, values for the ssYFP exocytosis assay, expressed as relative fluorescence units, are means ± SE.
RESULTS
ssYFP labels exocytotic, but not endocytotic, intracellular compartments.
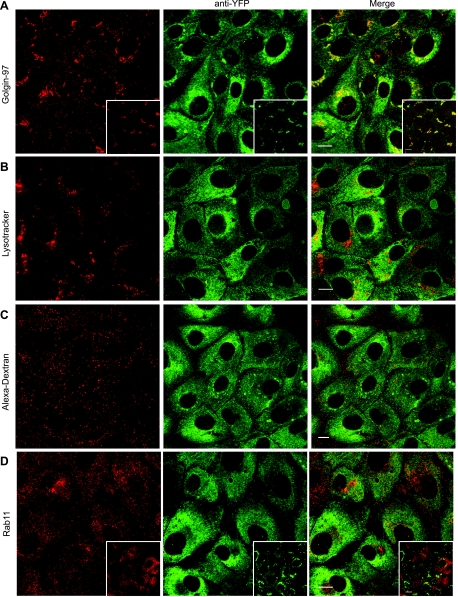
The intrinsic fluorescence of ssYFP appears as a perinuclear tubular and vesicular pattern, consistent with a soluble protein that has entered the secretory pathway (see 3-dimensional reconstruction in Supplemental Movie 1 in the online version of this article; Fig. 1, middle column). To verify that ssYFP expression is indeed restricted to elements of the secretory pathway, we investigated ssYFP colocalization with various organelle markers. The results of this analysis are summarized in Fig. 1 and Table 1. As expected, we observed significant colocalization of ssYFP and the TGN marker golgin-97 (33). Since this assay was developed to distinctly label exocytotic vesicles, it was important to determine whether endocytotic vesicles could be labeled by ssYFP. When ssYFP-expressing cells were loaded with the fluid-phase marker Alexa 555-dextran for 0, 5, 10, and 15 min to label endocytotic vesicles (37), no significant colocalization between the markers was observed (15 min; Fig. 1C, see 3-dimensional reconstruction in Supplemental Movie 2). Similarly, cells loaded for 0, 10, and 15 min with LysoTracker Red, a marker of late endosomes and lysosomes, also displayed little to no colocalization, indicating further that the ssYFP marker was not simply delivered to a degradative compartment (10 min; Fig. 1B). Rab11 is a marker of the recycling endosome (37), and in some systems, Rab11-positive vesicles that travel from the Golgi to the recycling endosome have been observed (2, 33). Therefore, we stained ssYFP cells with an anti-Rab11 antibody to determine whether ssYFP could enter recycling vesicles. Two main structures could be discerned: an apical cap of Rab11 (not shown), which may correspond to the apical recycling endosome, and a perinuclear patch. A small amount of Rab11 staining overlapped with ssYFP; therefore, we cannot exclude the possibility that some ssYFP enters the recycling endosome (Fig. 1D). Finally, some cells, in both native and AQP2-expressing cell lines, displayed large bright vesicles containing ssYFP, but their significance is unknown. The presence of these vesicles was inconsistent among cells, and although they did not costain with LysoTracker or endocytic markers, they were brightly stained with the ER/cis-Golgi resident protein BiP (18), suggesting that they represent enlargements of the ER (see supplemental materials).
Fig. 1.
Colocalization of secreted soluble yellow fluorescent protein (ssYFP) in exocytotic, but not endocytotic, compartments. LLC-AQP2-ssYFP cells were colabeled with fluorescent markers of intracellular compartments (red) and anti-YFP antibodies to highlight all forms [including unfolded or nonfluorescent (green)] of ssYFP. A: extensive colocalization of perinuclear ssYFP and trans-Golgi network marker golgin-97. Extensive colocalization of perinuclear ssYFP and golgin-97 is especially apparent when only native ssYFP fluorescence is visualized (inset). Endocytotic vesicles (B, 15 min of incubation with Alexa 555-dextran), late endosomes/lysosomes (C, 10 min of incubation with LysoTracker Red), and recycling endosomes (D, costaining with anti-Rab11) display no significant colocalization with ssYFP. Rab11 costaining with native ssYFP fluorescence is shown for comparison (D, inset). All colocalization was assessed on 3-dimensional stacks; a single midnuclear xy plane is shown for clarity. Scale bar, 10 μm.
Table 1.
Quantification of colocalization of ssYFP with subcellular compartment markers
| Marker | Compartment | ssYFP Colocalization, % | PCC |
|---|---|---|---|
| Golgin-97 | TGN | 60.9±3 (34.9±0.5) | 0.30±0.03 (0.48±0.02) |
| Alexa 555-dextran | Endocytotic vesicles | 3.8±2 | 0.05±0.02 |
| LysoTracker | Late endosomes and lysosomes | 3.5±2 | 0.03±0.01 |
| Rab11 | Recycling endosomes | 28.2±4 (17.2±4) | 0.09±0.02 (0.21±0.01) |
Values are means ± SE (n = 3). 3-Dimensional image stacks of immunostained secreted soluble yellow fluorescent protein (ssYFP) colabeled with the listed organelle markers were analyzed for each condition using Volocity's (Improvision) colocalization function (see Fig. 1 for representative image slices). Colocalization values are reported as percentage of above-threshold pixels in ssYFP channel that overlapped above-threshold pixels in compartment marker channel. Values in parentheses represent colocalization of native ssYFP fluorescence and the given compartment marker. Pearson's correlation coefficient (PCC) is an indication of colocalization significance, where higher values correspond to a greater significance (4). TGN, trans-Golgi network.
In conclusion, the staining pattern and colocalization of ssYFP with known compartment markers indicate that ssYFP enters the secretory pathway, largely labeling the biosynthetic/post-TGN pathway independently of the endosomal pathway.
AQP2 partially colocalizes with ssYFP.
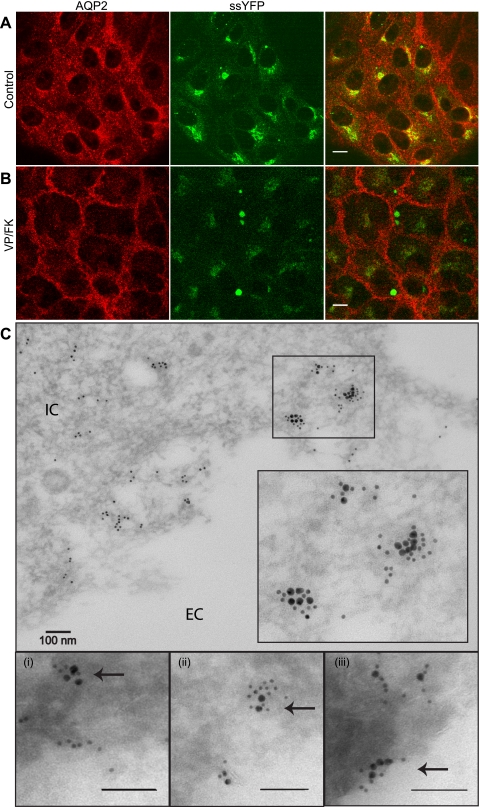
In LLC-AQP2 cells transfected with ssYFP, we determined whether ssYFP labels AQP2-containing vesicles. Indeed, immunofluorescence analysis indicates that 40.6 ± 2% of the AQP2 staining overlapped with ssYFP in control cells, particularly in the perinuclear area, but overlap was less in more peripheral regions [Fig. 2A; Pearson's correlation coefficient (PCC) = 0.21]. Only partial colocalization was expected, since ssYFP was intended to target a subpopulation of AQP2-bearing vesicles and would not label recently endocytosed or recycling AQP2, with the probable exception of AQP2 that has been resorted through the TGN. Furthermore, ssYFP is a bulk secretion marker and would therefore be expected to label other AQP2-negative exocytotic vesicles. In addition, AQP2 trafficking was undisturbed by ssYFP expression, since on stimulation of the cells with VP, a typical robust accumulation of AQP2 was still observed at the cell surface (Fig. 2B). Importantly, only 14.2 ± 3% of AQP2 colocalized with ssYFP in stimulated cells (Fig. 2B; PCC = 0.09), which is consistent with the idea that exocytosis of vesicles containing ssYFP and AQP2 is preferentially increased upon VP stimulation. Colocalization of ssYFP and AQP2 was confirmed by low-temperature-embedding immunogold electron microscopy using anti-AQP2 and anti-YFP antibodies (Fig. 2C).
Fig. 2.
Colocalization of ssYFP and aquaporin-2 (AQP2) in LLC-PK1 cells. A: partial colocalization of AQP2 (red) with ssYFP (green) in a perinuclear compartment. B: localization of AQP2 and ssYFP after 15 min of stimulation with vasopressin-forskolin (VP/FK). AQP2 is relocated to the plasma membrane, whereas some ssYFP remains inside the cell, especially in a perinuclear compartment. Images represent native ssYFP fluorescence and immunostained AQP2. Scale bar, 10 μm. C: immunoelectron-microscopic colocalization of AQP2 (15-nm gold) and ssYFP (10-nm gold), as indicated by close association of AQP2 and ssYFP in some intracellular clusters in some regions. Bottom: clusters of colocalized AQP2 and ssYFP (arrows) at higher magnification approaching the membrane (i), at the membrane (ii), and after fusion (iii). IC, intracellular; EC extracellular.
ssYFP is constitutively secreted into the extracellular medium.
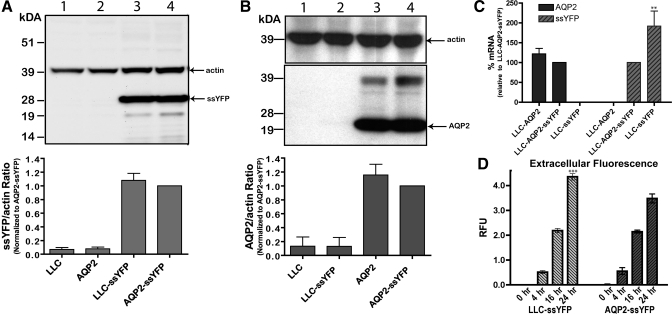
To investigate AQP2-specific exocytosis, we first selected clones of AQP2-expressing (LLC-AQP2-ssYFP) and AQP2-negative (LLC-ssYFP) cells that showed similar expression levels of ssYFP as assessed by Western blotting and densitometry [Fig. 3A; difference in ssYFP-to-actin ratio = 0.08 ± 0.01 (SE), n = 3, P = not significant (NS)]. To rule out the possibility that AQP2 protein expression is altered by the presence of ssYFP, we also confirmed that the LLC-AQP2-ssYFP clone expressed levels of AQP2 similar to those expressed by the parental LLC-AQP2 cells (Fig. 3B; difference in AQP2-to-actin ratio = 0.15 ± 0.15, n = 3, P = NS). RNA analysis by real-time PCR revealed similar expression levels of AQP2 mRNA in LLC-AQP2-ssYFP and parental LLC-AQP2 cells (defined as 100%; Fig. 3C; difference in ssYFP-to-actin ratio = 21.6 ± 14%, n = 3, P = NS) and a nearly twofold higher expression of ssYFP (difference in ssYFP-to-actin ratio 191.8 ± 38%, n = 3, P < 0.01) in LLC-ssYFP than LLC-AQP2-ssYFP (defined as 100%) cells, although this expression difference is not discernable at the protein level. In addition, AQP2 and ssYFP absolute mRNA levels were similar in the different cell lines (Fig. 3C).
Fig. 3.
Characterization of LLC-ssYFP and LLC-AQP2-ssYFP cell lines. A: representative Western blot double stained for ssYFP and actin (as a loading control). Note similar expression levels of ssYFP in LLC-ssYFP and LLC-AQP2-ssYFP cell lines (lanes 3 and 4, respectively). Lanes 1 and 2 represent parental, untransfected LLC-PK1 and LLC-AQP2 cell lines, respectively. B: Western blot double stained for AQP2, stripped and restained for actin (as a loading control). Similar AQP2 expression between untransfected parental and ssYFP-transfected cell lines indicates that YFP expression did not affect AQP2 expression (cf. lanes 3 and 4). Lanes 1 and 2 represent LLC-PK1 untransfected parental cell lines and LLC-ssYFP, respectively. All Western blots were quantified by densitometry and are expressed as average of ssYFP- or AQP2-to-actin ratio in 3 independent experiments normalized to expression in the AQP2-ssYFP cell line. C: real-time PCR quantification of mRNA. Note similar AQP2 expression in ssYFP-expressing cells and parental LLC-AQP2 controls. LLC-ssYFP cells express ∼2-fold more ssYFP than LLC-AQP2-ssYFP cells. P0 served as an internal control, and all values were normalized to corresponding LLC-AQP2-ssYFP level. D: ssYFP fluorescence in extracellular medium of AQP2-ssYFP, as well as LLC-ssYFP, cultures grown in phenol red-free DMEM. Note steady increase to ∼4-fold over a 24-h period (normalized to 0 h), indicating constitutive ssYFP secretion within both cell lines. RFU, relative fluorescence units. **P < 0.01. ***P < 0.001.
Both ssYFP-expressing cell lines were then seeded on 24-well plates and grown to confluence. The extracellular medium was collected after 0, 4, 16, and 24 h and analyzed using a plate-reader fluorimeter (see above). The extracellular ssYFP fluorescence steadily increased over a 24-h period at a similar rate for LLC-AQP2-ssYFP and LLC-ssYFP cells, except for the 24-h point, when LLC-ssYFP cell secretion was slightly, but significantly, higher (Fig. 3D; difference = 0.88 ± 0.22, n = 3, P < 0.001 at 24 h). It is probable that the difference at 24 h is due to the slightly higher expression of ssYFP in the LLC-ssYFP cell line, which at <24 h becomes insignificant. These experiments demonstrate that 1) ssYFP is constitutively secreted in both cell lines and 2) this constitutive secretion is independent of AQP2 expression under baseline conditions.
AQP2-containing cells show an increase in exocytosis in response to hormonal stimulation.
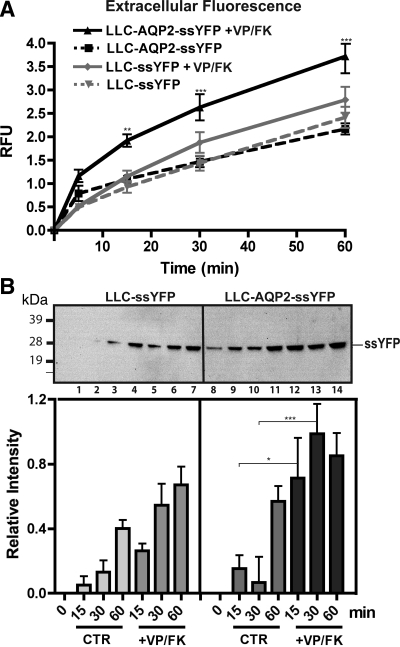
To determine whether the rate of exocytosis increases during stimulation of AQP2 cell surface accumulation, we incubated LLC-AQP2-ssYFP and LLC-ssYFP cells in HBSS for 0, 5, 15, 30, or 60 min with or without VP/FK, and the extracellular medium was collected for fluorimetric analysis. Such a mixture of VP and FK (a cAMP-elevating agent) has been previously shown to stimulate a maximal increase in water permeability and AQP2 cell surface accumulation in cultured LLC-PK1 cells (28, 53). To determine whether VP signaling was comparable in both cell lines, [3H]VP binding sites (an estimate of V2R levels) were quantified using a radioligand binding assay. LLC-ssYFP and LLC-AQP2-ssYFP cells were found to contain a similar number of [3H]VP binding sites (31,359 ± 3,234 and 32,939 ± 4,940 sites/cell, respectively, n = 3, P = NS). Additionally, cAMP concentration, as determined by an ELISA-based cAMP assay, reached similar levels in LLC-ssYFP and LLC-AQP2-ssYFP cell lines (2.93 ± 0.12 and 2.81 ± 0.20 pmol/well, respectively, n = 8, P = NS). Exocytosis significantly increased in AQP-expressing cells within the first 15 min of stimulation (to 1.92 ± 0.11, n = 5, P < 0.01, representing a 1.92-fold increase) and subsequently leveled off at 30 and 60 min (Fig. 4A). VP/FK also stimulated a slight increase in the exocytotic rate of LLC-ssYFP cells [from 0.93 ± 0.12 to 1.15 ± 0.12 (n = 5, P = NS) at 15 min, representing a 0.22-fold increase], and although this increase did not reach statistical significance at all time points, our results do not rule out the possibility that VP has a small effect on ssYFP secretion that is independent of AQP2 expression. These results are consistent with a VP/FK-stimulated temporary burst of exocytosis of AQP2-containing vesicles within the first 15 min (as seen by a rise in the slope of the stimulated curve) that ceases at later time points (as indicated by return of the slope of the stimulated curve to a level similar to the slope of the baseline curve, reflecting ongoing constitutive ssYFP secretion). To confirm the validity of our fluorescence assay, we performed parallel experiments, in which the extracellular medium was collected and analyzed by Western blotting, rather than fluorimetry. Similar trends were observed, although the differences between nonstimulated and stimulated conditions were half of those shown by the fluorescence data (0.42 ± 0.16, n = 3, P < 0.05, at 15 min), indicating that (as we expected) fluorescence detection is more sensitive but, more importantly, that little degradation or loss of YFP fluorescence in the extracellular medium occurs over the time frame of the experiment (Fig. 4B).
Fig. 4.
VP/FK increases exocytosis in AQP2-containing, but not control, cells. A: ssYFP in extracellular medium of LLC-ssYFP and LLC-AQP2-ssYFP cell lines. Note similar increase with time under baseline conditions. When VP/FK is applied, AQP2-containing cells show a large, but temporary, increase in ssYFP secretion within the first 15 min of stimulation. Relative fluorescence values are background subtracted and calculated relative to LLC-AQP2-ssYFP values at 15 min. B: increase of ssYFP in extracellular medium with (+VP/FK) or without (CTR) VP/FK over a 1-h period confirmed by Western blot (top) and quantification by densitometry of ssYFP bands in 3 independent Western blots (bottom). Values were calculated as increase relative to 0-min baseline control. Lanes 1–4 and 8–11 represent baseline secretion for 0, 15, 30, and 60 min of LLC-ssYFP and LLC-AQP2-ssYFP, respectively; lanes 5–7 and 12–14 represent VP/FK-stimulated secretion for 15, 30, and 60 min of LLC-ssYFP and LLC-AQP2-ssYFP, respectively. *P < 0.05. **P < 0.01. ***P < 0.001.
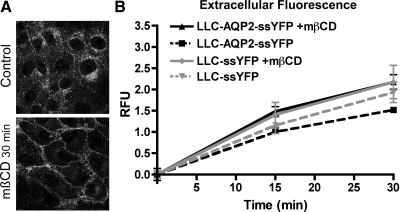
Next, we tested the specificity of this assay to monitor the exocytotic effect of VP by analyzing the delivery of ssYFP into the medium of cells in which AQP2 cell surface accumulation was achieved by blockade of the endocytotic pathway by mβCD (Fig. 5A). We previously showed that mβCD induces AQP2 cell surface accumulation of AQP2 in vitro (35) and in vivo (47) by inhibiting the endocytosis of constitutively recycling AQP2. Although we occasionally observed a slight trend to an increase in exocytosis with mβCD treatment, these differences were not significant for either cell line at all time points tested [increase of 0.49 ± 0.10 (n = 6, P = NS) and 0.25 ± 0.29 (n = 6, P = NS) at 15 min for LLC-AQP2-ssYFP and LLC-ssYFP, respectively; Fig. 5B]. We conclude, therefore, that ssYFP secretion was not significantly changed in the presence of mβCD, establishing that cell surface accumulation of AQP2 occurred, in this case, in the absence of the increase of the ssYFP signal that was observed after VP/FK stimulation.
Fig. 5.
VP exocytotic effect is specific. A: application of methyl-β-cyclodextrin (mβCD, 10 mM), an inhibitor of endocytosis, leads to accumulation of AQP2 at the cell surface (15 min incubation). B: mβCD did not lead to a significant increase in exocytosis as measured by ssYFP fluorimetry assay in LLC-ssYFP or LLC-AQP2-ssYFP cells. Relative fluorescence values were background subtracted and calculated relative to LLC-AQP2-ssYFP at 15 min.
Is S256 phosphorylation involved in the VP-induced AQP2 exocytotic burst?
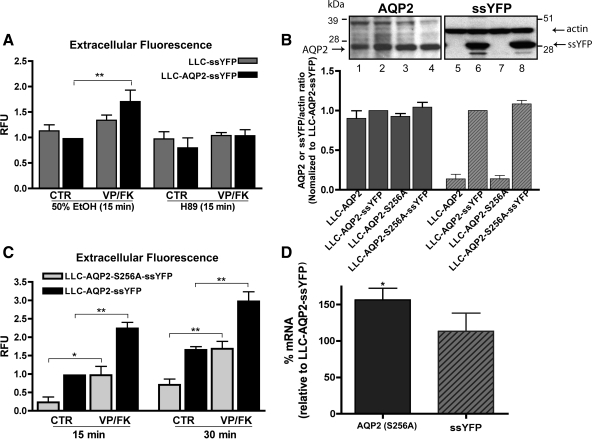
Phosphorylation of the COOH-terminal residue S256 by PKA has been postulated to play a permissive or facilitative role in VP-induced AQP2 cell surface accumulation (14, 16, 27). To investigate the role of S256 in AQP2-specific exocytosis, we first preincubated LLC-AQP2-ssYFP and LLC-ssYFP cells with the PKA inhibitor H-89 or vehicle (50% ethanol) and measured the VP/FK response. As expected, H-89 diminished the VP-induced exocytosis in cells expressing AQP2 [Fig. 6A; from a difference of 0.71 ± 0.22 (n = 5, P < 0.01) between control and VP/FK-treated cells to a difference of 0.24 ± 0.19 (n = 5, P = NS) between H-89- and H-89 + VP/FK-treated cells].
Fig. 6.
Role of S256 phosphorylation in constitutive and VP-induced AQP2 exocytosis. A: 15 min of preincubation with the PKA inhibitor H-89 blocked VP/FK-induced burst of exocytosis in AQP2-expressing cells. Extracellular medium fluorescence after 15 min was background subtracted and normalized to LLC-AQP2-ssYFP control. B: Western blot analysis shows similar expression of AQP2 (left, lanes 2 and 4) and ssYFP (right, lanes 6 and 8) in wild-type AQP2 and AQP2-S256A-expressing cells. Results of 3 independent blots were quantified by densitometry and shown as ratio of ssYFP or AQP2 to actin-loading controls (left) normalized to LLC-AQP2-ssYFP expression. C: constitutive exocytosis is reduced in LLC-AQP2-S256A-ssYFP cells. Although absolute value of the response to VP/FK was reduced compared with wild-type controls (LLC-AQP2-ssYFP), fold increase in ssYFP secretion was greater in the S256A mutant cell line. Relative fluorescence values were background subtracted and calculated relative to LLC-AQP2-ssYFP at 15 min. D: real-time PCR quantification of mRNA reveals 0.5-fold higher expression of AQP2-S256A than ssYFP (see Fig. 3B) and similar ssYFP expression levels in ssYFP and S256-expressing cell lines. P0 mRNA was used as an internal control, and all values were normalized to corresponding LLC-AQP2-ssYFP levels (set at 100%). *P < 0.05. **P < 0.01.
In complementary experiments, we generated ssYFP cells containing a phosphorylation-deficient mutant of AQP2 (LLC-AQP2-S256A-ssYFP) that expressed ssYFP at levels similar to those of the LLC-AQP2-ssYFP cell line (Fig. 6B right, lanes 6 and 8; average difference in ssYFP-to-actin ratio = 0.085 ± 0.06, n = 3, P = NS). Similar AQP2 expression was also confirmed by Western blotting (Fig. 6B, left; average difference in AQP2-to-actin ratio = 0.044 ± 0.06, n = 3, P = NS). Real-time PCR analysis showed a small, ∼0.5-fold, increase in AQP2 (156.3 ± 15%, n = 3, P < 0.05) but not ssYFP (113.3 ± 25%, n = 3, P = NS) mRNA expression in the mutant cell line compared with wild-type controls (defined as 100%; Fig. 6D, see also Fig. 3B). Interestingly, despite the slightly higher expression of mutant AQP2 in these cells, the baseline rate of ssYFP secretion was significantly lower (Fig. 6C; 0.23 ± 0.15, n = 5, P < 0.01 at 15 min), indicating that S256 may play a role in regulating the constitutive rate of AQP2 exocytosis. Under VP stimulation, while the absolute size of the exocytotic burst was smaller than in wild-type AQP2-expressing cells [Fig. 6C; from a difference of 1.24 ± 0.17 (n = 5, P < 0.001) in wild-type controls to a 0.74 ± 0.24 (n = 5, P < 0.05) at 15 min in the mutants], the fold increase in exocytosis was actually greater in the mutants than in the wild-type controls (∼4.2- vs. ∼2.2-fold increase).
DISCUSSION
In the present study, we describe the development and characterization of a rapid fluorescence-based assay to monitor exocytosis. This is achieved by expression of an exogenous soluble secreted protein in cells, the fluorescence of which can be detected in the extracellular medium. We have employed this new tool to label the exocytic arm of a well-known model system for regulated trafficking of membrane proteins, that of the major VP-responsive AQP2 water channel expressed in the kidneys. We report that a transient burst in exocytosis occurs upon antidiuretic hormone stimulation in AQP2-expressing, but not nontransfected control, cells; this finding is consistent with previous hypotheses regarding the mechanistic details underlying the cell surface accumulation of AQP2. We additionally show that our assay is specific, inasmuch as mβCD leads to cell surface accumulation of AQP2 by inhibiting endocytosis but does not produce a significant exocytotic signal. Finally, we observed that although phosphorylation of S256 in the COOH-terminal tail of AQP2 is an important event in its constitutive recycling, its importance in the exocytotic response to VP is less obvious, since VP induced a significant percent increase in the exocytotic signal, even in cells that expressed AQP2-S256A.
Exocytosis has been quantified previously in a variety of ways, in particular in specialized secretory cells, such as neuroendocrine and pancreatic cells, where regulated cargo can be detected upon secretion by labeling of the soluble cargo destined for secretion (25, 45). In neurons, electrophysiological analysis of the postsynaptic membrane response has been instrumental in the study of regulated exocytosis of neurotransmitters. Here we employ an exogenous marker, rather than a specifically packaged cargo, which could be subject to naturally occurring regulatory mechanisms. This exogenous marker passively labels the secretory pathway, allowing us to monitor bulk exocytosis, and could theoretically be applied to any cell type. Previously, a construct similar to ssYFP (a secreted soluble green fluorescent protein) was employed as a control in a study characterizing the vacuolar sorting pathway in plants (38), where it was also observed to enter the secretory pathway, highlighting the fact that ssYFP could probably be adapted for many cell types. Moreover, in contrast to the membrane-bound vesicular stomatitis virus glycoprotein-green fluorescent protein construct used extensively in seminal work on characterization of the secretory pathway (23, 32, 46), ssYFP requires only fluorimetry for initial analysis and, thus, could be adapted to become a less costly alternative for large-scale screening of modulators of the secretory pathway, inasmuch as microscopy is not required for initial-stage screening but can be applied later for refinement of target characterization.
One caveat of the assay is that AQP2 vesicles are not labeled exclusively and, although transcriptional changes are unlikely to have an influence because of the fast kinetics of the response, more rapid increases in the translation or decreases in the degradation of ssYFP in response to VP could theoretically produce the observed results. However, ssYFP does not contain any of the specific types of 5′- and 3′-untranslated region sequences that mediate many examples of this type of regulation (10, 29). Also, VP has been shown to increase AQP2 degradation (19, 20). Shunting of AQP2-bearing vesicles to late endosomes or lysosomes could, therefore, increase ssYFP degradation. Therefore, the VP-induced increase in ssYFP secretion that we report here may even be underestimated.
Other assays that have been employed to monitor AQP2 exocytosis include loading and rerelease of FM dyes (60) and measurements of membrane capacitance (34). Although the report of Lorenz et al. on membrane capacitance could theoretically distinguish between exocytosis and endocytosis, it is unclear whether this would be feasible in systems such as LLC-PK1 cells, where a massive endocytotic response to VP occurs due to V2R internalization (7), in contrast to the small endocytotic response observed in inner medullary collecting duct cells in their study (34). In comparison, ssYFP, a simple and readily detected marker, has the advantage that it does not rely on the endosomal/recycling pathway to introduce the label and, thus, more clearly separates exocytotic from endosome-derived vesicles that could fuse with the plasma membrane. Furthermore, AQP2 proteins that recycle through the TGN would also become labeled as they are repackaged along with newly synthesized protein. As mentioned above, there is evidence that at least one of the sorting steps of recycling AQP2 occurs at the TGN, but whether it occurs before or after transition through the Rab11-positive recycling endosome or whether there is some advantage to resorting at the level of the TGN remains unknown.
Additionally, we found that two distinct agents (VP and mβCD) can induce accumulation of AQP2 at the cell surface by different underlying mechanisms. It was proposed over a decade ago that exocytotic and endocytotic pathways could be involved in VP-induced cell surface accumulation of water channels (30), but the relative role of these events under various physiological conditions is unknown. Indeed, differential regulation of endocytosis vs. exocytosis in response to different stimuli has been observed in other systems, such as GLUT4 cell surface accumulation in muscle cells (59). Therefore, examination of other physiological stimuli known to modulate cell surface AQP2 accumulation or abundance, such as cGMP-raising agents (5, 8), hypertonicity (21, 22, 56), prostaglandins, dopamine (40, 61), and bradykinin (51), will increase our understanding of regulated membrane protein trafficking in this and other systems. In particular, it would be interesting to determine whether there are kinetic differences that may favor an increase of exocytosis or a decrease of endocytosis under different circumstances. Indeed, we recently showed that hypertonicity induces an MAP kinase-dependent increase in cell surface AQP2 expression, mainly by inhibiting endocytosis and not by increasing exocytosis (21).
Our data also provide insight into the role of S256 phosphorylation in AQP2 trafficking. Although it is clear that this event is required for the VP-stimulated cell surface accumulation of AQP2, the phosphorylation-deficient S256A AQP2 mutant protein can be inserted into the plasma membrane constitutively and can accumulate at the cell surface upon blockade of endocytosis (4–6, 8, 9). Using our new secretion assay, we observed first that the constitutive rate of exocytosis was significantly reduced in cells expressing the S256A mutation compared with cells bearing wild-type AQP2 or cells lacking AQP2. The simplest interpretation is that S256 is additionally involved in regulating the kinetics of the constitutive cycle and that loss of baseline phosphorylation slows (but does not prevent) constitutive exocytosis of AQP2-bearing vesicles, entrapping ssYFP within them. However, although the absolute value of the VP-induced exocytotic burst is reduced in cells expressing the S256A mutation of AQP2, the fold increase in secretion is actually higher in the mutant cell line because of a decreased baseline level of ssYFP secretion. Although it is more difficult to interpret the size of an exocytotic burst in the context of this reduced baseline secretion, our present data raise the intriguing possibility that S256 phosphorylation may not act as an “on-switch” for AQP2 exocytosis, as is generally believed. Rather, we suggest that S256 phosphorylation may play only a minor role in the regulated VP-induced exocytotic burst and may, instead, play a more significant role in the reduction of AQP2 endocytosis in response to VP. This idea is supported by our recent data showing that phosphorylation of S256 reduces the interaction of AQP2 with the accessory protein hsc/hsp70 which is involved in the endocytotic portion of the AQP2 recycling process (36). S256 phosphorylation may also regulate AQP2 interaction with MAL, another protein involved in endocytosis (26). Clearly, more work is required for a full understanding of the role of S256 phosphorylation in the AQP2 response to VP, a signaling process that is made even more complex by recent studies suggesting the potential involvement of other phosphorylation sites in the AQP2 COOH terminus in AQP2 trafficking, recycling, and membrane accumulation (6, 12, 24).
Despite the apparent minor effect of the S256A mutation on VP-induced ssYFP secretion, we found that the VP-induced exocytotic burst is abolished by the PKA inhibitor H-89, highlighting the importance of this kinase in the exocytotic process. PKA has also been reported to influence the SNARE machinery (1, 62), as well as cytoskeletal regulators, such as the Rho GTPases (31, 52), and its inhibition probably affects other VP-dependent processes in addition to S256 phosphorylation. VP is also known to induce a spike in intracellular Ca2+ (13, 41, 60), which can also influence exocytotic events, and the development of the present assay will greatly aid future studies aimed at delineating the exact intracellular mechanisms involved in VP-induced exocytosis.
An intriguing aspect of the present study is that although cell surface V2R expression, as well as cAMP elevation, is observed at similar levels in both cell lines, the exocytotic burst induced by VP stimulation in LLC-PK1 cells is detected at significant levels only when the cells are expressing AQP2, implying that the presence of AQP2 in some way is permissive for this process. As mentioned above, the association of AQP2 with other components of the trafficking pathway is most likely modulated by AQP2 phosphorylation and dephosphorylation. Thus the presence of AQP2 in some, but not all, vesicles within the secretory pathway appears to facilitate their exocytosis compared with non-AQP2-containing vesicles, at least in response to cAMP elevation. Consistent with the data presented here, a recent study revealed VP-induced changes in the actin cytoskeleton only in AQP2-expressing cells (43). Definition of the spatial and temporal interactions between AQP2 and other molecules that regulate the trafficking process is now required and is an ongoing theme in many laboratories.
In summary, we have developed a novel assay in which an exogenous fluorescent protein, ssYFP, is packaged into secretory vesicles and is released into the culture medium during vesicle exocytosis. Using this assay, we show that, as originally postulated, VP provokes a burst of exocytosis in AQP2-expressing, but not untransfected, cells. This implies that the presence of AQP2 on intracellular vesicles is necessary for this response. We were surprised to find that the stimulatory effect of VP on exocytosis may be only partially, if at all, dependent on phosphorylation of the COOH-terminal S256 residue in AQP2-expressing cells. In contrast, we confirmed that H-89-sensitive protein kinase activity is critical for this process. Taken together, our present and previous data support the notion that maximum cell surface accumulation of this water channel is achieved by regulation of the exocytosis and endocytosis pathways that are involved in AQP2 recycling, as predicted by Knepper and Nielsen (30) by mathematical modeling of collecting duct and toad bladder water permeability responses to VP before the discovery of AQP2.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-38452. P. Nunes is supported by a doctoral-level postgraduate scholarship from the National Sciences and Engineering Research Council, U. Hasler by a Swiss FSBMB Fellowship, as well as an ECOR Fellowship awarded by Massachusetts General Hospital, R. Bouley by a National Kidney Foundation Young Investigator Award, and H. A. J. Lu by NIDDK Grant K08 DK-075940-01. The microscopy core facility of the Massachusetts General Hospital Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (NIDDK Grant DK-57521) and the Center for the Study of Inflammatory Bowel Disease (NIDDK Grant DK-43341).
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baba T, Sakisaka T, Mochida S, Takai Y. PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2+-dependent exocytosis of neurotransmitter. J Cell Biol 170: 1113–1125, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 22: 439–455, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouley R, Hasler U, Lu HA, Nunes P, Brown D. Bypassing vasopressin receptor signaling pathways in nephrogenic diabetes insipidus. Semin Nephrol 28: 266–278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouley R, Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D. Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in “endocytosis-resistant” membrane domains after vasopressin treatment. Biol Cell 98: 215–232, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bouley R, Sun TX, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol 285: C750–C762, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Brockmann R, Beyer A, Heinisch JJ, Wilhelm T. Posttranscriptional expression regulation: what determines translation rates? PLoS Comput Biol 3: e57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278: F29–F42, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol 5: 3610–3616, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Haas IG BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia 50: 1012–1020, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hasler U, Nielsen S, Feraille E, Martin PY. Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol 290: F177–F187, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Hasler U, Nunes P, Bouley R, Lu HA, Matsuzaki T, Brown D. Acute hypertonicity alters aquaporin-2 trafficking and induces an MAPK-dependent accumulation at the plasma membrane of renal epithelial cells. J Biol Chem 283: 26643–26661, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16: 1571–1582, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of Golgi-to-plasma membrane transport intermediates in living cells. J Cell Biol 143: 1485–1503, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaether C, Salm T, Glombik M, Almers W, Gerdes HH. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur J Cell Biol 74: 133–142, 1997. [PubMed] [Google Scholar]
- 26.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 28.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keene JD RNA regulons: coordination of post-transcriptional events. Nat Rev Genet 8: 533–543, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F214–F224, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15: 510–519, 1996. [PMC free article] [PubMed] [Google Scholar]
- 32.Lippincott-Schwartz J, Smith CL. Insights into secretory and endocytic membrane traffic using green fluorescent protein chimeras. Curr Opin Neurobiol 7: 631–639, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic 6: 1142–1156, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz D, Krylov A, Hahm D, Hagen V, Rosenthal W, Pohl P, Maric K. Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells. EMBO Rep 4: 88–93, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuhashi N, Shimada T, Mano S, Nishimura, Hara-Nishimura I. Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol 41: 993–1001, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA 3rd, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8: 110–123, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Nejsum LN, Zelenina M, Aperia A, Frokiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Nickols HH, Shah VN, Chazin WJ, Limbird LE. Calmodulin interacts with the V2 vasopressin receptor: elimination of binding to the C terminus also eliminates arginine vasopressin-stimulated elevation of intracellular calcium. J Biol Chem 279: 46969–46980, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol 182: 587–601, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouli AE, Kennedy HJ, Schofield JG, Rutter GA. Insulin targeting to the regulated secretory pathway after fusion with green fluorescent protein and firefly luciferase. Biochem J 331: 669–675, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature 389: 81–85, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Russo LM, McKee M, Brown D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki S, Kuwahara M, Yamashita Y, Marumo F. Structure and function of AQP2. Nephrol Dial Transplant 15 Suppl 6: 21–22, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Tajika Y, Matsuzaki T, Suzuki T, Ablimit A, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol 124: 1–12, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Tamma G, Carmosino M, Svelto M, Valenti G. Bradykinin signaling counteracts cAMP-elicited aquaporin 2 translocation in renal cells. J Am Soc Nephrol 16: 2881–2889, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Toriano R, Ford P, Rivarola V, Tamarappoo BK, Verkman AS, Parisi M. Reconstitution of a regulated transepithelial water pathway in cells transfected with AQP2 and an AQP1/AQP2 hybrid containing the AQP2 C-terminus. J Membr Biol 161: 141–149, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Valenti G, Procino G, Carmosino M, Frigeri A, Mannucci R, Nicoletti I, Svelto M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci 113: 1985–1992, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005. [DOI] [PubMed] [Google Scholar]
- 56.van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Vossenkamper A, Nedvetsky PI, Wiesner B, Furkert J, Rosenthal W, Klussmann E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells. Am J Physiol Cell Physiol 293: C1129–C1138, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Wen H, Frokiaer J, Kwon TH, Nielsen S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol 10: 1416–1429, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Wijesekara N, Tung A, Thong F, Klip A. Muscle cell depolarization induces a gain in surface GLUT4 via reduced endocytosis independently of AMPK. Am J Physiol Endocrinol Metab 290: E1276–E1286, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Yip KP Epac-mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Zelenina M, Christensen BM, Palmer J, Nairn AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 278: F388–F394, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Zhou KM, Dong YM, Ge Q, Zhu D, Zhou W, Lin XG, Liang T, Wu ZX, Xu T. PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56: 657–669, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.