Abstract
Partially inbred lines of laboratory opossums differ in plasma LDL cholesterol concentration and cholesterol absorption on a high cholesterol diet. The aim of the present studies was to determine whether ezetimibe inhibits cholesterol absorption and eliminates the differences in plasma cholesterol and hepatic cholesterol metabolism between high and low responders on a high cholesterol diet. Initially, we determined that the optimum dose of ezetimibe was 5 mg/kg/day, and treated six high and six low responding opossums with this dose (with equal numbers of controls) for 3 weeks while opossums consumed a high cholesterol and low fat (HCLF) diet. Plasma and LDL cholesterol concentrations decreased significantly (P<0.05) in treated but not in untreated high responding opossums. Plasma cholesterol concentrations of untreated low responders increased slightly (P<0.05) but not in treated low responders. Percent cholesterol absorption was significantly higher in untreated high responders than in other groups. Livers from high responders with or without treatment were significantly (P <0.01) heavier than livers from low responders with or without treatment. Hepatic cholesterol concentrations in untreated high responders were significantly (P<0.05) higher than in low responders with or without treatment (P<0.001). The gall bladder bile cholesterol concentrations in untreated high responders were significantly (P<0.05) lower than in other groups. A decrease in biliary cholesterol in low responders treated with ezetimibe was associated with a decrease in hepatic expression of ABCG5 and ABCG8. These studies suggest that ezetimibe decreases plasma cholesterol levels in high responders mainly by decreasing cholesterol absorption and increasing biliary cholesterol concentrations. Since ezetimibe’s target is NPC1L1 and NPC1L1 is expressed in the intestine of opossums, its effect on cholesterol absorption may be mediated by inhibiting NPC1L1 function in the intestine.
Keywords: Cholesterol, Cholesterol absorption, Dietary response, Hyperlipidemia, LDL
INTRODUCTION
Responsiveness of plasma lipoprotein cholesterol to dietary lipids varies greatly among animal species and among the individuals of any one species, including humans (1, 2). High responding individuals increase their plasma cholesterol levels to a considerable extent when challenged with a high cholesterol and high fat (HCHF) diet. However, low responding individuals of the same species maintain or only moderately increase their plasma cholesterol levels when challenged with the same diet. High and low responding individuals of the same species have been developed by selective breeding for use in determining the metabolic and molecular mechanisms responsible for differences in plasma cholesterol response to dietary lipids. Selective breeding for high and low response, coupled with inbreeding, has produced partially inbred strains of laboratory opossums (Monodelphis domestica) that show extreme variability in diet-induced hyperlipidemia (3). However, these strains of laboratory opossums have quite similar plasma and lipoprotein cholesterol levels on a basal diet that is lower in cholesterol and fat content (3). Our studies have shown that diet-induced hyperlipidemia in opossums is mainly due to dietary cholesterol (4). Genetic analyses have indicated that the regulation of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) cholesterol concentration in laboratory opossums in response to dietary challenge is largely determined by a single major gene (5). This single major gene is responsible for most (80%) of the variability in VLDL and LDL cholesterol on the HCHF challenge diet (5). Previously, we conducted studies to develop a better understanding of the metabolic and molecular mechanisms that are responsible for diet-induced hyperlipidemia in laboratory opossums. The results of those studies suggested that cholesterol absorption and hepatic acyl-coenzyme A:cholesterol acyltransferase (ACAT) play a major role in diet-induced hyperlipidemia in laboratory opossums (6). NPC1L1 protein plays an important role in cholesterol influx into enterocytes (7) and ezetimibe inhibits cholesterol absorption by inhibiting the activity of NPC1L1 (8). The present studies were conducted to determine whether opossums express NPC1L1 in their intestines and livers, and whether the ezetimibe would decrease cholesterol absorption, decrease plasma and VLDL+LDL cholesterol and normalize hepatic cholesterol metabolism in the opossum model. Since NPC1L1 is the target protein for ezetimibe, these studies may provide evidence for a regulatory role of NPC1L1 in diet-induced hyperlipidemia in laboratory opossums.
METHODS
Experimental animals
At the Southwest Foundation for Biomedical Research (SFBR), an inbreeding program has produced 18 partially inbred strains of laboratory opossums (Monodelphis domestica) with inbreeding coefficients of >0.7. Among these strains, three high responding and three low responding lines have been selectively bred for their LDL cholesterol response to the HCHF diet. Two of these strains have been designated ATHH (high responding) and ATHE (low responding). Most but not all ATHH opossums are high responders, and most but not all ATHE opossums are low responders. High and low responding individuals from these two stocks were used for these studies. The animals were maintained in polycarbonate rodent cages under laboratory conditions that have been standardized for this species (9).
The protocol of these experiments was approved by the Institutional Animal Care and Use Committee of the SFBR. The SFBR is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and is registered with the U.S. Department of Agriculture.
Experimental diets
For these experiments, the animals were fed a basal diet or a high cholesterol and low fat (HCLF) experimental diet ad libitum. However, prior to the experiments, the animals were challenged with the HCHF diet for 4 to 8 weeks to confirm each individual’s dietary response as high for ATHH opossums and low for ATHE opossums. The basal diet is a commercial pelleted fox food (Reproduction Diet, Nutritionally Complete Fox Food Pellets, Milk Specialties Co., New Holstein, WI). The composition of these diets has been described previously (4). The fat content of the basal diet was 10% of dry weight, and the cholesterol content was relatively low (0.16% by dry weight basis) (4). The fat content of the HCLF experimental diet was 10.8% of dry weight and the cholesterol content was 0.71% by dry weight basis. The fat content of the HCHF diet was 18.8% of dry weight and the cholesterol content was 0.71% by dry weight basis. The experimental diets were prepared from the commercial fox food by adding water, lard and crystalline cholesterol as described earlier (4). The pellets were stored at −20°C to prevent spoilage and oxidation.
Experimental design
Experiment 1: Dose response
To determine the optimal dose of ezetimibe, we selected 12 ATHH high responding and 12 ATHE low responding laboratory opossums (each weighing approximately 100 g). The animals were selected on the basis of response to the HCHF diet. The animals were then returned to the basal diet for 8 weeks so that their plasma cholesterol levels would return to baseline. The animals were divided into four groups of six: 1) high responding, treated with ezetimibe, 2) high responding, untreated controls, 3) low responding, treated, and 4) low responding, untreated controls. The animals were fed the HCLF diet for 6 weeks and at the end of a 6-week period the animals were bled to determine the plasma lipoprotein cholesterol levels. During this time six animals from high responding groups died and thus the experiment began with four animals in group 1, two animals in group 2, six animals in group 3, and six animals in group 4. At the start of ezetimibe treatment, two animals in group 1 and two animals in group 2 were high responders to HCLF diet and the rest of the animals were low responders to that diet. Opossums in the treatment group were treated with a weekly escalating (in half log increments) dose of ezetimibe starting at 0.625 mg/kg/day for a week (week 1). Other doses were 1.344 (week 2), 2.216 (week 3), 3.654 (week 4), 5.025 (week 5) and 10.000 (week 6 and 7) mg/kg/day. The last dose (10 mg/kg/day) was given for 2 weeks before blood was collected. The drug was mixed into the HCLF diet on the basis of food consumption. Each animal was given the food on the basis of its weight each week. At the end of each dose period, animals were weighed and bled to determine plasma lipoprotein cholesterol, and triglyceride levels.
Experiment 2: Cholesterol absorption
For cholesterol absorption measurements, 12 ATHH high responding and 12 ATHE low responding opossums to the HCHF diet were selected. The selected animals were then maintained on the basal diet for 8 weeks to bring their plasma cholesterol levels back to baseline levels. Animals were then started on the HCLF diet. After consumption of the HCLF diet for 4 weeks, plasma cholesterol and triglyceride concentrations were measured. Then, the animals were divided into four groups as defined in the previous paragraph. Groups 1 and 3 were treated with ezetimibe at a dose of 5 mg/kg body weight per day, and groups 2 and 4 were used as the untreated controls. In this experiment, we maintained the animals for 4 rather than 6 weeks on the HCLF diet before starting them on ezetimibe because of the high mortality rate in the high responding group in the initial experiment. The animals were treated for 3 weeks with ezetimibe, and plasma cholesterol and triglyceride concentrations were measured after the end of the second and third weeks. Intestinal cholesterol absorption was measured during the third week. After measurement of cholesterol absorption, the animals were necropsied, and livers were removed for measurement of gene expression and free and esterified cholesterol concentrations.
Blood and tissue collection
After an overnight fasting the animals were exsanguinated by cardiac puncture under isofluorine anesthesia (9). The blood was placed in tubes containing EDTA. The liver was removed, and bile was aspirated from the gall bladder by syringe and placed in a small vial. The liver was placed in a plastic bag and frozen immediately in liquid nitrogen. Samples were stored at −80°C.
Plasma and lipoprotein cholesterol analysis
Plasma was obtained by centrifugation and total plasma and HDL cholesterol were measured by enzymatic methods with the Ciba-Corning Express Plus Analyzer. VLDL and LDL were precipitated by the Lipid Research Clinics procedure (10) and HDL cholesterol was measured in the supernatant. The VLDL+LDL cholesterol concentration was calculated as the difference between the total plasma cholesterol and HDL cholesterol concentrations. When the cholesterol level in a sample exceeded the value of the highest calibrator, 358 mg/dl, the sample was diluted with saline to bring it within the range of the calibrators and analyzed again. For samples that were diluted for the total cholesterol assay, the same dilutions were used for the precipitation of VLDL+LDL. Fasting plasma triglycerides in laboratory opossums are low and do not change upon consuming the HCHF diet; thus triglycerides do not affect the results of cholesterol assay.
Cholesterol absorption measurements
Cholesterol absorption was measured by the fecal isotope ratio method described by Turley et al. (11) for hamsters and by us for opossums (6). Opossums were given 2μCi of [3H]-β-sitosterol (American Radiolabeled Chemicals, St. Louis, MO) and 1μCi of [14C]-cholesterol (Amersham Pharmacia, Piscataway, NJ) in corn oil (200 μl) intragastrically by syringe without anesthesia (11). Opossums were placed in individual cages for 4 days. They were allowed to have access to food and water ad libitum. Feces were collected daily for 4 days and pooled, and a small amount (2 g) was used to extract sterols as described by Turley et al. (10). The petroleum ether extracts were transferred into scintillation vials and evaporated to dryness under nitrogen and the radioactivity of both isotopes was counted in a liquid scintillation counter (model LS-7500, Beckman, Palo Alto, CA). To correct for color-related quenching of 14C and 3H counts in fecal samples, quench curves were run using increasing amounts of fecal extracts (nonradioactive fecal samples from opossums) with standardized 14C and 3H-labeled toluene (Packard Instruments, Downers Grove, IL). The percent cholesterol absorption was calculated as follows: (14C/3H [dose]–14C/3H [fecal sample])/(14C/3H [dose]) × 100 = percent cholesterol absorbed.
Measurement of hepatic cholesterol concentrations
Liver samples (200–500 mg) were homogenized and extracted with chloroform and methanol (2:1) by the method of Folch et al. (12). The chloroform extract was evaporated to dryness and dissolved in 200 μl isopropanol and total and free cholesterol concentrations were measured by an enzymatic method using a kit (Wako Pure Chemicals USA, Inc., Richmond, VA). The cholesterol concentrations were expressed as milligram per gram of liver tissue.
Measurements of cholesterol and total bile acids in gall bladder bile
Bile obtained from the gall bladder was diluted ten-fold with methanol. A small aliquot of the sample was used to measure cholesterol by the enzymatic method as described above for hepatic cholesterol. Total bile acids in bile samples were measured by the 3α-hydroxysteroid dehydrogenase method as described by Turley and Dietschy (13). Each assay contained 1.5 ml of Tris-HCl buffer (0.133 M Tris and 0.666 mM EDTA) pH 9.5 (Sigma Chemical Co., St Louis, MO), 1 ml of hydrazine hydrate (1 M) pH 9.5 (Sigma), 0.3 ml of NAD+ (7 mM) pH 7.0 (Sigma), 0.1 ml of methanolic extract of bile (1:10 dilution) and 0.1 ml of 3α-hydroxysteroid dehydrogenase (Worthington Biochemicals Corp., Freehold, NJ) containing 2 units of enzyme activity per ml in Tris-HCl buffer (0.03 M) containing 1 mM EDTA, pH 7.2. For each assay a reagent blank was prepared by adding 0.1 ml of methanol in place of a methanolic extract of bile. For each bile sample an appropriate blank was prepared by adding 0.1 ml of buffer in place of the enzyme as described by Turley and Dietschy (13). The reaction mixtures were incubated at 30° C for an hour. The standard curve was prepared by using an equal amount of sodium taurocholate and sodium taurochenodeoxyocholate. The concentration of total bile acids has been expressed as μmol/ml of bile.
Northern blot analysis
To measure levels of NPC1L1 mRNA in the intestine, we used tissues collected from a previous experiment. Total RNA was isolated from the small intestines of three high and three low responding animals fed the basal diet, and from three high and three low responding animals fed the HCLF diet for 4 weeks. The small intestine was divided into 12 segments of equal lengths, and total RNA was isolated from the sixth segment (jejunum) from the proximal end. Total RNA was isolated from frozen, pulverized tissues using TRI Reagent (Molecular Research Center, Cincinnati, OH), and quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
The NPC1L1 cDNA (Accession No.) was synthesized by reverse transcription-polymerase chain reaction (RT-PCR) using the forward (5′-cctctcacattcaaggatgg3′) and reverse (5′-ctgtccagggagagcaaa-3′) primers, and cloned into the pCR4-TOPO vector. The cDNA insert in the recombinant plasmid was amplified by PCR, and the PCR products were radiolabeled for use as the NPC1L1 probe. Northern blotting was performed as described previously (14).
Real-time RT-PCR
Levels of ABCG5, ABCG8, and NPC1L1 mRNA were measured by real-time PCR using SYBR Green chemistry on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Total RNA isolated using the TRI Reagent was treated with DNase from the TURBO DNA-free kit (Applied Biosystems) according to the instructions of the manufacturer. Single-stranded cDNA was synthesized from 1 μg of DNase-treated RNA in a 20 μL reaction using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). The reverse transcription reaction was diluted 30-fold (for hepatic genes), 300-fold (for intestinal NPC1L1), and 90,000-fold (for 18S rRNA), then 3 μL of the diluted reaction was added to a mixture containing gene-specific primers and the Fast SYBR Green Master Mix (Applied Biosystems) for PCR amplification according to the manufacturer’s instructions. GAPDH mRNA (hepatic genes) or 18S rRNA (intestinal NPC1L1) was used as an internal control. mRNA levels were determined by the standard curve method, and the results were expressed in arbitrary units. A standard curve was generated for each gene by serial dilutions (10-fold) of a reference sample. The reference sample was prepared by pooling cDNAs from high and low responders on the HCLF diet. After the PCR amplification step, a dissociation curve analysis was performed to ensure that only a single product was amplified.
The following oligonucleotides were used for real-time RT-PCR: (a) forward ABCG5 primer, 5′-cagcagcgtgttgtattgga-3′; reverse ABCG5 primer, 5′-agccgcgcacagcaatacc-3′; (b) forward ABCG8 primer, 5′-acttgaccgtctgggagactt-3′; reverse ABCG8 primer, 5′-acactccccgcaggtactc-3′; (c) forward NPC1L1 primer, 5′-gcttatgatggtgccgtgaa-3′; reverse NPC1L1 primer, 5′-ccgaaggtcagctgtgatgt-3′; (d) forward GAPDH primer, 5′-ggagaaagctgccaaatacg-3′; reverse GAPDH primer, 5′-gaagagtgggtgtcgctgtt-3′; (e) forward 18S rRNA primer, 5′-ccgtcgtagttccgaccata-3′; reverse 18S rRNA primer, 5′-aagtttcagctttgcaaccatact-3′. Primers for each gene (except 18S rRNA) were selected from sequences in different exons.
Data analysis
Values in figures and tables are expressed as mean±SEM. Values for groups were compared by ANOVA. If significant differences were found, pair-wise comparisons were done using Bonferroni’s test. The baseline values and the values obtained after the treatment for each group were compared using paired t-test. Associations among the variables were determined by using Pearson’s correlation. Significance was set at P<0.05.
RESULTS
Optimum dose of ezetimibe in high and low responding laboratory opossums
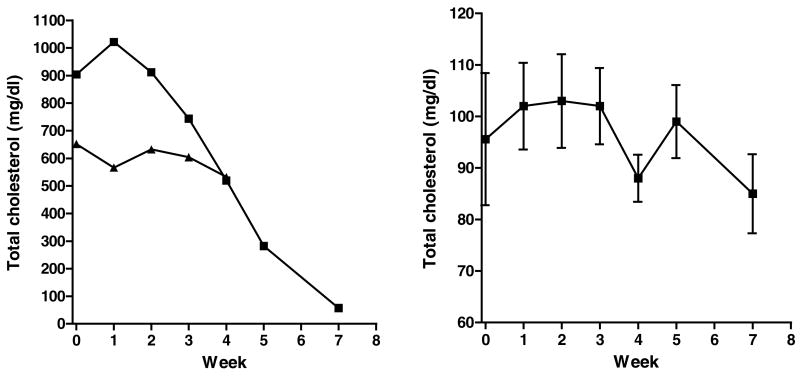
Figure 1 illustrates the effect of ezetimibe treatment on the total plasma cholesterol concentration of two high responding and eight low responding opossums. One of the high responders had a linear decrease in plasma cholesterol concentrations in response to ezetimibe treatment after the first week, from 1022 mg/dl at week 1 to 57 mg/dl after 7 weeks of treatment. The other high responder also had a decrease (from 652 mg/dl to 532 mg/dl) in response to ezetimibe treatment but this opossum died after 4 weeks of treatment. There was a trend of decrease in plasma cholesterol levels of low responding opossums treated with ezetimibe also, but these values were not significantly different from the baseline values (week 0) (P = 0.520).
Figure 1.
Response of total plasma cholesterol concentration to ezetimibe treatment of two high (individual values, left panel) and eight low (mean ± SEM, right panel) responding opossums on the HCLF diet (Experiment 1: Dose response). Before starting opossums on ezetimibe treatment, they consumed HCLF diet for 6 weeks. The dose of ezetimibe was escalated (in half log increments) weekly beginning at 0.625 mg/kg/day. Other doses for week 2, 3, 4, 5 and 6 were 1.344, 2.216, 3.654, 5.025 and 10.000 mg/kg/day, respectively.
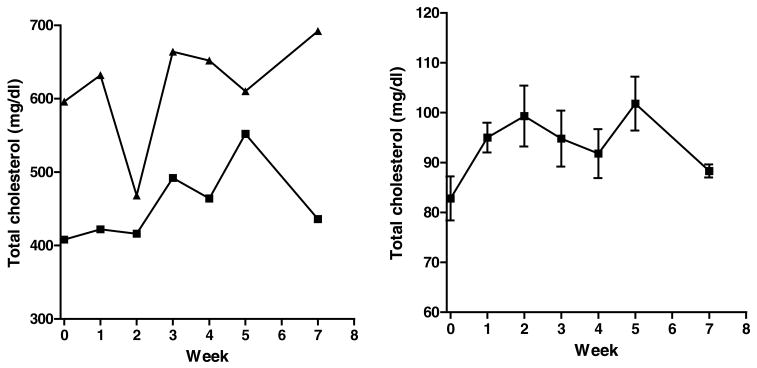
Total plasma cholesterol values for two high and six low responding untreated opossums are shown in Figure 2. The plasma cholesterol concentrations fluctuated considerably but did not decrease systematically from the baseline values during 7 weeks. Body weights of opossums treated with ezetimibe (body weights in grams: 92.0±5.0, 91.9±5.1, 91.4±4.8, 91.0±6.4, and 91.30±6.60 at week 0, 1, 3, 5, and 7, respectively, n=10) and untreated controls (96.8±8.6, 96.7±8.6, 94.6±8.8, 97.2±8.0, and 100.2±8.0 at week 0, 1, 3, 5, and 7, respectively, n=8) did not change with time on the HCLF diet. On the basis of these results, we decided to use dose of ezetimibe at 5 mg/kg/day for laboratory opossums and decided to start the treatment after 4 weeks of feeding the HCLF diet.
Figure 2.
Total plasma cholesterol concentration for untreated two high (individual values, left panel) and eight low (mean±SEM, right panel) responding opossums on the HCLF diet.
Plasma and lipoprotein cholesterol response to ezetimibe in high and low responding opossums
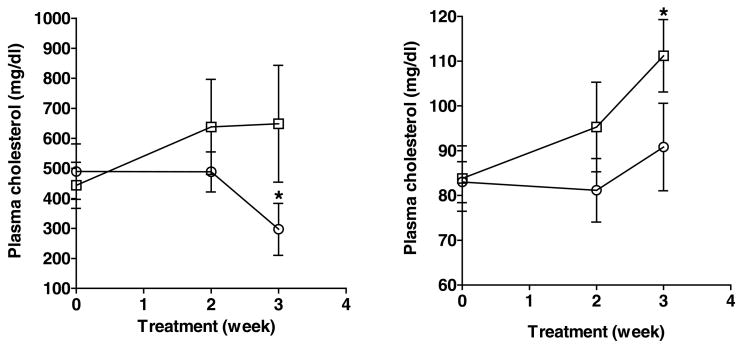
Table 1 presents plasma and lipoprotein cholesterol concentrations of high and low responding opossums assigned to the cholesterol absorption experiment after they had consumed the HCLF diet for 4 weeks. Plasma cholesterol and LDL cholesterol concentrations of low responding opossums differed significantly (P < 0.001) from those of high responding opossums. Ezetimibe treatment was started after 4 weeks of consuming the HCLF diet, at a dose of 5 mg/kg/day for 3 weeks; the animals continued to be fed the HCLF diet during these 3 weeks. Figure 3 illustrates plasma cholesterol values of these groups on the ezetimibe treatment. There was a significant (P = 0.004) decrease in plasma cholesterol concentrations of high responding opossums treated with ezetimibe for three weeks, whereas there was no significant (P > 0.05) change in plasma cholesterol concentrations of untreated high responding opossums. On the other hand, there was no significant (P > 0.05) change in plasma cholesterol concentrations of low responding opossums treated with ezetimibe, whereas there was a significant (P = 0.008) increase in plasma cholesterol concentrations of untreated low responding opossums. The change in plasma cholesterol concentrations was due to the change in plasma LDL cholesterol concentrations; there was no change in HDL cholesterol concentrations in these groups (HDL cholesterol: high responders treated with ezetimibe, 70.0±12.4 mg/dl at baseline and 60.0±6.8 mg/dl after three weeks of treatment; low responders treated with ezetimibe, 53.5±2.6 mg/dl at baseline and 60.3±5.1 mg/dl after three weeks of treatment; high responder controls, 59.5±6.1 mg/dl at baseline and 60.3±5.1 mg/dl after three weeks with no treatment; low responder controls, 59.8±5.8 mg/dl at baseline and 64.3±8.5 mg/dl after three weeks with no treatment).
Table 1.
Plasma lipoprotein cholesterol concentrations of low and high responding laboratory opossums selected for ezetimibe treatment after consuming the HCLF diet for 4 weeks (Experiment 2: Cholesterol absorption).
| Group assignment | Phenotype | Plasma cholesterol (mg/dl) | LDL cholesterol (mg/dl) | HDL cholesterol (mg/dl) |
|---|---|---|---|---|
| 1. ATHH treated | High responding | 489.20±92.33* | 419.20±100.88 | 70.00±12.35 |
| 2. ATHH untreated | High responding | 443.60±76.49 | 390.60±77.50 | 53.00±3.08 |
| 3. ATHE treated | Low responding | 83.00±4.58† | 29.50±2.38† | 53.50±2.63 |
| 4. ATHE untreated | Low responding | 83.83±3.73† | 28.33±5.77† | 59.5±6.12 |
Mean±SEM (n = 5 for high responding groups and n = 6 for low responding groups)
Values are significantly different from values of groups 1 and 2 (P<0.001)
Figure 3.
Effect of ezetimibe treatment (5 mg/kg/day) on plasma cholesterol concentrations of high (mean±SEM, n = 5, left panel) and low (n = 6, right panel) responding opossums (Experiment 2: Cholesterol absorption). There was a significant (P = 0.004) decrease in plasma cholesterol concentrations of high responding ATHH opossums treated with ezetimibe (O) (marked with an asterisk) but not in low responding ATHE opossums treated with ezetimibe (O). There was no change in plasma cholesterol concentrations of untreated high responding opossums (□) but there was a significant (P = 0.008) increase in plasma cholesterol concentrations of untreated low responding opossums (□) (marked with an asterisk).
Effect of ezetimibe treatment on cholesterol absorption and liver weights in high and low responding opossums
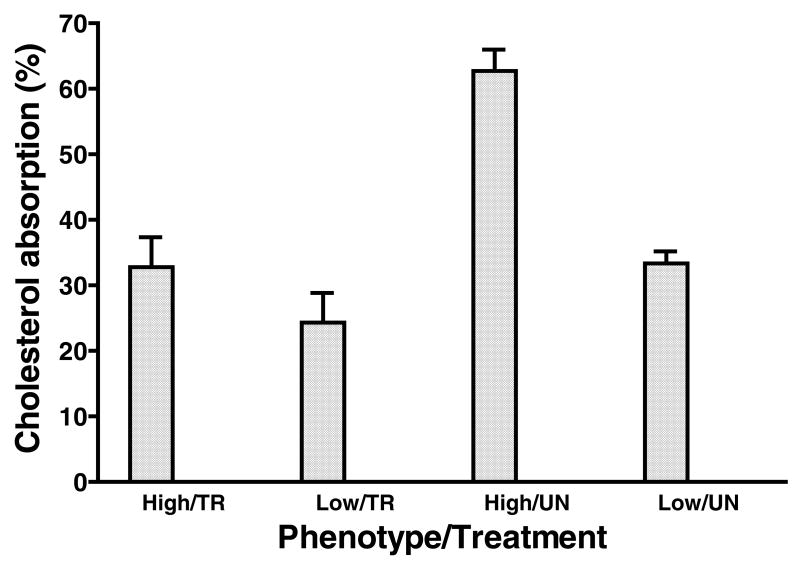
Figure 4 presents the cholesterol absorption data from high and low responding opossums on the HCLF diet in response to the ezetimibe treatment. Percent cholesterol absorption was highest in untreated high responding opossums (62.66±3.29) and was significantly higher than high responding opossums treated with ezetimibe (32.79±4.54, P < 0.001) and low responding opossums treated with (24.33±4.13, P < 0.001) or without (33.35±1.68) ezetimibe. However, there was no effect of ezetimibe on liver weights (Table 2). Livers from high responding opossums with (6.74±1.11 g) or without (6.78±0.99) treatment with ezetimibe, had significantly (P <0.01) higher weights than low responding opossums with (3.05±0.24) or without (2.97±0.25) treatment with ezetimibe.
Figure 4.
Percent cholesterol absorption (mean±SEM) in high responding ATHH and low responding ATHE opossums maintained on the HCLF diet and treated (TR) with ezetimibe (5 mg/kg/day) for 3 weeks or untreated (UN) controls (Experiment 2: Cholesterol absorption). N=5 for the high groups and n=6 for the low groups.
Table 2.
Liver weights and hepatic cholesterol concentrations in low and high responding laboratory opossums after treatment with or without ezetimibe while consuming the HCLF diet (Experiment 2: Cholesterol absorption).
| Group assignment | Phenotype | Liver weight (g) | Total cholesterol (mg/g liver tissue) | Free cholesterol (mg/g liver tissue) | Esterified cholesterol (mg/g liver tissue) |
|---|---|---|---|---|---|
| 1. ATTH treated | High responding | 6.74±1.11* | 7.52±2.219 | 2.12±0.929 | 5.40±1.611 |
| 2. ATHH untreated | High responding | 6.78±0.99 | 8.55±1.142 | 2.61±0.71 | 5.95±1.021 |
| 3. ATHE treated | Low responding | 3.05±0.24† | 3.15±0.183# | 0.78±0.059† | 2.35±0.198† |
| 4. ATHE untreated | Low responding | 2.97±0.25† | 3.71±0.253# | 0.68±0.253† | 3.03±0.312† |
Mean±SEM (n = 5 for high responding groups and n = 6 for low responding groups)
Value is significantly different from value of groups 1 and 2 (P<0.05)
Value is significantly different from value of groups 1 and 2 (P<0.05)
Effect of ezetimibe on liver cholesterol and gall bladder cholesterol and total bile acids in high and low responding opossums
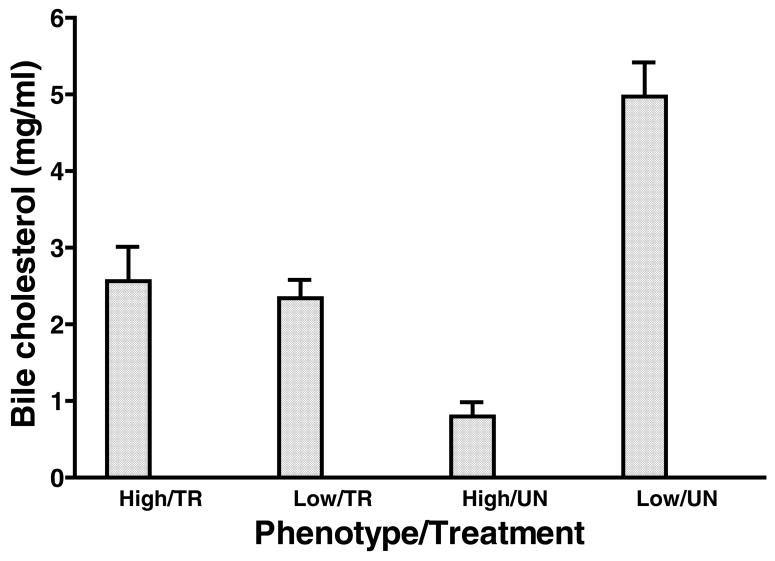
Table 2 presents hepatic cholesterol concentrations in high and low responding opossums with or without the treatment with ezetimibe. Hepatic total cholesterol concentrations in high responding opossums treated with ezetimibe were highly variable and were not different from other groups. However, hepatic total cholesterol concentrations were approximately three-fold higher in untreated high responding opossums than in low responding opossums with or without treatment with ezetimibe (P <0.001). Approximately 70–80% of the total hepatic cholesterol was present in esterified form in both high and low responding opossums and there was no effect of treatment with ezetimibe (Table 2). Figure 5 presents the gall bladder total cholesterol concentrations in high and low responding opossums with or without treatment with ezetimibe while consuming the high cholesterol diet. The gall bladder cholesterol concentration was lowest (0.798±0.185 mg/ml) in untreated high responding opossums and significantly different (P <0.03) from low (2.342±0.238 mg/ml) and high (2.564±0.450 mg/ml) responding opossums treated with ezetimibe. The gall bladder cholesterol concentration of untreated low responding opossums (4.973±0.447) was the highest and was significantly (P < 0.002) different from the other three groups. There was no difference in gall bladder cholesterol concentrations between high and low responding opossums treated with ezetimibe. There was also no difference in total bile acid concentration of high and low responding opossums treated with or without ezetimibe (bile acid concentration: high responders treated with ezetimibe, 8.58±1.87 μmol/ml; high responder controls, 9.31±3.00 μmol/ml; low responders treated with ezetimibe, 10.22±2.99 μmol/ml; low responder controls, 9.47±1.09 μmol/ml).
Figure 5.
Gall bladder bile cholesterol concentrations (mean±SEM) of high responding ATHH and low responding ATHE opossums maintained on the HCLF diet and treated with or without ezetimibe (5 mg/kg/day) for 3 weeks (Experiment 2: Cholesterol absorption). Gall bladder bile cholesterol concentrations in untreated low responding opossums were significantly (P <0.002) higher than those in the other groups. Gall bladder cholesterol concentrations in the untreated high responding group were significantly (P <0.001) lower than those in high and low responding opossums treated with ezetimibe. N=5 for the high groups and n=6 for the low groups.
Relationship between cholesterol absorption and liver and plasma variables
There was a significant association between cholesterol absorption and liver weight (r=0.468, P =0.028). There was also a significant association between cholesterol absorption and plasma (r=0.567, P =0.006) and VLDL+LDL (r=0.572, P =0.005) cholesterol concentrations at the time of cholesterol absorption measurement (7 weeks on the HCLF diet). However, there was no association between cholesterol absorption and HDL cholesterol concentrations.
Variables affecting liver metabolism
There was a strong and significant association between liver weight and hepatic total (r=0.758, P <0.001) esterified (r=0.620, P =0.002) and free (r=0.738, P <0.001) cholesterol concentrations. However, there was a significant and negative association between liver weight and gall bladder cholesterol concentration (r=−0.537, P =0.012). There was also a strong and negative association between gall bladder cholesterol and hepatic total (r=−0.604, P =0.004) esterified (r=−0.0547, P =0.010) and free (r=−0.498, P <0.022) cholesterol concentrations. There was a strong and significant correlation between liver weight and plasma (r=0.899, P <0.0001) and LDL (r=0.903, P <0.0001) cholesterol concentrations before the treatment was started with ezetimibe. After the treatment with ezetimibe, the association between liver weight and plasma and LDL cholesterol concentrations stayed significant, but was reduced as indicated by r values (from 0.899 to 0.484 for plasma and from 0.903 to 0.479 for LDL cholesterol concentrations).
Expression of NPC1L1 in intestine of opossums
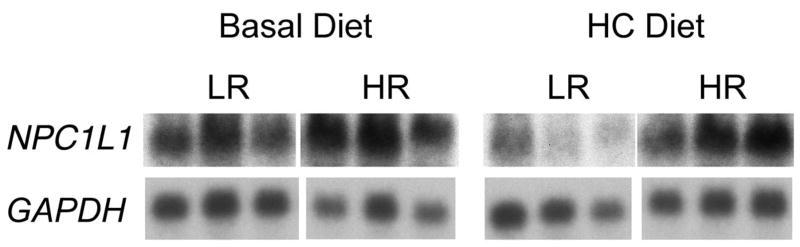
Figure 6 reflects NPC1L1 mRNA levels in the intestines (jejunum) of high and low responding opossums consuming the basal diet (left panel) or the HCLF diet (right panel). The levels in intestines did not differ between high and low responding opossums on the basal diet, but the levels in the intestines of two of the three low responders were lower than in the intestines of high responding opossums on the HCLF diet. These observations suggest that the expression of NPC1L1 in the intestine is variable. We quantified the levels of NPC1L1 mRNA in these high and low responding opossums on the HCLF diet by quantitative RT-PCR. The levels were normalized to 18S ribosomal RNA and expressed in arbitrary units. The values for high responders were higher (534±29, n=3) than low responders (430±66, n=3) but these were not significantly different (P =0.195).
Figure 6.
Northern blot of NPC1L1 in the intestines (jejunum) of three low responding (LR) and three high responding (HR) opossums on the basal diet (left panel) and three low responding (LR) and three high responding (HR) opossums on the high cholesterol (HC) diet (right panel). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Expression of ABCG5, ABCG8 and NPC1L1 in livers of opossums
Table 3 presents the values for mRNA levels of ABCG5, ABCG8 and NPC1L1 in livers of opossums treated with or without ezetimibe while they consumed the HCLF diet. Expression of ABCG8 in the livers of untreated low responding opossums was significantly higher than that in the livers of untreated high responding opossums. Ezetimibe treatment significantly decreased the expression of both ABCG5 and ABCG8 in the livers of low responding opossums but not in the livers of high responding opossums. On the other hand, ezetimibe treatment significantly increased the expression of NPC1L1 in the livers of high responding opossums but not in the livers of low responding opossums.
Table 3.
mRNA levels of ABCG5, ABCG8 and NPC1L1 in livers of low and high responding laboratory opossums after treatment with or without ezetimibe while consuming the HCLF diet (Experiment 2: Cholesterol absorption).
| Group assignment | Phenotype | ABCG5 | ABCG8 | NPC1L1 |
|---|---|---|---|---|
| 1. ATHH treated | High responding | 1.16±0.08* | 0.94±0.05 | 1.43±0.26 |
| 2. ATHH untreated | High responding | 1.22±0.18 | 0.80±0.15 | 0.74±0.12§ |
| 3. ATHE treated | Low responding | 1.10±0.06 | 1.13±0.06 | 1.86±0.26 |
| 4. ATHE untreated | Low responding | 1.61±0.19† | 1.73±0.19†,# | 1.85±0.15 |
Mean±SEM (normalized to GAPDH mRNA levels), n = 5 for high responding groups and n = 6 for low responding groups.
Value is significantly different from value of groups 3 (P <0.05).
Value is significantly different from values of groups 1, 2 and 3 (P <0.05).
Value is significantly different from values of groups 1, 3, and 4 (P <0.05).
DISCUSSION
The results demonstrate that ezetimibe treatment of high responding opossums rapidly decreases plasma and VLDL+LDL cholesterol. This reduction in plasma and VLDL+LDL cholesterol in high responding opossums is due in part to a 50% decrease in intestinal cholesterol absorption (Figure 4). Even though treated low responding opossums did not differ significantly from untreated controls in percent cholesterol absorption, they had the lowest level of cholesterol absorption and also the lowest plasma and VLDL+LDL cholesterol concentrations. Thus, a small decrease in percent cholesterol absorption due to ezetimibe treatment in low responding opossums may also cause a reduction in their plasma and VLDL+LDL cholesterol levels. Our observations also showed that high and low responding opossums express NPC1L1 in their intestines on the HCLF diet, but the expression is variable in low responding opossums (Figure 6). Since NPC1L1 protein is required for cholesterol absorption through the intestine and is the target of ezetimibe (7, 8), the decrease in percent cholesterol absorption in high responding opossums treated with ezetimibe may be due to the inhibition of NPC1L1 function in the intestine (15).
Low responding opossums also express NPC1L1 in their intestine however, ezetimibe does not have a significant effect on percent cholesterol absorption in low responding opossums. Our previous studies have shown that low responding opossums decrease their percent cholesterol absorption by 50% on the HCHF diet, whereas high responding opossums cannot down-regulate their percent cholesterol absorption on the same HCHF diet (6). It is therefore, likely that low responding opossums have another mechanism that down-regulates cholesterol absorption on a high cholesterol diet with or without high fat, which may involve other proteins such as cholesterol esterase (16), phospholipase A2 (17) acyl coenzyme A:cholesterol acyltransferases (18), 3-hydroxy-3-methylglutyral coenzyme A (19), scavenger receptor B1 and ABC transporters (ABCG5, ABCG8, ABCB4, and ABCB11) (20–22).
Another aim of our studies was to investigate whether the reduction in cholesterol absorption caused by the ezetimibe treatment would normalize plasma cholesterol concentrations and modulate hepatic cholesterol metabolism. The results demonstrate that treatment of high responding opossums with ezetimibe for 3 weeks decreased plasma cholesterol concentration and altered the hepatic cholesterol metabolism. The major change of hepatic cholesterol metabolism was in cholesterol concentration of gall bladder bile in high responding opossums treated with ezetimibe. High responding opossums treated with ezetimibe had higher cholesterol concentrations in their bile than untreated high responding opossums. Another change in hepatic cholesterol metabolism in high responding opossums treated with ezetimibe was observed in hepatic cholesterol concentration. Untreated high responding opossums had significantly higher hepatic cholesterol concentrations than low responding opossums with or without ezetimibe treatment (Table 2). However, high responding opossums treated with ezetimibe did not differ significantly in hepatic cholesterol concentrations from low responding opossums with or without the ezetimibe treatment. Gall bladder biliary cholesterol concentration was strongly and negatively associated with hepatic cholesterol concentration and liver weight. Increase in biliary cholesterol concentration after ezetimibe treatment in high responding opossums modulated hepatic cholesterol concentration. These studies therefore, demonstrate that the main defect of hepatic cholesterol metabolism in high responding opossums is an inability to increase the output of biliary cholesterol when fed the HCLF diet.
Since high responding opossums as compared to low responding opossums have higher intestinal cholesterol absorption and lower secretion of cholesterol in their bile, most of the dietary cholesterol has to be secreted in plasma lipoproteins or has to be stored in hepatocytes. On a high cholesterol diet, plasma cholesterol concentration increases rapidly, probably saturating the system. Consequently, a large amount of cholesterol has to be stored in the liver. Since a limited amount of cholesterol can be stored in hepatocytes, liver size (weight) of high responding opossums increases as excess dietary cholesterol accumulates there. Ezetimibe treatment decreases cholesterol absorption and increases secretion of cholesterol into bile and thus, it decreases cholesterol accumulation in the liver.
The effect of ezetimibe on biliary cholesterol concentration in low responding opossums was different from that in high responding opossums. The ezetimibe treatment of low responding opossums decreased biliary cholesterol concentration, whereas the ezetimibe treatment of high responding opossums increased biliary cholesterol concentration. ABCG5 and ABCG8 have been shown to play a major role in the secretion of cholesterol into bile (23, 24). To determine whether ezetimibe treatment affects the expression of ABCG5 and ABCG8 in the liver, we measured the expression of these genes in the livers of high and low responding opossums. These results clearly demonstrated that ezetimibe treatment of low responding opossums causes a decrease in ABCG5 and ABCG8 mRNA levels. Thus, the decrease in biliary cholesterol secretion in low responding opossums may be due to decreased expression of ABCG5 and ABCG8 in their livers. The increase in biliary cholesterol secretion in ezetimibe-treated high responding opossums may be due to increased excretion of cholesterol and bile acids from the body caused by a decrease in cholesterol absorption.
The human liver has been shown to express NPC1L1 in large amounts (5, 15, 25). A recent report has further shown that hepatic NPC1L1 regulates biliary cholesterol concentration and is a target of ezetimibe (26). We quantified NPC1L1 mRNA levels in the livers of high and low responding opossums treated with or without ezetimibe. The livers of low responding opossums with or without the treatment with ezetimibe had significantly higher NPC1L1 mRNA levels than the livers of untreated high responding opossums. The ezetimibe treatment significantly increased the NPC1L1 mRNA levels in the livers of high responding opossums but not in the livers of low responding opossums. The relevance of increased NPC1L1 mRNA levels in the secretion of biliary cholesterol in high responding opossums is not clear from these results. Further studies are needed to establish the role of NPC1L1 in the secretion of cholesterol in the bile of high and low responding opossums.
Untreated low responding opossums had a much higher concentration of cholesterol in their gall bladder bile than any other group. Our previous studies have shown that low responding opossums have higher hepatic activity of sterol 27-hydroxylase and higher concentration of 27-hydroxycholesterol in plasma and liver in response to the HCHF diet (27). Our previous studies have also shown that hepatic expression of sterol 27-hydroxylase, ABCG5 and ABCG8 in low responding opossums was higher than hepatic expression of these genes in high responding opossums fed the HCLF diet (14). Cholestenoic acid, a metabolite of 27-hydroxycholesterol, has been suggested to be the naturally occurring ligand for liver X receptor α, which has been shown to regulate the expression of ABCG5 and ABCG8 (28, 29). Treatment of mice expressing ABCG5 and ABCG8 with liver X receptor agonist produced a considerable increase in biliary cholesterol concentration and a decrease in cholesterol absorption but not in mice lacking ABCG5 and ABCG8 (30). Thus, the increase in hepatic sterol 27-hydroxylase may up-regulate the expression of ABCG5 and ABCG8 in low responding opossums as compared with high responding opossums and may increase hepatic biliary cholesterol secretion.
Acknowledgments
Supported by a grant from Merck and Schering Plough, NIH grant R01 DK65058, and a grant from the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation
We thank Scott Kelly, Susan Mahaney, and Cynthia Mermeia for their technical help.
Abbreviations used
- VLDL
very low density lipoprotein
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- HCHF
high cholesterol and high fat
- HCLF
high cholesterol and low fat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGill HC, Jr, Kushwaha RS. Individuality of lipemic responses to diet. Can J Cardiol. 1995;11(Suppl G):15G–27G. [PubMed] [Google Scholar]
- 2.Kushwaha RS, McGill HC., Jr Mechanisms controlling lipemic responses to dietary lipids. World Rev Nutr Diet. 1997;80:82–125. doi: 10.1159/000059583. [DOI] [PubMed] [Google Scholar]
- 3.Rainwater DL, VandeBerg JL. Dramatic differences in lipoprotein composition among gray short-tailed opossums (Monodelphis domestica) fed a high cholesterol/saturated fat diet. Biochim Biophys Acta. 1992;1126:159–166. doi: 10.1016/0005-2760(92)90286-5. [DOI] [PubMed] [Google Scholar]
- 4.Kushwaha RS, VandeBerg JF, VandeBerg JL. Effect of dietary cholesterol with or without saturated fat on plasma lipoprotein cholesterol levels in the laboratory opossum (Monodelphis domestica) model for the diet-induced hyperlipidemia. Br J Nutr. 2004;92:63–70. doi: 10.1079/BJN20041167. [DOI] [PubMed] [Google Scholar]
- 5.Rainwater DL, Kammerer CM, Singh ATK, et al. Genetic control of lipoprotein phenotypes in the laboratory opossums, Monodelphis domestica. GeneScreen. 2001;1:117–124. [Google Scholar]
- 6.Kushwaha RS, VandeBerg JF, Rodriguez R, VandeBerg JL. Cholesterol absorption and hepatic cholesterol acyl-coenzyme A:cholesterol acyltransferase activity play major roles in lipemic response to dietary cholesterol and fat in laboratory opossums. Metabolism. 2004;53:817–822. doi: 10.1016/j.metabol.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1-like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O’neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci U S A. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VandeBerg JL. The laboratory opossum, Monodelphis domestica. UFAW Handbook on the Care and Management of Laboratory Animals. In: Poole T, English P, editors. Terrestrial Vertebrates. Vol. 7. Oxford, UK: Blackwell Science Ltd.; 1999. pp. 193–209. [Google Scholar]
- 10.Lipid Research Clinics Program: The Lipid Research Clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 11.Turley SD, Daggy BP, Dietschy JM. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology. 1994;107:444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloan-Stanley G. Simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;256:497–509. [PubMed] [Google Scholar]
- 13.Turley SD, Dietschy JM. Re-evaluation of the 3α-hydroxysteroid dehydrogenase assay for total bile acids in the bile. J Lipid Res. 1978;19:924–928. [PubMed] [Google Scholar]
- 14.Chan J, Donalson LM, Kushwaha RS, Ferdinandusse S, VandeBerg JF, VandeBerg JL. Differential expression of hepatic genes involved in cholesterol homeostasis in high- and low-responding strains of laboratory opossums. Metabolism. 2008;57:718–724. doi: 10.1016/j.metabol.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies JP, Scott C, Oishi K, Liapis A, Loannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda I, Matsuoka R, Hamada T, Mitsui K, Imabayashi S, Uchino A, Sato M, Kuwano E, Itamura T, Yamada K, Tanaka T, Imaizumi K. Cholesterol esterase accelerates intestinal cholesterol absorption. Biochim Biophys Acta. 2002;1571:34–44. doi: 10.1016/s0304-4165(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 17.Mackay K, Starr JR, Lawn RM, Ellsworth JL. Phosphatidylcholine hydrolysis is required for pancreatic cholesterol esterase and phospholipase A2-facilitated cholesterol uptake into intestinal Caco-2 cells. J Biol Chem. 1997;272:13380–13389. doi: 10.1074/jbc.272.20.13380. [DOI] [PubMed] [Google Scholar]
- 18.Alegret M, Llaverias G, Silvestre JS. Acyl coenzyme A:cholesterol acyltransferase as hypolipidemic and antiatherosclerotic drugs. Methods Find Exp Clin Pharmacol. 2004;26:563–568. doi: 10.1358/mf.2004.26.7.863738. [DOI] [PubMed] [Google Scholar]
- 19.Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control of streptozotosin diabetic rats. Metabolism. 2007;56:430–438. doi: 10.1016/j.metabol.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Duan LP, Wang HH, Wang DQ. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J Lipid Res. 2004;45:1312–1323. doi: 10.1194/jlr.M400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.vanBerg-Henegouwen GP, Venneman NG, Portincasa P, Kosters A, van Erpecum KJ, Groen AK. Relevance of hereditary defects in lipid transport proteins for the pathogenesis of cholesterol gallstone disease. Sacnd J Gastroenterol Suppl. 2004;241:60–69. doi: 10.1080/00855920410011022. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Li-Hawkins J, Hammer RE, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittenburg H, Carey MC. Biliary cholesterol secretion by the twinned sterol half-transporters ABCG5 and ABCG8. J Clin Invest. 2002;110:605–609. doi: 10.1172/JCI16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies JP, Levy B, Loannou YA. Evidence for a Niemann-pick C (NPC) gene family identification and characterization of NPC1L1. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 26.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Loannou YA, Davies JP, Nilsson L-M, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushwaha RS, VandeBerg JF, Jackson EM, VandeBerg JL. High and low responding strains of laboratory opossums differ in sterol 27-hydroxylase and acyl-coenzyme A:cholesterol acyltransferase activities on a high cholesterol diet. J Nutr Biochem. 2001;12:664–673. doi: 10.1016/s0955-2863(01)00183-8. [DOI] [PubMed] [Google Scholar]
- 28.Fu X, Menke JG, Chen Y, Zhou Z, MacNaul Kl, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 29.Repa JJ, Berge KE, Pomajzl C, et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, York J, Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by LXR agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]