Abstract
The early stage of age-related macular degeneration (AMD) is characterized by the formation of subretinal pigment epithelium (RPE) deposits as a result of the dysregulation in the turnover of extracellular matrix (ECM) molecules. However, the mechanism involved remains unclear. Hypertension (HTN) is an important risk factor for AMD, and angiotensin II (ANG II) is the most important hormone associated with HTN. However, the relevance of ANG II receptors and ANG II effects on RPE have not been investigated yet. Therefore, the expression and regulation of ANG II receptors as well as the ECM turnover were studied in human RPE. ANG II receptors were expressed and upregulated by ANG II in human RPE. This regulation resulted in functional receptor expression, since an increase in intracellular concentration of calcium was observed upon ANG II stimulation. ANG II also increased matrix metalloproteinase (MMP)-2 activity and MMP-14 at the mRNA and protein levels as well as type IV collagen degradation. These ANG II effects were abolished in the presence of the ANG II receptor subtype 1 (AT1) receptor antagonist candesartan. In contrast, ANG II decreased type IV collagen via both AT1 and AT2 receptors, suggesting a synergistic effect of the two receptor subtypes. In conclusion, we have confirmed the presence of ANG II receptors in human RPE and their regulation by ANG II as well as the regulation of ECM molecules via ANG II receptors. Our data support the hypothesis that ANG II may exert biological function in RPE through ANG II receptors and that ANG II may cause dysregulation of molecules that play a major role in the turnover of ECM in RPE basement membrane and Bruch's membrane, suggesting a pathogenic mechanism to explain the link between HTN and AMD.
Keywords: calcium, hypertension, Bruch's membrane
early age-related macular degeneration (AMD) is one of the most common irreversible causes of severe loss of vision in elderly population (23, 39). Dysfunction of retinal photoreceptors is the ultimate cause of vision loss. However, the initial pathogenic target of AMD is the retinal pigment epithelium (RPE) and the subjacent extracellular matrix (Bruch's membrane, or BrM) (25). Although age is the major determinant for developing AMD, clinical studies have revealed a number of systemic and environmental risk factors (23, 49, 54). Among them, hypertension (HTN) is of special relevance (29, 34, 37, 39). However, the mechanism(s) by which HTN may affect AMD remains unclear. Interestingly, different reports have related that angiotensin II (ANG II), the final product of the renin-angiotensin system (RAS) and the most important hormone associated with HTN, may contribute to chronic diseases (17, 43, 58, 69).
Expression and localization of all components of the RAS have been well determined in adult eye tissues including the RPE (8, 62, 71, 75, 41), where the mediators of the RAS are locally released, conferring on the RPE the ability to modulate this system at tissue level. Furthermore, ANG II levels in the eye are higher than in plasma, indicating that there is a local production of ANG II in the eye (16, 56). Therefore, even in the absence of HTN, local production of ANG II in the retina is important.
The effects of ANG II are mediated via two receptor subtypes named AT1 and AT2, which belong to a group of seven-transmembrane-domain G protein-coupled receptors and have different functional properties and signal transduction mechanisms (51, 52 ). ANG II binding to the AT1 receptor activates G protein-coupled phospholipase C and inositol 1,4,5-trisphosphate, which increases intracellular Ca2+ levels (17). On the other hand, the signaling mechanisms of the AT2 receptor are diverse, and only a few of them have been characterized (17). Although ANG II receptor expression has been confirmed in the retinal vasculature (22, 63), retina (5, 62, 75), and the pigment epithelium-choroid complex (65, 67), nothing is known about the regulation of ANG II receptor subtype expression and their function in the RPE, which is a cell type of pivotal importance in the progression of AMD (25).
Among its many actions, ANG II promotes interstitial extracellular matrix (ECM) turnover by regulation of the ECM proteins (12, 20, 33, 66, 70, 73, 74, 77). For example, ANG II has been shown to stimulate synthesis of fibronectin, collagen, tissue inhibitor of metalloproteinase (TIMP)-2, and matrix metalloproteinase (MMP)-2 in mesangial and proximal tubule cells through activation of transforming growth factor-β secretion (62, 27). ANG II also activates transforming growth factor-α-mediated MMP-2 release in endothelial cells (3). In addition, ANG II increases collagen type I and III production via NADPH oxidase complex in cardiac fibroblasts (44), MMP-14, MMP-2, and MMP-9 expression and activity in MKN-28 cells, arteries, and aorta (24, 70, 74), as well as TIMP-1 expression and concentration in smooth muscle cells and coronary endothelial cells (33, 76). The RPE actively contributes to maintain a normal turnover of ECM components. However, the regulatory effects of ANG II on ECM proteins expression in the RPE are unknown.
Numerous biochemical and anatomical changes occur in BrM as part of aging retina in the absence of apparent disease, including collagenous thickening, calcification, and lipid infiltration (25). However, the accumulation of specific deposits composed by blebbing from the basolateral RPE surface and other debris between the RPE basement membrane and BrM (sub-RPE deposit) is a very prominent histopathological feature of eyes with AMD (9, 25, 35, 79). In addition, recent studies performed on AMD patient eyes examined by electron microscopy have reported that blebbing from the basolateral RPE surface traversed the RPE basement membrane and deposited in the inner and outer collagenous layers of BrM (61). However, the mechanism(s) by which these sub-RPE deposits traverse the RPE basement membrane remains unexplored. In this regard, MMP-2 is the most abundant and significant MMP enzyme synthesized by the RPE, and there is evidence that MMP-2 is crucial for degradation of ECM proteins in the RPE basement membrane and BrM (4, 19, 80). We hypothesize that the progression of the sub-RPE deposits into BrM requires degradation of ECM proteins such as type IV and I collagens and laminin, which are critical components of RPE basement membrane and adjacent BrM (19, 4), and that ECM turnover upregulation via MMP-2 activation is necessary for the breakdown of these physical barriers. ANG II-mediated regulation of genes that are expressed in RPE and are important for the ECM turnover might contribute to this process and provide a pathogenic mechanism to explain the link between HTN and AMD.
In the present study we aimed at characterizing the expression and function of ANG II receptors in the RPE and at investigating ANG II contribution to the development of early AMD. Our study confirms that both ANG II receptor subtypes were expressed and regulated by ANG II in human RPE and provides evidence that these receptors are functional in this type of cells. Importantly, our results suggest a potential role for ANG II in the regulation of ECM turnover through its receptors and support an unrecognized link among ANG II, ECM turnover, and ANG II receptor activation, which may participate in the pathophysiology of early AMD by dysregulating the ECM turnover in the RPE basement membrane and BrM.
MATERIALS AND METHODS
Compounds and drugs.
Rabbit polyclonal anti-AT1 (N-10) antibody, goat polyclonal anti-AT2 (N-19) antibody, mouse monoclonal anti-cytokeratin-18 (DA-7) antibody, and horseradish peroxidase-linked donkey anti-rabbit or donkey anti-goat antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-type IV collagen (M61403) antibody was purchased from Biodesign International (Temecula, CA). Rabbit polyclonal anti-laminin (AB19017) antibody and mouse monoclonal anti-endothelial cell (CD146) antibody were purchased from Chemicon International (Temecula, CA). Goat polyclonal anti-type I collagen (1310-01) antibody was purchased from Southern Biotechnology (Birmingham, AL). A protein quantification kit and agarose were purchased from Bio-Rad (Hercules, CA). PCR Master Mix was purchased from Promega (Madison, WI), and all primers were obtained from the DNA Core Facility, University of Miami (Miami, FL).
The commercial sources of products were otherwise as follows. Gentamicin, phosphate-buffered saline (PBS), laminin, Dulbecco's modified Eagle's medium-Ham's F12 (DMEM/F12), fetal bovine serum (FBS), HEPES, l-glutamine, penicillin/streptomycin, Tris-buffered saline (TBS), and Na2HCO3 were purchased from Invitrogen-GIBCO (Carlsbad, CA). Methanol was obtained from VWR (West Chester, PA). Collagen type IV from human placenta, ANG II, AT2 receptor antagonist PD123319, ethidium bromide, NaCl, KH2PO4, CaCl2, MgSO4, glucose, bovine serum albumin (BSA), EDTA, glycine, SDS, and Tris were purchased from Sigma-Aldrich (St. Louis, MO). The fluorescent calcium indicator fura-2 acetoxymethyl ester (fura-2 AM) was purchased from Molecular Probes (Eugene, OR). AT1-receptor antagonist candesartan (CD) was generously provided by Dr. Raij from the Veterans Administration Medical Center (Miami, FL).
Isolation of human RPE.
Human RPE monolayer was isolated from male humans as previously described (45). Three pairs of male human eyes (59, 67, and 71 yr old) not suitable for transplantation were obtained from Lions Eye Bank (Miami, FL) within 12 h after death. The eyes were rinsed two times with 10% gentamicin. The anterior segment was removed, and the vitreous/retina was separated from the RPE and choroid. The RPE monolayer was dissected free from BrM and then minced into smaller fragments under a dissecting microscope (Leica MZ6; Spectra Services, Ontario, NY). The fragments were transferred into empty individual Eppendorf tubes or into Eppendorf tubes containing 300 μl of cold lysis buffer or (1×) PBS. All tissues were stored at −80°C until mRNA and protein extraction. An aliquot from the isolated RPE was stained with a monoclonal anti-cytokeratin-18 antibody and with a monoclonal anti-endothelial cell (CD146) antibody to ensure that there was no contamination with endothelial cells.
Cell culture conditions.
Human ARPE-19 cells were purchased from American Type Culture Collection (Manassas, VA), grown in maintenance medium [DMEM/F12 (1:1 vol/vol) supplemented with 10% FBS, 1 mM l-glutamine, 100 μg/ml penicillin/streptomycin, and 0.348% Na2HCO3] in a 5% CO2 humidified air incubator at 37°C. The cells were then subcultured, propagated, and maintained in the same medium. For experiments, cells were seeded at subconfluent density in six-well plates and grown to confluence. All experiments were performed using confluent cells from passages 20–21.
Treatment with angiotensin II alone or in combination with AT1 and AT2 receptor antagonists.
Confluent cells were plated at subconfluent density (2 × 105 cells or 106 cells) in six-well plates or in T-75 (75 cm2) flasks. The cells were grown for 4 days to confluence. At this time, the cells were prepared for the experiment by changing the maintenance medium to the assay medium (maintenance medium without phenol red) for 2 days. This medium was then replaced with assay medium that was supplemented with 1% FBS instead of 10% FBS for 1 day. Subsequently, the medium was changed to the assay medium supplemented with 0.1% FBS. At this time, cells were treated with different concentrations of ANG II for 3, 6, 12, and 24 h. In some experiments, in addition to ANG II, ARPE-19 cells were exposed to 1 and 100 nM CD, an AT1 receptor blocker, 1 and 100 nM PD123319 (PD), an AT2 receptor blocker, and/or a combination of both inhibitors for 30 min before ANG II stimulation. The culture medium was removed, and the cells were washed two times with PBS. After that, fresh assay medium supplemented with 0.1% FBS was added for 24 h. The cell homogenates and supernatants were collected for RNA extraction using a RNeasy mini kit (Qiagen, Valencia, CA) and for protein quantification using a kit from Bio-Rad based on the Bradford method.
Reverse transcriptase-polymerase chain reaction.
Total RNA extraction and reverse transcriptase (RT) reaction were performed as described previously (45). Two microliters of the cDNA solution were used as a template for polymerase chain reaction (PCR). PCR amplifications were performed in a total volume of 50 μl, following a standard PCR protocol. AmpliTaq DNA polymerase (Perkin Elmer, Waltham, MA) was used as the thermostable enzyme. The specificity of each reaction was monitored in control reactions, where amplifications were performed on samples after omission of RT. The PCR for ANG II receptor subtypes AT1 and AT2 was performed using specific pairs of primers previously described by Hou et al. (32) and carried out with the following profile: 2 min at 95°C for 1 cycle, 1 min at 95°C and 1 min at 60°C for 35 cycles, and 7 min at 72°C. For the amplification of the human collagen type IV (chain α1), collagen type I (chain α1), and laminin (chain α4), primers previously described were used (13, 55, 59). Collagen type IV (chain α1) gene was amplified by a first step of 15 s at 95°C, followed by 1 min at 60°C for 40 cycles, and 7 min at 72°C. For collagen type I (chain α1), PCR procedure was conducted using 30 amplification cycles (denaturation for 1 min at 94°C, annealing for 1 min at 60°C, and extension for 2 min at 72°C). Amplification of laminin (chain α4) was carried out with the following profile: 1 min at 60°C for 1 cycle, 15 s at 95°C and 1 min at 60°C for 25 cycles, and 7 min at 72°C. To ensure that similar amounts of cDNA were used for PCR, we assessed samples for expression of GAPDH as a standard housekeeping gene. Amplification of this gene was assessed as described previously (45). Primer sequences and size of amplicons for each targeted gene are described in Table 1. PCR products were separated on 2% agarose gels containing 0.05% ethidium bromide. Bands were scanned and quantified by densitometry using ImageJ 1.17 software (National Institutes of Health, Bethesda, MD). The intensity of PCR bands is expressed as relative intensity to GAPDH.
Table 1.
Sequence of primers used for gene amplification
| Target Gene | Forward Primer | Reverse Primer | Size, bp |
|---|---|---|---|
| AT1 | GATGATTGTCCCAAAGCTGG | TAGGTAATTGCCAAAGGGCC | 255 |
| AT2 | AAGAAGAAATCCCTGGCAAGC | CTTGGTCACGGGTTATCCTGT | 302 |
| Collagen I (α1) | CTCCGGCTCCTGCTCCTCTTA | GCACAGCACTCGCCCTCCC | 225 |
| Collagen IV (α1) | ACTCTTTTGTGATGCACACCA | AAGCTGTAAGCGTTTGCGTA | 150 |
| Laminin (α4) | CTCCATCTCACTGGATAATGGTACTG | GACACTCATAAAGAGAAGTGTGGACC | 362 |
AT1, angiotensin II receptor subtype 1; AT2, angiotensin II receptor subtype 2.
Western blot analysis.
Either small dissected pieces from freshly isolated RPE or ARPE-19 cell layers were homogenized in lysis buffer, and then centrifuged at 15,000 g for 30 min at 4°C. Supernatant was collected and protein determined as described earlier. Protein extracts (20–40 μg) were denatured with SDS sample buffer followed by 5 min of boiling and then separated on 8–12% Tris-glycine gel (Novex, San Diego, CA). After electrophoresis, the proteins were transferred in 1× transfer buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, and 20% methanol, pH 8.4) to a 0.45-μm Immobilon-P polyvinyl difluoride membrane (Millipore, Billerica, AL) in a mini-PROTEAN II transfer cell (Bio-Rad) set at a constant voltage of 120 mV for 2 h. Membranes were then blocked in a 5% nonfat dry milk-TBS solution for at least 1 h at room temperature. Blots were incubated overnight at 4°C with one of the following antibodies: rabbit polyclonal anti-AT1 (N-10), goat polyclonal anti-AT2 (N-19), rabbit polyclonal anti-MMP-14, mouse monoclonal anti-type IV collagen (M61403), goat polyclonal anti-type I collagen (1310-01), rabbit polyclonal anti-laminin (AB19017), or mouse monoclonal anti-α-actin. Membranes were washed three times with TBS, incubated with horseradish peroxidase-linked donkey anti-rabbit, donkey anti-goat, or donkey anti-mouse antibodies for 2 h at room temperature, and then washed four times with TBS. Immunoreactive bands were determined by exposing the nitrocellulose blots to a chemiluminescent solution and exposing to X-Omat AR film (Eastman Kodak, Rochester, NY). Three independent experiments were performed in triplicate.
Zymography.
MMP-2 activity was assessed by zymography. Culture medium was collected after treatment and then centrifuged at 13,000 g for 30 min at 4°C. Insoluble material was removed and the supernatant collected. Protein concentration was determined as described earlier, and MMP-2 activity assessed using 10% gelatin zymography gels (Novex) as described previously (47). Briefly, 10 μg of protein extracts from each experimental condition were used. Gels were incubated 18 h in 50 mM Tris buffer, allowing determination of total proteolytic MMP-2 activity with no interference from their associated tissue inhibitors. Densitometry was performed using the Image J 1.17 densitometry program as described earlier. Each zymogram was repeated at least four times. Inhibition of gelatinase activity was assayed by incubating gels with 1 mM EDTA, a metalloproteinase inhibitor (data not shown).
Calcium mobilization induced by angiotensin II.
To determine whether ANG II increases intracellular concentration of calcium ([Ca2+]i) by activating its specific receptors, we determined [Ca2+]i using the fluorescent calcium indicator fura-2 AM as previously described (7, 28). ARPE-19 cells (105 cells/ml) cultured in DMEM/F-12 containing 10% FBS without phenol red were subcultured on 20 × 7-mm glass coverslips for 24 h. Confluent cell-coated coverslips were rinsed with HEPES buffer (10 mM HEPES, 145 mM NaCl, 2.5 mM KH2PO4, 1 mM CaCl2, 1 mM MgSO4, and 10 mM glucose, pH 7.4) containing 0.1% BSA before loading with fura-2 AM (1 μM in HEPES buffer) for 40 min at 37°C. After two washes with PBS, coverslips were mounted into a spectrofluorimeter perfusion chamber maintained at 37°C and perfused at a rate of 6 ml/min with HEPES buffer equilibrated at 37°C. Fura-2-loaded ARPE-19 cells were alternately excited at 340 and 380 nm every 3 s. Calcium images were first recorded at 3-s intervals for up to 35 min with serum-free medium alone and then with the solutions tested. Fluorescence measurements were made using a Spex Fluorilog spectrofluorometer (Spex Industry, Edison, NY) set for alternative dual-wavelength excitation at 340 and 380 nm. The light emitted at 520 nm was collected by a photomultiplier and passed to a Spex system microcomputer, which averaged the emission collected over a 0.50-s period at each excitation wavelength. Correction for autofluorescence was performed as described previously (26), and [Ca2+]i was calculated as [Ca2+]i = Kd × [(R − Rmin)/(Rmax − R)] × λ, where Kd (224 nM) is the dissociation constant of the complex fura-2-Ca2+ and Rmin, Rmax, and λ are constant parameters that depend on the optical system used. In our experimental conditions, they were Rmin = 0.9, Rmax = 18, and λ = 4. The effects of ANG II on [Ca2+]i were determined by calculating the average magnitude of the peak [Ca2+]i responses.
Real-time quantitative RT-PCR.
MMP-2 and MMP-14 mRNA expression were determined using real-time PCR. Total RNA was extracted using the RNeasy mini kit. The primer probe mixture was purchased from Applied Biosystems (Foster City, CA) and used as specified by the manufacturer. For each molecule, 1–3 μg of total RNA was used to generate cDNA with a SuperScript III First Strand synthesis kit (Novex-Invitrogen, San Diego, CA). With the use of SYBR green master mix and an ABI Prism 7900HT detection system (Applied Biosystems), cDNA was amplified for 40 cycles of 15 s at 95°C and 60 s at 60°C. Samples were run in triplicate to ensure amplification integrity. The primer-probe sets used were as follows: MMP-14, (forward) 5′-TGGTGGCTGTGCATGAGTTG-3′ and (reverse) 5′27-GTGACCCTGACTTGTTCCATA-3′; and GAPDH, (forward) 5′-TGCACCACCAACTGCTTAG- 3′ and (reverse) 5′-GGATGCAGGGATGATGTTC-3′. The MMP-2 primer-probe set was described previously (46). Signals for MMP-2 and MMP-14 in each sample were standardized against the GAPDH mRNA signal. As standards, each PCR was run on a fivefold dilution range of 2 pg of plasmid containing the appropriate template. Data are expressed as percentages of untreated cells and represent means ± SE of three independent experiments run in triplicate.
Degradation of collagen type IV induced by angiotensin II.
We evaluated whether increased release of active MMP-2 by ANG II into the supernatant might have caused degradation of collagen type IV. For this study, six-well plates containing thin inserts (1.0 μm) of collagen type IV (Becton Dickinson Labware, Bedford, MA) were used. Human collagen type IV was solubilized in sterile 0.02 N acetic acid (2.5 mg/ml; pH 3.0), poured three times into insert plates (50 μg/cm2 each time), and incubated at 37°C. The insert plates were washed with PBS in sterile conditions. An equal amount of medium from ARPE-19 cells treated with ANG II alone or in combination with its receptor blockers was placed on top of cell culture inserts, and plates were incubated at 37°C for 24 h. The medium was discarded, and the inserts were washed three times with PBS. Inserts were washed for 1 h in 2.5% Triton X-100 and incubated for 24 h in 50 mM Tris buffer. The inserts were stained with Coomassie blue, air dried, and mounted, and images were examined under a microscope (Axiophot; Carl Zeiss Meditec, Oberkochen, Germany). Collagen degradation intensity was measured from digital images at ×40 magnification (Photoshop 6.0; Adobe Systems, Mountain View, CA) as described previously (47). Results are expressed as a percentage of the control (medium from untreated cells).
Statistical analyses.
Data are expressed as a percentage of control. Results are means ± SE of three to four independent experiments performed in triplicate (as indicated). One-way ANOVA and Dunnett multiple comparison post hoc tests were performed. A Kruskal-Wallis test and post hoc Wilcoxon-Mann-Whitney U-test were used for zymography and Western blot densitometric analysis. P < 0.05 was considered to be significant. All analyses were performed with Prism 4 software (GraphPad Software, San Diego, CA).
RESULTS
Human RPE expresses both ANG II receptor subtypes.
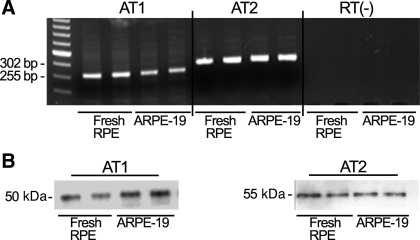
We first determined whether human RPE expresses ANG II receptors as assessed by RT-PCR and Western blot analysis. As shown in Fig. 1, we were able to detect both AT1 and AT2 receptor transcripts (shown are representative 255- and 302-bp amplicons of human AT1 and AT2 mRNA; Fig. 1A) and protein signals at ∼50 and 55 kDa using AT1 and AT2 antisera, respectively (Fig. 1B). These findings demonstrate that under basal conditions, human RPE expresses both ANG II receptors.
Fig. 1.
Human retinal pigment epithelium (RPE) expresses both angiotensin II (ANG II) receptors at the mRNA and protein levels. Total RNA and protein were collected from freshly dissected human RPE and cultured ARPE-19 cells. A: agarose gel from a representative experiment. ANG II receptor subtype 1 (AT1) and 2 (AT2) transcription was analyzed by RT-PCR. Representative amplicons of AT1 (302 bp), AT2 (255 bp), −RT, and molecular mass standard were run in parallel. B: ARPE-19-derived AT1 and AT2 protein expression evaluated by Western blot using AT1 (N-10) antiserum (left) and AT2 (N-19) antiserum (right).
Regulation of AT1 and AT2 receptors mRNA by ANG II.
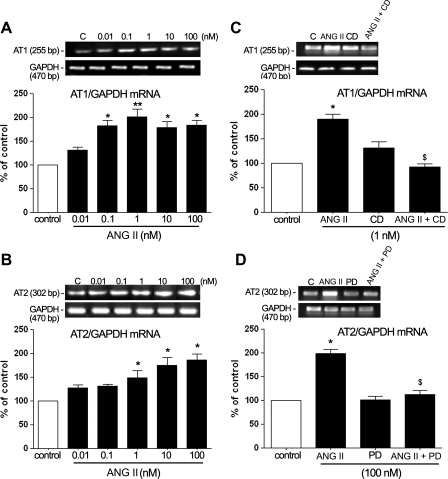
ANG II has been shown previously to regulate the levels of both ANG II receptor subtypes in nonocular tissues (17). Presently, we investigated whether ANG II modulates AT1 and AT2 expression in cultured ARPE-19 cells. To determine the range of effectiveness of ANG II, we treated the cells with ANG II (0.01–10 nM) for 24 h and studied the modulation of AT1 and AT2 expression by ANG II. ANG II from 0.1 to 100 nM increased AT1 mRNA levels. The maximal increase was achieved with 1 nM ANG II ∼1.9-fold (190 ± 11.5%, P < 0.01). Treatment with 0.1 nM ANG II increased AT1 mRNA levels ∼1.8-fold (180.35 ± 12.76%, P < 0.05), whereas 10 and 100 nM ANG II increased mRNA levels ∼1.7-fold (170.42 ± 9.8%, P < 0.05, and 174.42 ± 10.1%, P < 0.05, respectively). However, at concentration of 0.01 nM, there was no difference in AT1 mRNA expression compared with control (Fig. 2A).
Fig. 2.
Levels of AT1 and AT2 ANG II receptor mRNA are upregulated by ANG II in cultured ARPE-19 cells, and these effects are inhibited by selective AT1 and AT2 antagonists. ARPE-19 cells were incubated with different concentrations of ANG II alone for 24 h (A and B) or in combination with candesartan (CD; 1 nM), an AT1 receptor antagonist, or PD123319 (PD; 100 nM), an AT2 receptor antagonist, for 30 min before ANG II stimulation (C and D) and then washed with PBS and incubated in assay medium (0.1% FBS) for 24 h. Expression of AT1, AT2, and GAPDH mRNAs was analyzed using semiquantitative RT-PCR. AT1 and AT2 mRNA expression was normalized to GAPDH. C, control. Top, agarose gel from a representative experiment; number at left represents the molecular size of PCR product. Bottom, average of results of 4 independent experiments. Data are expressed as percentages of control and are means ± SE of 4 independent experiments run in triplicate on cultured cells. *P < 0.05; **P < 0.01 vs. control. $P < 0.01 vs. 1 or 100 nM ANG II effects.
We also showed that ANG II regulated AT2 mRNA. Minimal changes in AT2 mRNA expression were observed at 0.01 and 0.1 nM ANG II. The AT2 mRNA level increased ∼1.45-fold (142 ± 20%, P < 0.05) in the presence of 1 nM ANG II. The maximal increase of AT2 mRNA level was achieved with 10 and 100 nM ANG II ∼1.75-fold (170 ± 21.2%, P < 0.01) and 1.8-fold (179 ± 12.8%, P < 0.01), respectively (Fig. 2B).
The maximal upregulation of AT1 mRNA by ANG II (1 nM) and AT2 mRNA by ANG II (100 nM) was prevented by pretreatment with the selective AT1 antagonist CD (Fig. 2C) and by the selective AT2 receptor antagonist PD, respectively (Fig. 2D). Minimal changes in either AT1 or AT2 mRNA expression were observed after pretreatment with CD and PD in combination (data not shown). Thus the levels of AT1 and AT2 mRNA were regulated by ANG II in ARPE-19 cells, and these effects were inhibited by selective AT1 and AT2 antagonists.
ANG II-stimulated [Ca2+]i signaling in ARPE-19 cells.
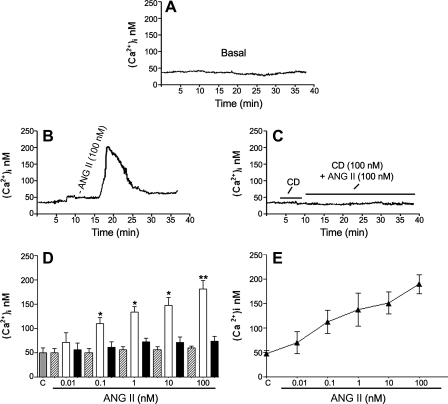
A number of studies have shown that ANG II produces an increase in [Ca2+]i in nonocular tissues (21, 72, 81), albeit its effect in RPE cells has not been reported. To determine whether ANG II increases [Ca2+]i by activating its specific receptors, we measured responses to ANG II (0.01–100 nM) in cultured ARPE-19 cells. The level of [Ca2+]i was ∼50 ± 12 nmol under basal conditions (Fig. 3A). In contrast, ANG II induced a transient rise in [Ca2+]i, reaching a transient peak between 4 and 6 min after stimulation, which then declined to a nonsignificant stable level slightly above the baseline by 25 min (Fig. 3B). The transient peak levels as well as all responses were dose dependent (Fig. 3, D and E). Pretreatment with the AT1 receptor antagonist CD (Fig. 3C), but not the AT2 antagonist PD (data not shown), prevented the ANG II-induced [Ca2+]i responses, suggesting that the ANG II-mediated increase in [Ca2+]i in ARPE-19 cells is mediated by the AT1 receptor and that this receptor is coupled to [Ca2+]i signaling.
Fig. 3.
ANG II increases intracellular calcium. Fluorescence measurement shows intracellular calcium ([Ca2+]i) responses in ARPE-19 cells under basal conditions and during ANG II (100 nM) stimulation with or without CD (100 nM), a selective AT1 receptor antagonist added 5 min before and during ANG II stimulation. Representative recordings of intracellular calcium mobilization in response to ANG II show traces under basal conditions (A), stimulated with ANG II (100 nM) alone (B), and stimulated with ANG II (100 nM) in combination with 100 nM CD (C) added 5 min before and during ANG II stimulation antagonist. D: averaged [Ca2+]i during ANG II stimulation at different concentrations: baseline (hatched bars), [Ca2+]i peaks (open bars), and 25 min after stimulation (solid bars). Bars represent means ± SE (n = 4). *P < 0.05; **P < 0.01. E: dose response of ANG II-elicited peak.
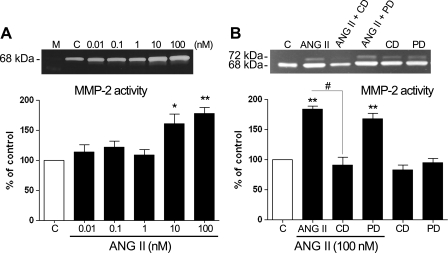
Modulation of MMP-2 activity by ANG II.
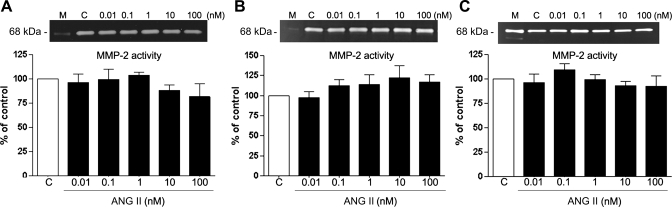
MMP-2 is known to be a key enzyme that is released to the medium and implicated in ECM remodeling in the retina (2, 5). Alterations in the activity of this enzyme, among other factors, are involved and believed to be crucial in the early stages of AMD (19, 46, 78). ANG II has been shown to regulate MMP-2 in nonocular tissues (20, 24, 33, 73). We decided to analyze whether ANG II might alter MMP-2 activity and protein expression in RPE cells. ARPE-19 cells were cultured as described in materials and methods and treated with various concentrations (0.01–100 nM) of ANG II for periods of 3, 6, 12, and 24 h. Conditioned media and cells were then collected and subjected to zymography and/or Western blot analysis. No significant effect of ANG II on MMP-2 activity was observed at any assayed concentration of ANG II for 3, 6, or 12 h (Fig. 4, A–C). However, increases in MMP-2 activity of ∼1.5-fold (157 ± 10.71%, P < 0.05) and ∼1.9-fold (198.12 ± 14.28%, P < 0.01) were seen in cells treated with 10 and 100 nM ANG II, respectively, for 24 h (Fig. 5A). On the basis of these results, we focused our attention on the 24-h incubation time with ANG II alone or in the presence of AT1 and AT2 receptor antagonists. The MMP-2 activity increase induced by treatment with ANG II (10 and 100 nM) for 24 h was completely abolished by the AT1 receptor antagonist CD and unaffected by the AT2 receptor antagonist PD, as shown in Fig. 5B (data not shown for 10 nM ANG II).
Fig. 4.
Matrix metalloproteinase (MMP)-2 activity (in the supernatant) does not appear to be significantly modified after treatment with increasing doses of ANG II for 3, 6, and 12 h. ARPE-19-derived MMP-2 protein activity was evaluated by zymography in the presence of various concentrations of ANG II for 3 (A), 6 (B), and 12 h (C). Top, gelatin zymogram in the supernatant from a representative experiment: lane M, marker; lane C, control; number at left represents protein molecular mass in kDa. Bottom, average of results of 4 independent experiments run in triplicate on cultured cells. Data represent the relative amount of the 68-kDa form and are means ± SE.
Fig. 5.
Exposure to ANG II upregulates MMP-2 protein activity in the supernatant. A: ARPE-19-derived MMP-2 protein activity evaluated by zymography in the presence of various concentrations of ANG II for 24 h. B: effect of CD (100 nM) and PD (100 nM) on ANG II-induced MMP-2 protein activity. ARPE-19 cells were exposed to the AT1 or AT2 receptor blocker alone or in combination with ANG II (100 nM). CD abolished the ARPE-19-derived MMP-2 protein activity increase induced by ANG II. Top, gelatin zymogram in the supernatant from a representative experiment; number at left represents protein molecular mass in kDa. Bottom, average results of 4 independent experiments run in triplicate on cultured cells. Data represent the relative amount of the 68-kDa form and are means ± SE. *P < 0.05; **P < 0.01 vs. control. #P < 0.05 vs. 100 nM ANG II.
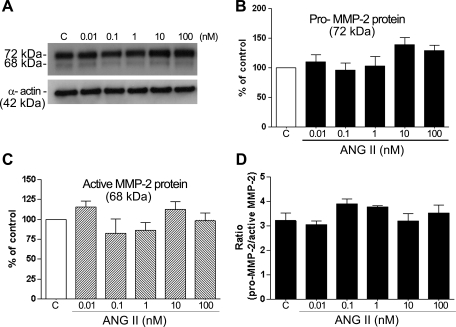
To elucidate whether the increase in active MMP-2 in the supernatant is related to a proportionate increase in cell-associated active MMP-2 protein, we used Western blot analysis and compared the impact of ANG II on secreted pro-MMP-2 protein (72 kDa) and cleaved, active MMP-2 protein (68 kDa). We confirmed that neither cell-associated pro-MMP-2 protein nor active MMP-2 protein appeared to be significantly modified after treatment with ANG II for 24 h (Fig. 6, A–C). In addition, the ratio of the cell-associated latent pro-MMP-2 to active MMP-2 protein was greater than 2.8 for untreated and treated RPE cells (Fig. 6D).
Fig. 6.
Exposure to ANG II does not change MMP-2 protein expression in the cellular lysate. ARPE-19-derived MMP-2 protein expression was evaluated by Western blot in the presence of various concentrations of ANG II for 24 h. A: immunoblotting in the cellular lysate from a representative experiment. Number at left represents protein molecular mass in kDa. B–D: average results of 4 independent experiments run in triplicate on cultured cells. MMP-2 protein expression was normalized to α-actin. Data represent the relative amount of pro-MMP-2 protein (B), active MMP-2 protein (C), and the ratio between pro-MMP-2 and active MMP-2 protein forms (D).
Real-time RT-PCR was also performed to determine the impact of ANG II on MMP-2 mRNA expression. Minimal modification in levels of mRNA was observed after ANG II treatment (Table 2). Together, these data show that ANG II (10 and 100 nM) increased MMP-2 activity via AT1 receptor activation and suggest that this increase occurred without any change in MMP-2 protein or mRNA expression.
Table 2.
Regulation of MMP-2 and MMP-14 mRNA by ANG II in ARPE-19 cells
|
mRNA |
||||
|---|---|---|---|---|
| MMP-2, ng | %Change | MMP-14, ng | %Change | |
| Control | 100 | 100 | ||
| ANG II (0.01 nM) | 103.6±11.1 | 3.7 | 611.3±16.3 | 511.3† |
| ANG II (0.1 nM) | 128.8±16.2 | 23.8 | 569.7±22.6 | 469.7† |
| ANG II (1 nM) | 110.9±15.7 | 10.9 | 486.6±19.7 | 386.6* |
| ANG II (10 nM) | 134.0±17.5 | 34.0 | 463.3±8.7 | 363.3* |
| ANG II (100 nM) | 122.7±6.6 | 22.7 | 620.5±5.6 | 520.5† |
Values are means ± SE of at least 3 independent experiments. ANG II, angiotensin II; MMP, matrix metalloproteinase.
P < 0.05;
P < 0.01vs. nontreated control cells.
ANG II modulates MMP-14 protein and mRNA expression in ARPE-19 cells.
MMP-2 is secreted as a partially active proenzyme (72 kDa) that is subsequently processed into an active 68-kDa form, as mentioned above. The regulation of MMP-2 activity is complex with possible loci of regulation being at the level of transcription, translation, or posttranslational processing by formation of a trimer composed by TIMP-2, which binds to MMP-14 before association with pro-MMP-2. Concentrations of both MMP-14 and TIMP-2 are critical for MMP-2 activation (68).
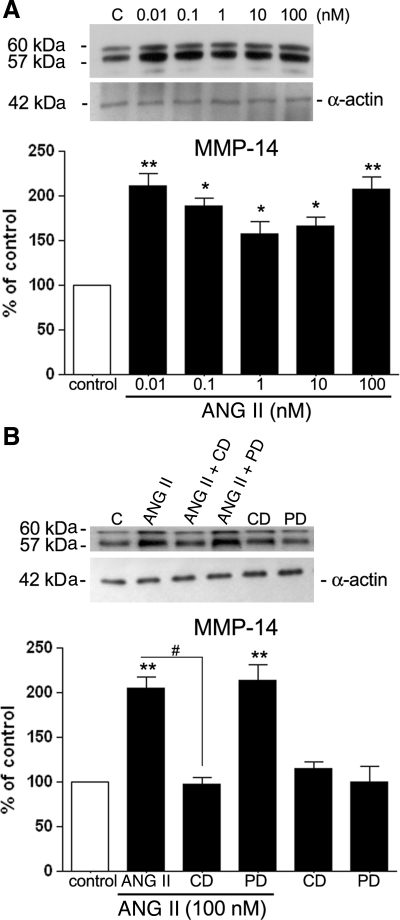
To elucidate whether the increase in active MMP-2 in the supernatant is related to a proportionate increase in MMP-14, we cultured and treated ARPE-19 cells with various concentrations of ANG II as described in materials and methods. After treatment, cells were collected for semiquantitative RT-PCR and Western blot analyses of the endogenous regulator MMP-14. We observed a significant increase in MMP-14 protein expression in ARPE-19 cells exposed to ANG II from 0.01 to 100 nM for 24 h (Fig. 7A). Treatment with 0.01 and 100 nM ANG II for 24 h induced an approximately twofold increase in MMP-14 protein (0.01 nM ANG II: 213 ± 15.6%, P < 0.01; 100 nM ANG II: 197 ± 13.1%, P < 0.01). An ∼1.70-fold (176.5 ± 5.85%, P < 0.05) increase in expression of this protein was observed after treatment with 0.1 nM ANG II. In addition, MMP-14 protein levels increased ∼1.5-fold (150 ± 12.2 and 159 ± 6.2%, P < 0.05) were observed after incubation with 1 and 10 nM ANG II, respectively (Fig. 7A).
Fig. 7.
ANG II upregulates MMP-14 protein in the cellular lysate, and this effect is abolished by CD. A: ARPE-19-derived MMP-14 protein evaluated by Western blot in the presence of various concentrations of ANG II for 24 h. B: effect of CD (100 nM) and PD (100 nM) on ANG II-induced MMP-14 protein expression. ARPE-19 cells were exposed to the AT1 or AT2 receptor blocker alone or in combination with ANG II (100 nM). Top, immunoblotting from a representative experiment showing change in protein expression; number at left represents protein molecular mass in kDa. Bottom, average results of 4 independent experiments run in triplicate on cultured cells. MMP-14 protein expression was normalized to α-actin. Data represent the relative amounts of the 57- and 60-kDa forms and are means ± SE. *P < 0.05; **P < 0.01 vs. control. #P < 0.05 vs. 100 nM ANG II.
Our results also showed that the increase in MMP-14 protein observed after incubation with 100 nM ANG II for 24 h (∼1.98-fold P < 0.01) was completely blocked by the AT1 receptor blocker 100 nM CD before the ANG II stimulation (∼58.2 ± 1.6% decrease vs. ANG II effect, P < 0.01). In contrast, this increase in MMP-14 protein expression by 100 nM ANG II was not modified by exposure to the AT2 receptor blocker PD (100 nM) before the ANG II stimulation (Fig. 7B), confirming AT1 receptor-mediated enhancement of MMP-14 protein expression.
Real-time RT-PCR was also performed to determine the impact of ANG II on MMP-14 mRNA expression. The changes observed in MMP-14 protein expression were accompanied by a significant increase in MMP-14 mRNA expression (Table 2). Maximal increases of ∼5.2-fold (611.31 ± 16.3%, P < 0.001, and 620.5 ± 5.6%, P < 0.001) in MMP-14 mRNA expression were observed after treatment with 0.01 and 100 nM ANG II, respectively, for 24 h (Table 2). In addition, treatment with ANG II (0.1 nM) induced an ∼4.6-fold increase in MMP-14 mRNA expression (569.7 ± 22.6%, P < 0.01), whereas an ∼3.7-fold increase in MMP-14 mRNA expression (486.6 ± 19.7%, P < 0.01, and 463 ± 8.7%, P < 0.01) was observed after treatment with 1 and 10 nM ANG II, respectively (Table 2). Together, these data suggest that 10 and 100 nM ANG II-induced increases of MMP-2 activity occurred concomitantly with an increase in MMP-14 mRNA.
Regulation of extracellular matrix proteins by ANG II in ARPE-19 cells.
Early pathological events leading to AMD involve an imbalance between synthesis and degradation of ECM molecules present in the RPE basement membrane and adjacent BrM. Collagens type IV and I and laminin are crucial components of RPE basal lamina and adjacent BrM (4, 19). In addition, these ECM molecules are recognized as a substrate and cleaved by MMP-2. Therefore, we decided to investigate the potential effects of ANG II on these molecules.
ARPE-19 cells were cultured and treated with different doses of ANG II for 3, 6, 12, and 24 h or only for 24 h as described in materials and methods. After treatment, cells were collected for RT-PCR and Western blot analysis.
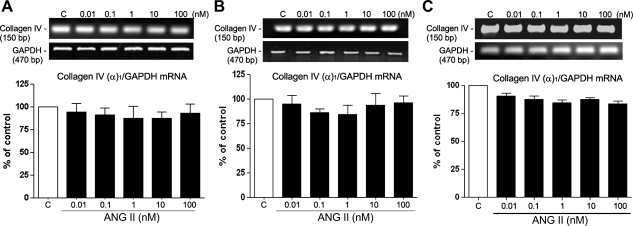
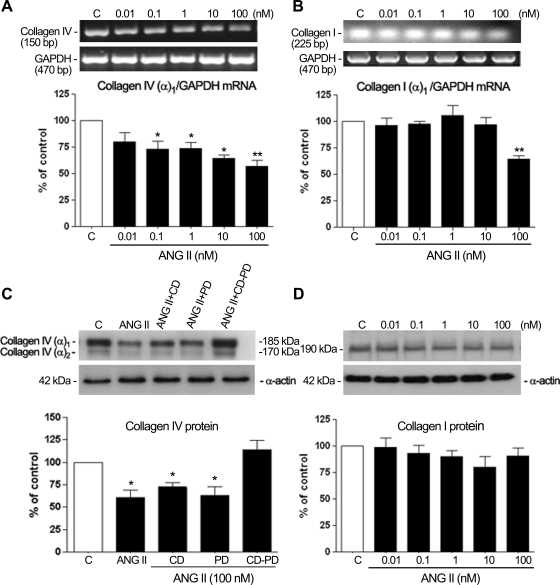
RT-PCR analysis of collagen type IV (chain α1) revealed no changes in transcription at any assayed concentration of ANG II for 3, 6, or 12 h (Fig. 8, A–C). However, a significant decrease in transcription was revealed when cells were incubated with increasing concentrations of ANG II (0.1–100 nM) for 24 h (Fig. 9A). A decrease of nearly 30% (32.6 ± 14%, P < 0.05, and 30.8 ± 8.7%, P < 0.05) was detected with 0.1 and 1 nM ANG II, respectively, and nearly 40% (41.6 ± 4.53%, P < 0.05) after treatment with 10 nM ANG II (Fig. 9A). The maximal decrease of ∼45% (46.3 ± 6.7%, P < 0.01) was achieved with 100 nM ANG II. Our results also revealed a significant decrease in the level of type I collagen chain α1 transcripts when cells were incubated with 100 nM ANG II (Fig. 9B).
Fig. 8.
Collagen type IV (in the cellular lysate) does not appear to be significantly modified after treatment with increasing doses of ANG II for 3, 6, and 12 h. Cells were exposed to increasing concentrations of ANG II for 3 (A), 6 (B), and 12 h (C). Top, collagen type IV (α1-subunit) mRNA evaluated by RT-PCR analysis. Agarose gel from a representative experiment shows amplicons for collagen type IV (150 bp) and the housekeeping gene product GAPDH (470 bp), which was used to normalize values. Bottom, average results of 3 independent experiments normalized to the loading control. Results are means ± SE.
Fig. 9.
Collagen IV (chain α1) and collagen I (chain α1) transcript levels and protein evaluated by RT-PCR and Western blot analysis in the presence of different concentrations of ANG II alone for 24 h (A, B, and D) or in the presence of ANG II (100 nM) alone or in combination with the AT1 receptor blocker, the AT2 receptor blocker, or the combination of both blockers for 24 h (C) in ARPE-19 cells. Top, agarose gel or Western blot from a representative experiment; numbers at left and right represent the molecular size of PCR product or the protein molecular mass in kDa. Bottom, average results of 3 independent experiments. Collagen IV (chain α1) and collagen I (chain α1) mRNA expression was normalized to GAPDH and protein expression to α-actin. Results are means ± SE. *P < 0.05; **P < 0.01 vs. control.
Protein analysis of type IV collagen in the cell lysates by Western blotting showed two bands in the upper molecular mass range of ∼185 and 170 kDa for collagen IV, probably corresponding to different combinations of α1 (IV) and α2 (IV) chains (Fig. 9C), which were only regulated by 100 nM ANG II. These immunoblottings revealed a clear diminution of type IV after 24 h of treatment with 100 nM ANG II. In contrast, protein analysis of type I collagen showed a single band of ∼190 kDa (Fig. 9D), which was not regulated by ANG II treatment.
Treatment of cells with 100 nM ANG II in the presence of AT1 and AT2 receptor antagonists alone or in combination (Fig. 9C) confirmed that a decrease in type IV collagen of ∼1.4-fold was seen in cells treated with an alternate method of the AT2 receptor stimulation, namely, a combination of ANG II and CD. Stimulation of the AT1 receptors alone, with a combination of ANG II and PD, caused a 1.5-fold decrease in type IV collagen protein. The stimulation of both receptors with ANG II in the absence of antagonists caused an ∼1.6-fold decrease in type IV collagen protein. Interestingly, the action of ANG II was totally inhibited by combination of both inhibitors (Fig. 9C), suggesting a synergistic effect of the two receptor subtypes on collagen IV protein in cell lysates.
RT-PCR and Western blot analysis for laminin confirmed that neither transcriptional nor protein expression of laminin appeared to be significantly modified after treatment with various concentrations of ANG II for 24 h (data not shown).
Regulation of extracellular matrix turnover by angiotensin II.
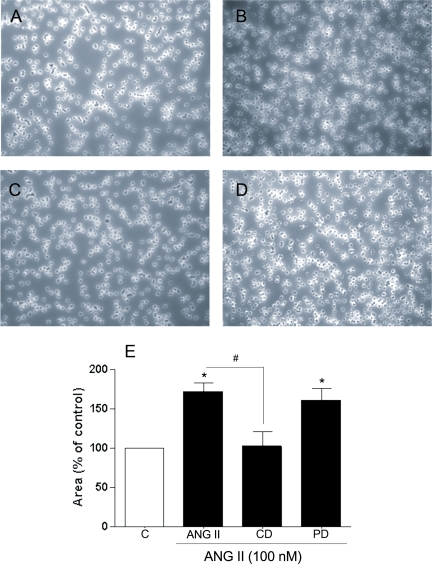
To elucidate whether the increase in active MMP-2 in the supernatant after treatment with 100 nM ANG II for 24 h is correlated with an increase in degradation of collagen IV, we incubated collagen IV matrix inserts for 24 h with medium from ARPE-19 cells treated with ANG II (100 nM) alone or in combination with the ANG II receptor blockers for 24 h. Collagen IV degradation increased by ∼65% (∼65.8 ± 2.7% decrease, P < 0.05) after treatment with medium of ARPE-19 cells treated with 100 nM ANG II for 24 h (Fig. 10E). This effect was significantly inhibited by the AT1 receptor antagonist CD (∼43.3 ± 3.2% decrease, P < 0.05 vs. ANG II effect) and unaffected by the AT2 receptor antagonist PD, as shown in Fig. 9D. These results suggest that ANG II via AT1 receptor might degrade collagen IV through modifications of MMP-2 activity in ARPE-19 cells.
Fig. 10.
Collagen type IV matrix inserts stained with Coomassie blue after exposure for 24 h to medium of ARPE-19 cells treated with ANG II (100 nM) alone (B) or in the presence of AT1 or AT2 receptor blockers (C and D). A: control. Magnification, ×40. E: average results of 3 independent experiments run in triplicate. Results are means ± SE. *P < 0.05 vs. control. #P < 0.01 vs. 100 nM ANG II.
DISCUSSION
Early AMD is characterized by the accumulation of specific deposits between the RPE basement membrane and BrM (1). To date, little is known about the mechanisms underlying early AMD pathogenesis, and dysregulation of the ECM turnover is believed to be a critical factor (19, 23). This pathology is of multifactorial nature involving factors other than age (23, 52, 54). In particular, HTN constitutes a risk factor for AMD (29, 36, 38, 40). However, the mechanism(s) associating the HTN with AMD remains unexplored. Interestingly, the localization of ANG II and its receptors in eye (16, 56, 65, 67) has opened the possibility for a prominent pathological role of ANG II such as some studies have demonstrated in other tissues (12, 20, 33, 66, 77). Our study demonstrated the presence of both ANG II receptor subtypes in human RPE and their transcriptional regulation by ANG II in ARPE-19 cells. In addition, we showed that ANG II has the ability to alter the normal RPE physiology, leading to degradation of collagen and potentially favoring dysregulation of the ECM turnover.
A previous report exploring the localization of the ANG II receptors AT1 and AT2 in the whole human retina used immunohistochemistry to show expression of the ANG II receptors in the pigment epithelium-choroidal complex (67). Our study utilized molecular biology techniques directly applied on isolated human RPE cells. Using this approach, we were able to confirm that the ANG II receptors are expressed in RPE cells as both mRNA and protein. However, the relative expression level of these receptors in the RPE remains to be elucidated.
Based on the fact that ANG II receptors AT1 and AT2 confer to ANG II an active physiological role, regulation of these receptors becomes essential in determining ANG II action. Investigating the regulation of these receptors expression may help to better understand how they participate in either physiological or pathological events.
Because concentration of ANG II in blood and eye can vary over a relatively large range, tissue differences in the dose dependence of ANG II effect must be considered. In this study, we found that in the complete absence of ANG II or in the presence of 0.01 nM ANG II, ARPE-19 cells expressed very low levels of both ANG II receptor subtypes. Treatment with 0.1 nM ANG II increased the expression of AT1 receptor but did not modify the AT2 receptor expression. In contrast, higher ANG II concentrations (from 1 to 100 nM) increased the expression of both ANG II receptor subtypes, but in a different manner: AT2 receptor was upregulated in a dose-dependent manner, whereas AT1 reached a plateau. These effects were ANG II receptor mediated, given that ANG II receptor antagonists CD and PD completely abolished the effects of ANG II on the transcriptional regulation of AT1 and AT2 receptors, respectively. This view is further supported by abundant evidence that ANG II/ANG II receptors are involved in pathological events in other tissues (3, 33, 62, 76).
Once we verified that ANG II receptors were expressed in human RPE and regulated at the transcriptional level by ANG II in ARPE-19 cells, we aimed at evaluating whether these receptors were functional in this type of cells. In this regard, it was reported that a characteristic intracellular consequence of activating these receptors is an increase in the levels of intracellular calcium (30, 50). Our data show that stimulation of the AT1 receptor by ANG II is transduced into an intracellular signal, in particular an increase in the levels of intracellular calcium. This result constitutes clear evidence that the AT1 receptor expressed in human ARPE-19 is functionally active and might be efficiently coupled to the phospholipase C (PLC) transduction pathway. Compared with these results, stimulation of the AT2 receptor by ANG II was not accompanied by mobilization of intracellular calcium. These data suggest differences in AT2 receptor coupling in ARPE-19 cells. AT2 receptor could be specifically coupled to the cytosolic phospholipase A2 (cPLA2), as has been shown recently in renal mesangial and proximal tubular epithelium cells, and not to the PLC pathway (15). Therefore, selective up- or downregulation of only AT2 receptor transduction pathway remains a possibility to be investigated.
The RPE is a central element in the pathogenesis of AMD. Dysfunctional RPE cells may alter the normal turnover of the ECM components modifying the sub-RPE deposit formation as well as the RPE basement membrane and BrM degradation, all of which lie at the origin of the early AMD (1, 42, 78). Although the cellular mechanism(s) for dysregulation of ECM turnover in AMD remains unknown, evidence suggests that MMPs and their tissue inhibitors may play an important role (42, 47, 36, 57). In this regard, we and others have shown that RPE synthesizes MMPs, especially MMP-2 and MMP-14, and that MMP-2 synthesis, release, and activity can be regulated by physiological stimuli. Our study showed that ANG II increased MMP-2 activity and that the AT1 blocker CD abolished the ANG II effect on MMP-2 activity, indicating that ANG II, via AT1 receptor, could induce matrix turnover by enhancing the release activity of MMP-2. We did not find a direct relationship between MMP-2 enzymatic activity and protein. In addition, the ratio of secreted pro-MMP-2 protein to cleaved, active MMP-2 was greater than 2.8 for untreated and treated ARPE-19 cells, suggesting that MMP-2 regulation occurs by gene transcription, translational regulation, and posttranslational activation of proenzymes.
The activation of MMP-2 (cleavage of the pro- form) involves the formation of a trimer composed of TIMP-2 binding to MMP-14 before association with pro-MMP-2. Given that pro-MMP-2 activation occurs only at low TIMP-2 concentrations relative to MMP-14, which permits availability of active MMP-14 to activate the pro-MMP-2 bound in the ternary complex (31), our study sought to explore whether ANG II might influence MMP-14 regulation. In agreement with previous observations in abdominal aortas (20), we detected an increase in MMP-14 at the mRNA and protein levels. In addition, this effect was abolished by an AT1 blocker. It is interesting to note that ANG II-induced MMP-14 is only correlated with MMP-2 activity at elevated levels of ANG II. However, surprisingly, there was no direct correlation between MMP-14 protein expression and MMP-2 activity at lower doses of ANG II. It is possible that an increase in TIMP-2 may occur when cells are treated with lower doses of ANG II and that excess of TIMP-2 relative to MMP-14 may eventually block all active MMP-14, therefore inhibiting proteolysis of pro-MMP-2 to active MMP-2. Whether an increase in TIMP-2 results from activation by low levels of ANG II deserves further investigation.
Our work also sought to analyze whether ANG II might influence the normal balance in ECM turnover by affecting the synthesis and/or degradation of collagens and laminin, crucial components of the RPE basement membrane and BrM. Our study showed a significant decrease in collagen type IV mRNA after exposure to physiological doses of ANG II and a significant decrease in collagen type IV protein levels via AT1 and AT2 receptors after exposure to 100 nM ANG II, suggesting a synergistic effect of the two receptor subtypes on collagen type IV degradation in these cells. This outcome contrasts with observations on other tissues where the ANG II induced increase in collagen type IV (14, 60). This disparity suggests that some of the effects induced by ANG II may be specific to the particular type of cell targeted. Nonetheless, our study challenged the RPE cells with ANG II for 3, 6, 12, and 24 h, and we cannot rule out the possibility that longer exposition times might induce an increase in collagen type IV. In addition, our results clearly demonstrated a significant decrease in collagen type I mRNA levels without change in the protein expression after treatment with 100 nM ANG II for 24 h. This effect on collagen I protein might be due to the half-life of collagen I in ARPE-19 cells. Treatment longer than 24 h may be necessary to evidence an effect on the regulation at the transcriptional level. Unlike collagen types I and IV, the other major component investigated, laminin, remained unaffected after ANG II stimulation of RPE cells.
Interestingly, this work also demonstrated for the first time that ANG II-induced MMP-2 activity is correlated not only with increased MMP-14 but also with type IV collagen degradation. Experiments in vivo in aged hypertensive mice using infusion of ANG II in osmotic minipumps need to be performed to correlate our in vitro findings in the current study.
In previous works by our laboratory we hypothesized that the RPE is the key target cell in deposit formation. Especially, we have demonstrated that RPE cells that are repeatedly exposed to oxidant stress showed blebbing of the cell membrane material as well as ECM turnover dysregulation (46, 47). In addition, mice exposed to oxidant stimulus showed sub-RPE deposits with blebs within the BrM (47). Based on these observations and our data from this study, we speculate that sub-RPE deposits formed in response to the oxidative injury might need an additional injury for these deposits to traverse the RPE basement membrane. In this regard, ANG II might be this second injury. Therefore, in vivo studies with an oxidant stimulus followed by exposure to ANG II remain a possibility to be investigated.
Together, these data provide evidence that ECM turnover increases in ARPE-19 cells after 24-h exposure to elevated eye levels of ANG II. In addition, it may help to explain, at least in part, the breakdown of RPE basement membrane that would allow the migration of sub-RPE through the BrM in AMD. In addition, ANG II might potentially contribute to diminish the formation of sub-RPE deposits. Interestingly, our study shows that ANG II receptor blockers may prevent these changes and suggests the possibility of ANG II as a therapeutical target in AMD.
GRANTS
This study was supported by National Eye Institute Grants R01 EY015249-01A1, EY015249-01A1S1, and EY014801.
Acknowledgments
Dr. Raul Corredor's technical support is appreciated.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol 44: 1–29, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Ahir A, Guo L, Hussain AA, Marshall J. Expression of metalloproteinases from human retinal pigment epithelial cells and their effects on the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci 43: 458–465, 2002. [PubMed] [Google Scholar]
- 3.Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-α. Am J Physiol Cell Physiol 286: C779–C7784, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Atkison SJ, Petterson ML, Butler MJ, Murphy G. Membrane type 1 matrix metalloproteinase and gelatinase A synergistically degrade type I collagen in a cell model. FEBS Lett 491: 222–226, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Aumailley M, Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med 76: 253–265, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Baramova EN, Bajou K, Remacle A, L'Hoir C, Krell HW, Weidle UH, Noel A, Foidart JM. Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett 405: 157–162, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bascands JL, Pecher C, Rouaud S, Emond C, Tack I, Bastie MJ, Burch R, Regoli D, Girolami JP. Evidence for existence of two distinct bradykinin receptors on rat mesangial cells. Am J Physiol Renal Fluid Electrolyte Physiol 264: F548–F556, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Berka JL, Stubbs AJ, Wang DZM, Dinocolantonio R, Alcorn D, Campbell DJ, Skinner SL. Renin-containing Muller cells of the retina display endocrine features. Invest Ophthalmol Vis Sci 36: 1450–1458, 1995. [PubMed] [Google Scholar]
- 9.Burns RP, Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch's membrane. Trans Am Ophthalmol Soc 78: 206–225, 1980. [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt CR, Pumfery AM, Micales B, Bindley CD, Lyons GE, Sramek SJ, Wallow IH. Renin mRNA is synthesized locally in rat ocular tissues. Curr Eye Res 13: 755–763, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Jerdon JA, Glaser BM. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci 27: 1615–1621, 1986. [PubMed] [Google Scholar]
- 12.Castoldi G, Di Gioia CR, Pieruzzi F, d'Orlando C, Van de Greef WM, Busca G, Sperti G, Stella A. ANG II increases TIMP-1 expression in rat aortic smooth muscle cells in vivo. Am J Physiol Heart Circ Physiol 284: H635–H643, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Chen CP, Yang YC, Su TH, Chen CY, Aplin JD. Hypoxia and transforming growth factor-beta 1 act independently to increase extracellular matrix production by placental fibroblasts. J Clin Endocrinol Metab 90: 1083–1090, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Lee JS, Iglesias-de la Cruz MC, Wang A, Izquierdo-Lahuerta A, Gandhi NK, Danesh FR, Wolf G, Ziyandeh F. Angiotensin II stimulates alpha3(IV) collagen production in mouse podocytes via TGF-b and VEGF signaling: implications for diabetic glomerulopathy. Nephrol Dial Transplant 20: 1320–1328, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Cui XL, Ding Y, Alexander LD, Bao C, Al-Khalili OK, Simonson M, Eaton DC, Douglas JG. Oxidative signaling in renal epithelium: critical role of cytosolic phospholipase A2 and p38(SAPK). Free Radic Biol Med 41: 190–192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci 35: 1008–1018, 1994. [PubMed] [Google Scholar]
- 17.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The angiotensin II. Pharmacol Rev 52: 415–472, 2000. [PubMed] [Google Scholar]
- 18.De Jong PT Age-related macular degeneration. N Engl J Med 355: 1474–1485, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Deryugina EI, Bourdon MA, Reisfeld RA, Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res 58: 3743–3750, 1989. [PubMed] [Google Scholar]
- 20.Eagleton MJ, Ballard N, Lynch E, Srivastava SD, Upchurch GR Jr, Stanley JC. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during angiotensin II induced aneurysm formation. J Surg Res 135: 345–51, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 289: F1012–F1019, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari-Dileo G, Davis EB, Anderson DR. Angiotensin II binding receptors in retinal and optic nerve head blood vessels. An autoradiographic approach. Invest Ophthalmol Vis Sci 32: 21–26, 1991. [PubMed] [Google Scholar]
- 23.Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med 342: 483–492, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50: 213–218, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Green WR Histopathology of age-related macular degeneration. Mol Vis 5: 27, 1999. [PubMed] [Google Scholar]
- 26.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–345, 1985. [PubMed] [Google Scholar]
- 27.Han SY, Jee YH, Han KH, Kang YS, Kim HK, Han JY, Kim YS, Cha DR. An imbalance between MMP-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early nephropathy. Nephrol Dial Transplant 21: 2406–2416, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Hansen LH, Gromada J, Bouchelouche P, Whitmore T, Jetliner LJ, Kings Vogel W, Nishimura E. Glucagon-mediated Ca2+ signaling in BHK cells expressing cloned human glucagons receptors. Am J Physiol Cell Physiol 274: C1552–C1556, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Hayreh SS, Servais GE, Virdis PS. Macular lesions in malignant arterial hypertension. Ophthalmology 198: 230–246, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Helou CM, Marchetti J. Morphological heterogeneity of renal glomerular arterioles and distinct [Ca2+]i responses to ANG II. Am J Physiol Renal Physiol 273: F84–F96, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Barrantes S, Toth M, Bernardo MM, Yurkov M, Gervasi DC, Raz Y, Sang QA, Fridman R. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem 275: 12080–12089, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Hou M, Pantev E, Moller S, Erlinge D, Edvinsson L. Angiotensin II type 1 receptors stimulate protein synthesis in human cardiac fibroblasts via a Ca2+-sensitive PKC-dependent tyrosine kinase pathway. Acta Physiol Scand 168: 301–309, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Yu LF, Zhong J, Qizo MM, Jiang FX, Du F, Tian XL, Wu YL. Angiotensin II type 1 receptor expression in human gastric cancer and induces MMP2 and MMP9 expression in MKN-28 cells. Dig Dis Sci 53: 163–168, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol 118: 351–358, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am J Ophthalmol 101: 342–353, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Jacot TA, Striker GE, Stetler-Stevenson M, Striker LJ. Mesangial cells from transgenic mice with progressive glomerulosclerosis exhibit stable, phenotypic changes including undetectable MMP-9 and increased type IV collagen. Lab Invest 75: 791–799, 1996. [PubMed] [Google Scholar]
- 37.Jonas JB, Hayreh SS, Martus P. Influence of arterial hypertension and diet-induced atherosclerosis on macular drusen. Graefes Arch Clin Exp Ophthalmol 241: 125–134, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Kigasawa K, Ishikawa H, Obazawa H, Minamoto T, Nagai Y, Tanaka Y. Collagen production by cultured human retinal pigment epithelial cells. Tokai J Exp Clin Med 23: 147–151, 1998. [PubMed] [Google Scholar]
- 39.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy. Ophthalmology 110: 1273–1280, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol 137: 486–495, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Kohler KWST, Jurlikes B, Guenther E, Zrenner E. Angiotensin II in the rabbit retina. Vis Neurosci 14: 63–71, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Kugler A Matrix metalloproteinases and their inhibitors. Anticancer Res 19: 1589–1592, 1999. [PubMed] [Google Scholar]
- 43.Lee M, Bohm M, Paul M, Ganden D. Tissue renin-angiotensin systems: their role in the cardiovascular disease. Circulation 101: 1362–1365, 2000. [PubMed] [Google Scholar]
- 44.Lijnen P, Papparella I, Petrov V, Semplicini A, Fagard R. Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. J Hypertens 24: 757–766, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Marin-Castaño ME, Elliot SJ, Potier M, Karl M, Striker LJ, Striker GE, Csaky KG, Cousins SW. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci 44: 50–59, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Marin-Castaño ME, Csaky KG, Cousins SW. Nonlethal oxidant injury to human retinal pigment epithelium cells causes cell membrane blebbing but decreased MMP-2 activity. Invest Ophthalmol Vis Sci 46: 3331–3340, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Marin-Castaño ME, Striker GE, Alcazar O, Catanuto P, Espinosa-Heidmann DG, Cousins SW. Repetitive nonlethal oxidant injury to retinal pigment epithelium decreased extracellular matrix turnover in vitro and induced sub-RPE deposits in vivo. Invest Ophthalmol Vis Sci 47: 4098–4112, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Marmor MF Structure and function of the retinal pigment epithelium. Int Ophthalmol Clin 15: 115–130, 1975. [DOI] [PubMed] [Google Scholar]
- 49.McConnell V, Silvestri G. Age-related macular degeneration. Ulster Med J 74: 82–92, 2005. [PMC free article] [PubMed] [Google Scholar]
- 50.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Mukoyama M Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem 268: 24539–24542, 1993. [PubMed] [Google Scholar]
- 52.Murphy TJ, Wayne Alexander R, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351: 233–236, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Newsome DA, Pfeffer BA, Hewitt AT, Robey PG, Hassell JR. Detection of extracellular matrix molecules synthesized in vitro by monkey and human retinal pigment epithelium: influence of donor and multiple passages. Exp Eye Res 46: 305–321, 1988. [DOI] [PubMed] [Google Scholar]
- 54.Nowak JZ Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58: 353–363, 2006. [PubMed] [Google Scholar]
- 55.Obayashi K, Akamatsu H, Okano Y, Matsunaga K, Masaki H. Exogenous nitric oxide enhances the synthesis of type I collagen and heat shock protein 47 by normal human dermal fibroblasts. J Dermatol Sci 41: 121–126, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 82: 619–628, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, Striker LJ. Age-related changes in α1- and α2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol Renal Fluid Electrolyte Physiol 263: F951–F957, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Ortega M, Ruperez M, Esteban V, Egido J. Molecular mechanisms of angiotensin II-induced vascular injury. Curr Hypertens Rep 5: 73–79, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci 46: 3604–3615, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez-López E, Rodriguez-Vita J, Cartier C, Rupérez M, Esteban V, Carvajal G, Rodrígues-Díez R, Plaza JJ, Egido J, Ruiz-Ortega M. Inhibitory effect of interleukin-1β on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am J Physiol Renal Physiol 294: F149–F160, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci 48: 968–977, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol 163: 879–887, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato T, Niwa M, Himeno A, Tsutumi K, Amemiya T. Quantitative receptor autoradiographic analysis for angiotensin II receptors in bovine retinal microvessels: quantitation with radioluminography. Cell Mol Neurobiol 13: 233–245, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Suzuki F, Oike Y, Ishida S. Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci 47: 2686–2692, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Savaskan E, Löffler KU, Meie F, Müller-Spahn F, Flammer J, Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res 36: 312–320, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Seeger H, Lippert C, Wallwiener D, Mueck AO. Valsartan and candesartan can inhibit deteriorating effects of angiotensin II on coronary endothelial function. J Renin Angiotensin Aldosterone Syst 2: 141–143, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Senanayake P, Drazba J, Shadrach K, Milsted A, Rungger-Bandle E, Nishiyama K, Miura S, Kamik S, Sears JE, Hollyfield JG. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci 48: 3301–3311, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338, 1995. [DOI] [PubMed] [Google Scholar]
- 69.Taylor WR Hypertensive vascular disease and inflammation: mechanical and humoral mechanisms. Curr Hypertens Rep 1: 96–101, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Uchiyama-Tanaka Y, Matsubara H, Nozawa Y, Murasawa S, Mori Y, Kosaki A, Maruyama K, Masaki H, Shibasaki Y, Fujiyama S, Nose A, Iba O, Hasagawa T, Tateishi E, Higashiyama S, Iwasaka I. Angiotensin II signaling and HB-EGF shedding via metalloproteinase in glomerular mesangial cells. Kidney Int 60: 2153–2163, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Wagner J, Danser AHJ, Derkx FHM, de Jong TV, Paul M, Mullins JP, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol 80: 159–163, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D, Hirase T, Inoue T, Node K. Atorvastatin inhibits angiotensin II-induced T-type Ca2+ channel expression in endothelial cells. Biochem Biophys Res Commun 347: 394–400, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintu SG, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol 167: 1429–1442, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiss RH, Ramirez A. TGF-beta- and angiotensin-II-induced mesangial matrix protein secretion is mediated by protein kinase C. Nephrol Dial Transplant 13: 2804–2813, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Wheeler-Schilling TH, Kohler K, Sautter M, Guenther E. Angiotensin II receptor subtypes gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci 11: 3387–3394, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Wolf G Angiotensin II and tubular development. Nephrol Dial Transplant 17: 48–51, 2002. Review. [DOI] [PubMed] [Google Scholar]
- 77.Wolf G, Ziyadeh NF. Renal tubular hypertrophy induced by angiotensin II. Semin Nephrol 17: 448–454, 1997. [PubMed] [Google Scholar]
- 78.Young RW Pathophysiology of age-related macular degeneration. Surv Ophthalmol 31: 291–306, 1987. [DOI] [PubMed] [Google Scholar]
- 79.Zhu ZR, Goodnight R, Nishimura T, Sorgente N, Ogden TE, Ryan SJ. Experimental changes resembling the pathology of drusen in Bruch's membrane in the rabbit. Curr Eye Res 7: 581–592, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation: role in cell invasion across collagen barrier. J Biol Chem 276: 16248–16256, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]