Abstract
Vaccination therapy of AD animal models and patients strongly suggests an active role of brain mononuclear phagocytes in immune-mediated clearance of amyloid-β peptides (Aβ) in brain. Although Aβ uptake by macrophages can be regulated by pro- and anti-inflammatory cytokines, their effects on macrophage-mediated Aβ degradation are poorly understood. To better understand this mechanism of degradation, we examined whether pro- and anti-inflammatory cytokines affect the degradation of Aβ using primary cultured human monocyte-derived macrophages (MDM) and microglia using pulse-chase analysis of fibrillar and oligomer 125I-Aβ40 and Aβ42. Initial uptake of fibrillar Aβ40 and Aβ42 was 40% and its degradation was saturated by 120 hrs in both MDM and microglia, compared to an initial uptake of oligomeric Aβ less than 0.5% and saturation of degradation within 24 hrs. Interferon-γ (IFN-γ) increased the intracellular retention of fibrillar Aβ40 and Aβ42 by inhibiting degradation, whereas interleukin-4 (IL-4), IL-10, and transforming growth factor-β1 (TGF-β1), but not IL-13 and IL-27, enhanced degradation. Fibrillar Aβ degradation in MDM is sensitive to lysosomal and insulin degrading enzyme (IDE) inhibitors but insensitive to proteasomal and neprilysin inhibitors. IFN-γ and TNF-α directly reduced the expression of IDE and chaperone molecules (Hsp70 and Hsc70), which are involved in refolding of aggregated proteins. Co-culture of MDM with activated, but not naïve T cells, suppressed Aβ degradation in MDM, which was partially blocked by a combination of neutralizing antibodies against pro-inflammatory cytokines. These data suggest that pro-inflammatory cytokines suppress Aβ degradation in MDM, whereas select anti-inflammatory and regulatory cytokines antagonize these effects.
Introduction
Immunotherapy against β-amyloid peptide (Aβ) deposition has been an emerging therapeutic approach to combat Alzheimer’s disease (AD). Immunization of transgenic mice expressing platelet derived growth factor B-chain promoter-driven familial AD β-amyloid precursor protein (APP) mutant with aggregated Aβ resulted in significant clearance of Aβ deposition at both pre and post-symptomatic stages (1), and restored cognitive function (2). Both active and passive Aβ immunotherapies have led to efficient clearance of Aβ deposition in APP mouse brain (3). Although clinical trials of Aβ vaccination therapy (AN1792) have been halted due to meningoencephalitis observed in 18 of 298 enrolled patients (4), antibody development significantly reduced cognitive decline in AD patients (5), demonstrating its potential for treatment of the disease.
In addition, non-Aβ vaccination, such as using myelin oligodendrocyte glycoprotein (MOG), proteolipid protein (PLP), or glatiramer acetate (GA) with specific adjuvants, induces Th1-cell response, microglial activation, and clearance of Aβ deposition in APP mouse brain (6, 7). These studies suggest that anti-Aβ specific antibodies as well as Th1/2-cell mediated activation of innate immunity play important roles in Aβ clearance in brain, although the exact mechanisms are not completely understood. One proposed mechanism of Aβ clearance through immune activation is through secretion of pro-inflammatory cytokines from Th1 cells, such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and CD40 ligand (CD40L). Indeed, a number of inflammatory molecules, such as IFN-γ, interleukin (IL)-1β, transforming growth factor (TGF)-1β, and TNF-α are upregulated in APP mice (Tg2576) (8–10). However, APP mice lacking either CD40L or IFN-γ receptor type I showed reduced Aβ depostion, microgliosis, and β-processing of APP (11, 12). Thus, the effect of pro-inflammatory cytokines in CNS is not consistent with the result of vaccination studies on APP mice. While the role of cytokines on macrophage phagocytosis (Aβ or beads uptake) has been studied (13, 14), the role of T-cells on Aβ degradation in macrophages is poorly characterized. Here we demonstrate the effect of T cells and their related cytokines on Aβ degradation in human primary cultured macrophages and microglia. Pro-inflammatory cytokines inhibited Aβ degradation in MDM, whereas select anti-inflammatory and regulatory cytokines (IL-4, IL-10, and TGF-β1), enhanced Aβ degradation. IFN-γ and TNF-α directly suppressed Aβ degradation enzyme expression.
Material and Methods
Isolation of human monocyte-derived macrophages (MDM)
Human monocytes were recovered from peripheral blood mononuclear cells of donors after leukopheresis and purified by counter-current centrifugal elutriation (15). Monocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat-inactivated human serum, 2 mM L-glutamine, gentamicin (50 μg/ml), ciprofloxacin (10 μg/ml), and macrophage colony-stimulating factor (MCSF, 1000 U/ml, Wyeth Pharmaceutical, Cambridge, MA). Monocytes were cultivated for 7 days and then referred to as MDM as described (16, 17).
Purification of T-cells and trasnswell co-culture system
Peripheral blood leukocytes (PBL) were obtained from leukopheresis of donors and purified by countercurrent centrifugal elutriation. T cells were isolated from PBL by negative selection using a magnetic based Pan T cell isolation kit (Miltenyi Biotec). The cells were then stimulated with anti-CD3 and anti-CD28 for 24 hr, and subjected to co-culture with MDM (500,000 cells/well of 24-well plate, Fisher Scientific) after pulse-labeling of MDM with fibrillar 125I-Aβ and removal of unbound Aβ fraction using a Transwell insert (Fisher), where T cells were plated (5 × 105 cells/well) in MDM tissue culture media, for the pulse-chase study.
Isolation of human microglia
Human microglia were isolated as described (18, 19). Fetal brain tissue (gestational age, 14 to 16 weeks) was obtained from the Birth Defects Laboratory, University of Washington, Seattle, in full compliance with the ethical guidelines of the NIH and the Universities of Washington and Nebraska Medical Center. The tissue was washed with cold Hanks balanced salt solution (Invitrogen, Carlsbad, CA) supplemented with Ca2+ and Mg2+ and then digested with 0.25% trypsin (Sigma, St. Louis, Mo.) for 30 min at 37°C. Trypsin was neutralized with fetal bovine serum (FBS), and the tissue was further dissociated to obtain single-cell suspensions. The cells were resuspended in DMEM supplemented with a mixture containing 10% heat-inactivated FBS, 1,000 U of purified recombinant human macrophage colony stimulating factor (MCSF) per ml, penicillin and streptomycin (50 μg/ml), and 100 μg of neomycin per ml. The mixed culture was maintained under 5% CO2 for 7 days, and the medium was fully replaced to remove any cell debris. The microglia cells released with further incubation were collected and purified by preferential adhesion. Microglia were cultured as adherent monolayers at a density of 100,000 cells/well in 48-well plates, and floating cells were removed after 4 hs. The purity of microglia was confirmed by immunocytochemistry using anti-CD68 (>98%) and anti-glial fibrillar acidic protein (astrocyte marker, <2%). Replicate cultures were used for the pulse-chase study.
Prepareation of fibrillar and oligomeric Aβ
Iodinated Aβ40 and Aβ42 were prepared using IODO-beads (Pierce, Rockford, IL), synthetic Aβ40 and Aβ42 peptide (Invitrogen, Carlsbad, CA), and iodine-125 (125I)(Amersham Bioscience, Piscataway, NJ) according to kit instructions. 125I-Aβs were aggregated with stirring at 37°C for 3 days, followed by centrifugation at 40,000 × g for 20 min at 4°C. The precipitated fraction was used as fibrillar Aβ, after the confirmation of its structure by atomic force microscopy (AFM), for pulse chase study. Oligomeric Aβ40 and Aβ42 were prepared as described with minor modification (20). Briefly, 125I-Aβ40 or Aβ42 were incubated in PBS at 4°C for 24hs, followed by centrifugation at 14,000 × g for 10 min at 4°C. The supernatant fraction was used as oligomeric Aβ40 or Aβ42, after confirmation of the strucure by AFM, for the pulse-chase study.
Atomic Force Microscopy (AFM)
For visualization of fibrillar or oligomeric Aβ by AFM, aggregated Aβ40 or Aβ42 was deposited on a freshly split mica film, glued to a glass slide and dried under an argon gas flow. Images were taken in air, height, amplitude and phase modes using a Molecular Force Probe 3D controller (Asylum Research Inc., Santa Barbara, CA) as described (21, 22).
Pulse-chase analysis
The kinetics of Aβ degradation were investigated by pulse-chase analysis using fibrillar or oligomeric 125I-Aβs as described (23, 24). Briefly, human MDM (500,000 cells/well) or microglia (100,000 cells/well) were pulse-labeled with fibrillar or oligomeric 125I-Aβ (200,000 cpm/well) at the final concentration of 1 μM in tissue culture media for 1 hr at 37°C, washed three times with PBS, and chased with fresh tissue culture media up to 120 hr in the presence or absence of T cells in the Transwell insert, with recombinant cytokines (IFN-γ, TNF-α, IL-13, IL-27; eBioscience, San Diego, CA, IL-4, IL10; R&D Systems, Minneapolis, MN, TGF-β1; PeproTech, Rocky Hill, NJ), anti-cytokine neutralizing antibodies, or inhibitors. At each time point, media were collected and cells lysed in lysis buffer (1M NaOH) for counting intracellular Aβ. 99% of this fraction was undigested Aβ as determined by trichloroacetic acid (TCA) precipitation. The collected media were subsequently mixed with TCA, (final concentration of 10%) for polypeptide precipitation, and centrifuged at 3,000 × g for 15 min at 4°C. The radioactivity level of both the TCA-soluble (degraded Aβ) and the precipitated (non-degraded Aβ) fractions were determined for calculating the fractions of intracellular Aβ, extracellular intact Aβ, and extracellular degraded Aβ
Laser-scanning Confocal Microscopy
MDM were plated onto poly-D-lysine-coated round coverslips (BD Biosciences, San Jose, CA) at a density of (50,000 cells/slip) and were placed on 24-well tissue culture plates (Fisher Scientific) 24 hrs prior to the study. The cells were incubated with aggregated Aβ42 (1 μM) for 3 days at 37°C, and fixed with acetone/methanol (1:1 v/v) for 20 min. at −20°C. The cells were permeabilized with 0.5% Triton-X 100 and blocked with 3% normal goat serum and 0.2% BSA/0.05% Tween-20 in PBS, and double stained with 6E10 anti-Aβ monoclonal (1: 250 dilution, mouse monoclonal, Signet, Dedham, MA) or anti-Aβ polyclonal (1:500 dilution, Zymed/Invitrogen, Carlsbad, CA), and a panel of markers: anti-lysosomal membrane glycoprotein 1 (LAMP1, lysosomal marker, rabbit polyclonal, 1:100 dilution, Affinity Bioreagents, Golden, CO); anti-insulin degrading enzyme (IDE, rabbit polyclonal, 1:200 dilution, Oncogene Science, San Diego, CA); anti-20S α4 subunit (proteasome marker, mouse monoclonal clone HC6, 1:100 dilution, Biomol International, Plymouth Meeting, PA); anti-heat shock protein 70 (HSP70, aggresome marker, mouse monoclonal clone 2A4, 1:100 dilution, Abcam, Cambridge, MA); anti-mannose-6-phosphate receptor (M6P-R, late-endosome marker, mouse monoclonal clone 2G11, 2μg/ml dilution, Abcam); anti-vimentin (interfilament marker, mouse monoclonal clone Vim 3B4, 1:200 dilution, DakoCytomation, Glostrup, Denmark); anti-β–coat protein (β-COP, cis-Golgi marker, rabbit polyclonal, 1:2000 dilution, Abcam), and anti-Hsc70 (5 μg/ml, rat monoclonal, Stressgen, Victoria, BC), followed by washing with PBS/Tween-20 and labeling with corresponding Alexa 488 or 568-conjugated secondary antibody for the detection of primary antibodies. The coverslips were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA) and subjected to confocal imaging using a Nikon TE 2000U Sweptfield confocal system (Nikon Instruments, New York, NY).
Immunoblots
MDM lysates (2,000,000/well) were subjected to standard immunoblotting for anti-IDE (1: 6,000; rabbit polyclonal antibody, Oncogene Science, San Diego, CA), anti-HSC70 (1: 3,000; rat monoclonal, Stressgen, Victoria, BC), anti-HSP70 (1: 100,000; mouse monoclonal clone 2A4, Abcam, Cambridge, MA), or anti-β-actin (mouse monoclonal clone AC-15, 1: 5,000,000; Sigma, St. Louis, MO) antibodies. Horseradish peroxidase-conjugated secondary antibodies were employed against mouse and rabbit IgG (1:2000 dilution, Vector Laboratories), and developed using chemiluminescence. The images were digitally captured and the band intensities were quantified by Typhoon imaging system (Amersham Pharmacia Biotech). Data were presented as a ratio of target/β-actin band intensity.
Statistics
All experiments were repeated at least three times with different donors, and all data were normally distributed. In the case of multiple mean comparisons, the data were analyzed by analysis of variance, followed by Newman-Keuls multiple comparison tests using statistics software (Prism 4.0, Graphpad Software, San Diego, CA). In the case of single mean comparison, data were analyzed by Student’s t-test. p values less than 0.05, 0.01, or 0.001 were regarded as significant difference.
Results
Pulse-chase study of 125I-Aβ and 125I-AcLDL
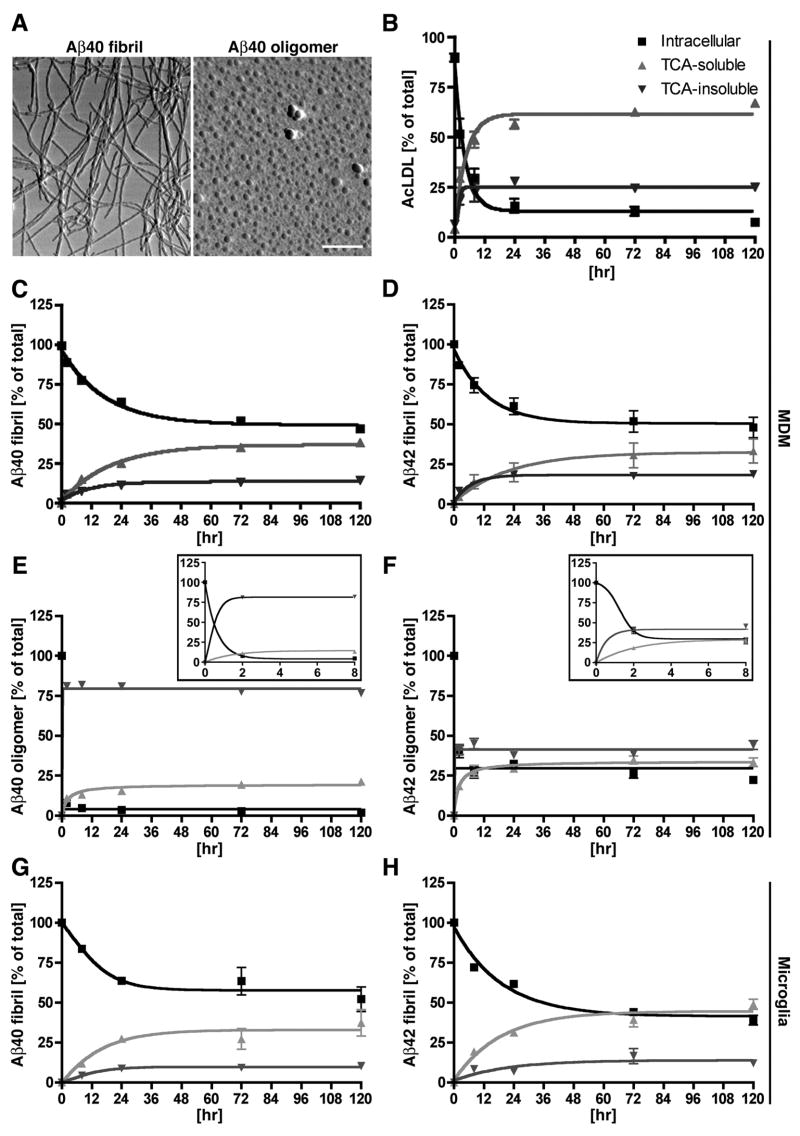
Human MDM were incubated with fibrillar 125I-Aβ40 or 125I-Aβ42, oligomeric 125I-Aβ40 or 125I-Aβ42, or soluble 125I-AcLDL for 1 hr at 37°C, followed by washing and incubation with tissue culture media for 0 to 120 hrs. The aggregated Aβ was specifically prepared as either fibril or oligomer form in this preparation as confirmed by atomic force microscopy (Fig. 1A). At each time point, both media and cells were collected and subjected to TCA protein precipitation as described in Material and Methods section. The TCA soluble fraction of cell lysates did not contain a detectable amount of intact Aβ, as determined by Aβ ELISA or immunoblotting (data not shown). We also confirmed that a negligible amount of degraded 125I-labeled protein exists in the cells as examined using TCA-soluble fraction of the cell lysate. Thus, we waived the TCA precipitation step for the analysis of intracellular retention of 125I-Aβ and 125I-AcLDL in this study. At each time point, the percentages of intracellular and extracellular TCA-soluble/insoluble fractions were calculated. AcLDL is a positive control for scavenger receptor-mediated endocytosis, and has been used previously in comparison with Aβ (23). The degradation of AcLDL was saturated within 24 hrs with approximately 10% intracellular retention, 65% extracellular TCA-soluble fraction, and 25% extracellular TCA-insoluble fraction (Fig. 1 B). The degradation of fibrillar Aβ40 and Aβ42 was similarly saturated around 120 hrs with approximately 50% intracellular retention, 40% extracellular TCA-soluble fraction, and 10% extracellular TCA-insoluble fraction (Fig. 1 C–D). On the other hand, oligomeric Aβ40 and Aβ42 were distinctly different from fibrillar Aβ40 and Aβ42 both in initial binding and clearance. While fibrillar Aβ40 and Aβ42 show similar initial uptake (40–41% of total input), oligomeric Aβ40 and Aβ42 uptake was almost negligible (0.22 and 0.58%, respectively) even as compared to monomeric Aβ40 and Aβ42 (Table I). Degradation of oligomeric Aβ40 and Aβ42 was quickly saturated within 24 hrs (Fig. 1 E–F insets) with approximately 5 or 30% intracellular retention, 75 or 40% extracellular TCA-soluble fraction, and 20 or 30% extracellular TCA-insoluble fraction, respectively (Fig. 1 E–F). These data suggest that phagocytized oligomeric Aβ40 degradation is very similar to that of AcLDL, while oligomeric Aβ42 shows enhanced intracellular retention compared to oligomeric Aβ40, indicative of rapid aggregation of oligomeric Aβ42 after its phagocytosis. Since the uptake efficiency of oligomeric Aβ40 or Aβ42 is negligible as compared to those of fibrillar Aβ40 or 42, we studied the degradation of fibrillar Aβ for the rest of the study. We also tested Aβ degradation in primary cultured human microglia (Fig. 1G–H). Fibrillar Aβ40 or Aβ42 degradation was saturated in 120 hrs with approximately 60 or 42% intracellular retention, 10% or 15% extracellular TCA-soluble fraction, and 30% or 43% extracellular TCA-insoluble fraction, respectively (Fig. 1E–F). These data suggest that the fibrillar Aβ degradation is similar between Aβ40 and Aβ42, and between human MDM and microglia in this system.
FIGURE 1. AcLDL and Aβ phagocytosis in BM-derived macrophages.
A, AFM images of Aβ40 fibrils (left) and oligomers (right). Scale bar, 500 nm. B–F, MDM (500,000 cells/well of 96-well plate) were incubated with 125I-AcLDL (B), 125I-Aβ40 fibril (C), 125I-Aβ42 fibril (D), 125I-Aβ40 oligomer (E) or 125I-Aβ42 oligomer (F) for pulse labeling. After 1 hr incubation, cells were chased with fresh media at 37°C for 0–120 hrs. At each time point, the intracellular (black square), TCA-soluble (light gray triangle; degraded form), and TCA-precipitated (dark gray inverted triangle; intact form) fraction of 125I-AcLDL, 125I-Aβ40 or 125I-Aβ42 in the media was counted and presented as % total count of all fractions at each time point. Insets in E and F are high-magnified areas between 0 to 8 hrs. GH, human microglia (100,000 cells/well of 48-well plate) were incubated with 125I-Aβ40 fibril (G) or 125I-Aβ42 fibril (H), followed by the same procedure as MDM.
TABLE 1.
Labeling efficiency of Aβ in human MDM
| Sample | Efficiency (%) |
|---|---|
| Fibrillar Aβ40 | 41.20 +/− 1.00 |
| Fibrillar Aβ42 | 40.32 +/− 1.64 |
| Oligomeric Aβ40 | 0.22 +/− 0.02 |
| Oligomeric Aβ42 | 0.58 +/− 0.12 |
| Monomeric Aβ40 | 1.38 +/− 0.13 |
| Monomeric Aβ42 | 10.60 +/− 0.50 |
Differential role of pro-inflammatory cytokines on Aβ degradation
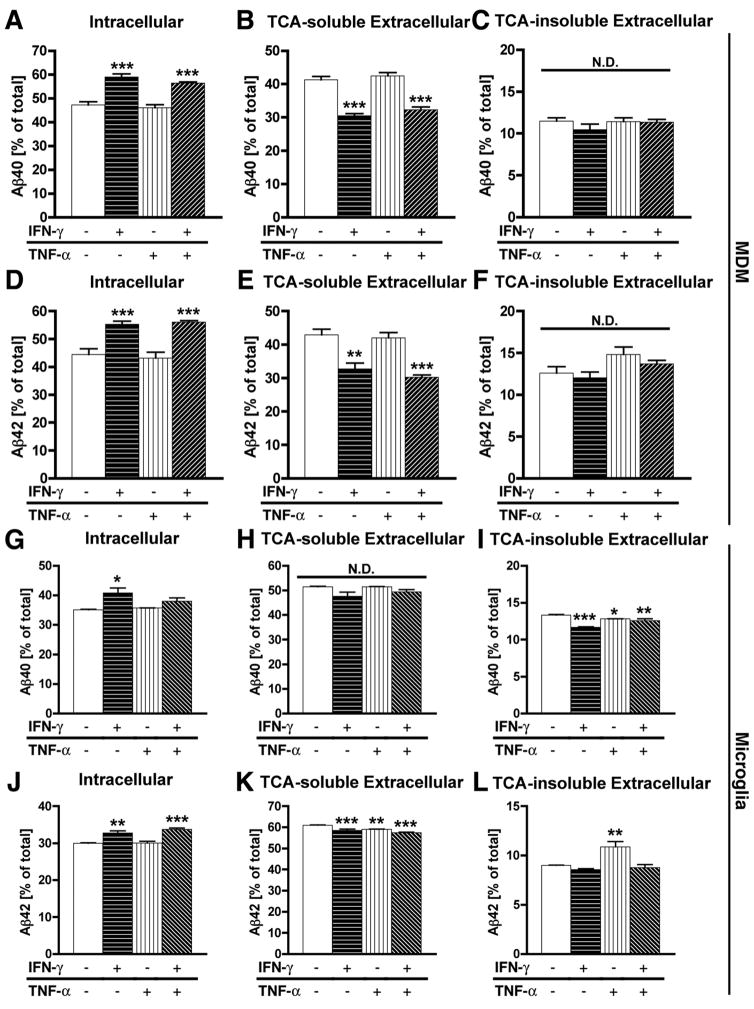
Next, we tested a panel of pro-inflammatory cytokines on Aβ clearance in MDM. MDM were pulse-labeled by fibrillar 125I-Aβ40 or 125I-Aβ42, and incubated with a combination of cytokines during the chase period, followed by fractionation and quantification of Aβ degradation (Fig. 2 A–F). IFN-γ with or without TNF-α significantly enhanced intracellular retention of fibrillar Aβ40 or Aβ42 (Fig. 2A, D) and significantly reduced extracellular TCA-soluble fraction (Fig. 2B, E), whereas extracellular TCA-insoluble fraction was not significantly changed (Fig. 2C, F). These data suggest that IFN-γ suppresses both fibrillar Aβ40 and Aβ42 degradation in MDM.
FIGURE 2. The effect of pro-inflammatory cytokines on Aβ degradation.
MDM (A–F) or microglia (G–L) were pulse-labeled with aggregated 125I-Aβ40 (A–C, G–I) or 125I-Aβ42 (D–F, J–L), and chased with fresh tissue culture media for 120 hrs in the presence or absence of a combination of human IFN-γ and TNF-α (all cytokines at final 10 ng/ml). After chasing, the total cell lysate was collected and subjected to γ-counting, which represents the intracellular 125I-Aβ retention (A, D, G, J), extracellular TCA-soluble 125I-Aβ (B, E, H, K) and insoluble 125I-Aβ fractions (C, F, I, L) which were collected and counted by the γ–counter. Each fraction was presented as % total 125I-Aβ (a sum of each fraction for each group). *, **, *** denotes p < 0.05, 0.01, 0.001 vs. control MDM or microglia group (open column) as determined by ANOVA and Newman-Keuls post hoc. N.D.; no statistical difference.
We have also tested the effect of IFN-γ and TNF-α on fibrillar Aβ40 or Aβ42 degradation in human primary microglia (Fig. 2 G–L). Although the effects of IFN-γ and TNF-α were not as strong as those in MDM, IFN-γ significantly enhanced intracellular retention of fibrillar Aβ40, and reduced extracellular TCA-insoluble fraction (Fig. 2 G, I), whereas no effect was observed on the extracellular TCA-soluble fraction (Fig. 2H). Intracellular retention of fibrillar Aβ42 was also enhanced by IFN-γ (Fig. 2J), and its extracellular TCA-soluble fraction was reduced by either IFN-γ, TNF-α, or IFN-γ plus TNF-α stimulation (Fig. 2K), whereas extracellular TCA-insoluble fraction was enhanced only by TNF-α stimulation (Fig. 2L). Thus, in microglia, we observed enhanced intracellular retention of both fibrillar Aβ40 and Aβ42 by IFN-γ as compared to control MDM. However, the cytokine response was generally weaker than that in MDM, which attributes to some difference in degradation pattern between fibrillar Aβ40 and Aβ42 in this study.
Differential role of anti-inflammatory and regulatory cytokines on Aβ degradation
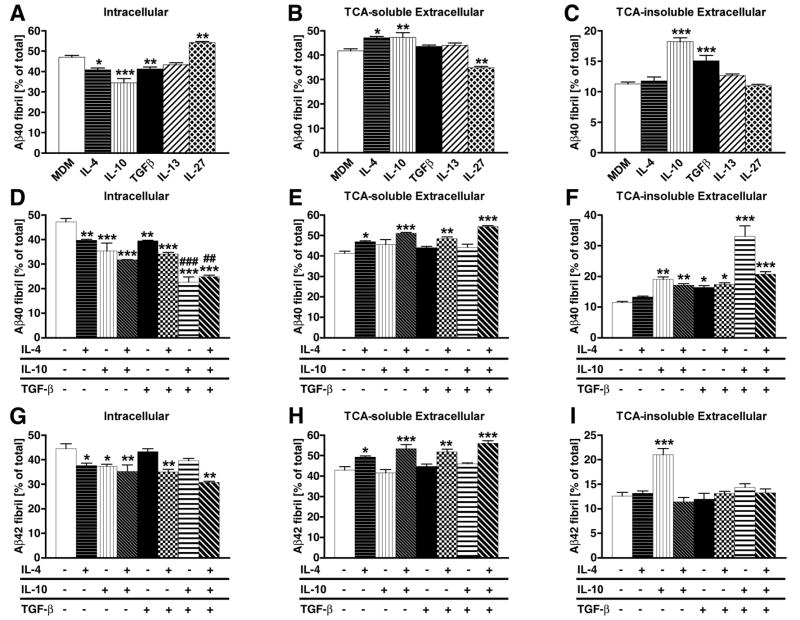
We also tested a panel of anti-inflammatory and regulatory T cell-related cytokines on Aβ clearance in MDM: IL-4, IL-10, TGF-β1, IL-13, and IL-27. In our initial screen, we found that intracellular retention of fibrillar Aβ40 was significantly reduced by IL-4, IL-10, and TGF-β1, whereas IL-13 had no effect and IL-27 enhanced the retention (Fig 3A). Extracellular TCA-soluble fraction was significantly enhanced by IL-4 and IL-10, unaltered by TGF-β1 and IL-13, and suppressed by IL-27 (Fig. 3B). Extracelllar TCA-insoluble fraction was significantly enhanced by IL-10 and TGF-β1, and unaltered by IL-4, IL-13, and IL-27 (Fig. 3C). These data suggest that IL-4, IL-10, and TGF-β1 enhance Aβ degradation in MDM, whereas IL-27 reduces Aβ degradation. Human microglia, however, did not show significant response to any of the anti-inflammatory or regulatory cytokines in our system (data not shown), suggesting the poor expression of receptors or intracellular signaling molecules for these cytokines, or some disconnection of intracellular signaling to modulation of Aβ degradation in cells. Since microglial response to pro- or anti-inflammatory and regulatory cytokines was not as robust as in MDM, we focused our efforts on MDM for the rest of the study.
FIGURE 3. The effect of anti-inflammatory cytokines on Aβ degradation.
A–C, MDM were pulse-labeled with aggregated 125I-Aβ40, and chased with fresh tissue culture media for 120 hrs in the presence or absence of human IL-4, IL-10, IL-13, IL-27, and TGF-β1 (all cytokines at final 10 ng/ml). D–I, MDM were pulse-labeled with aggregated 125I-Aβ40 (D–F) or 125I-Aβ42 (G–H), and chased with fresh tissue culture media for 120 hrs in the presence or absence of a combination of human IL-4, IL-10, and TGF-β1 (all cytokines at final 10 ng/ml). After chasing, the total cell lysate was collected and subjected to γ-counting, which represents the intracellular 125I-Aβ retention (A, D, G), extracellular TCA-soluble 125I-Aβ (B, E, H) and insoluble 125I-Aβ fractions (C, F, I) which were collected and counted by the γ–counter. Each fraction was presented as % total 125I-Aβ (a sum of each fraction for each group). *, **, *** denotes p < 0.05, 0.01, 0.001 vs. control MDM group (open column) as determined by ANOVA and Newman-Keuls post hoc.
To understand the co-stimulatory effects of these molecules, we studied the effects of IL-4, IL-10, and TGF-β1 on fibrillar Aβ40 and Aβ42 degradation in MDM. IL-4 and IL-10 (separately or together) significantly reduced intracellular retention (Fig. 3D) and enhanced TCA-soluble fraction of Aβ40 (Fig. 3E). However, co-stimulation of MDM with TGF-β1 and IL-10 in the presence or absence of IL-4 most significantly enhanced Aβ40 degradation as compared to MDM control (Fig. 3D–E). Stimulation with IL-10 or TGF-β1 tended to enhance TCA-insoluble extracellular fraction of Aβ40 (Fig. 3F). Intracellular retention of fibrillar Aβ42 was also suppressed by IL-4, IL-10, IL-4 plus IL-10, TGF-β1 plus IL-4, and TGF-β1 plus IL-4 and IL-10 (Fig. 3G). TCA-soluble extracellular fraction of Aβ42 was most enhanced by TGF-β1 plus IL-4 and IL-10 (Fig. 3H), and IL-10 predominantly enhanced TCA-insoluble extracellular fraction of Aβ42 (Fig. 3I). Overall, both fibrillar Aβ40 and Aβ42 degradation were enhanced by IL-4, IL-10, and TGF-β1, and combination of all three was most potent, suggesting a synergistic effect for Aβ degradation. Since the degradation patterns of fibrillar Aβ40 and Aβ42 were similar in time course and response to cytokines in MDM, we focused on the characterization of fibrillar Aβ40 on MDM for the rest of the study.
Localization of phagocytized Aβ in MDM
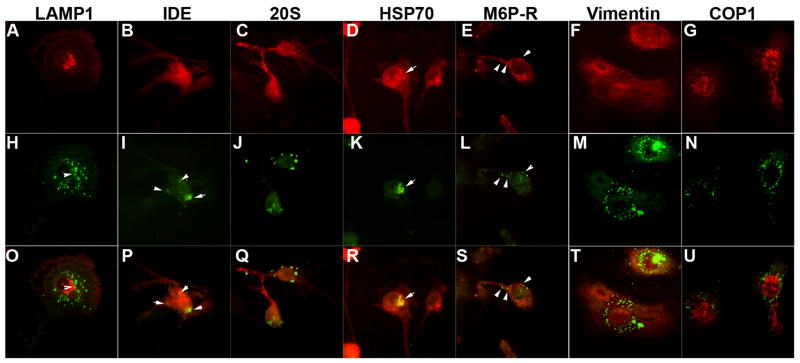
We have previously demonstrated that phagocytized Aβ is frequently localized in the aggresome in primary mouse bone marrow-derived macrophages (24). Although fluorescent-labeled Aβ is located in endosomes/lysosomes in murine microglia (25), its localization has not been shown in human MDM. Thus, we investigated the intracellular localization of fibrillar Aβ40 after the initial uptake by MDM using immunofluorescence and laser-scanning confocal microscopic imaging of aggregated Aβ with a panel of intracellular markers (Fig. 4 A–U). In contrast to murine microglia, there was little co-localization of Aβ40 aggregates (Fig. 4H and O) with lysosomal marker (LAMP1, Fig. 4A and O, red). We observed co-localization of Aβ aggregates with insulin degrading enzyme (IDE, Fig. 4 B, I and P), an aggresomal marker (Hsp70, Fig. 3D, K and R), and partial co-localization with an endosomal marker (M6P-R, Fig. 4E, L, and S). There was no co-localization with proteasomal marker (20S, Fig. 4C, J, and Q), interfilament marker (vimentin, Fig. 4F, M, and T), or cis-Golgi marker (COPI, Fig. G, N and U). These data suggest that processing of Aβ aggregates occurs in endosomes, aggresomes, and IDE in MDM, with a short lifespan of Aβ aggregates in lysosome.
FIGURE 4. Confocal imaging of Aβ aggregates in MDM.
Primary cultured MDM was incubated with 1 μM of filamentous Aβ40 for 1 hr, chased with tissue culture media for 5 days, and subjected to confocal microscopy imaging of immunofluorescence stained by organella markers/enzymes (A–G, O–U, red) and anti-Aβ antibody (H–N, either mono- or poly-clonal, green). O–U, Merged images of corresponding images in A–G and O–U. Arrows indicate co-localized staining between two colors. Original Magnification: 400X. LAMP1, lysosomal membrane glycoprotein 1 (lysosomal marker); IDE; insulin degrading enzyme; 20S (20S proteasome marker); HSP70, heat shock protein 70 (chaperone molecule, aggresome marker); M6P-R, mannose-6-phosphate receptor (late-endosome marker); Vimentin (interfilament marker); COPI, coat protein I (cis-Golgi marker).
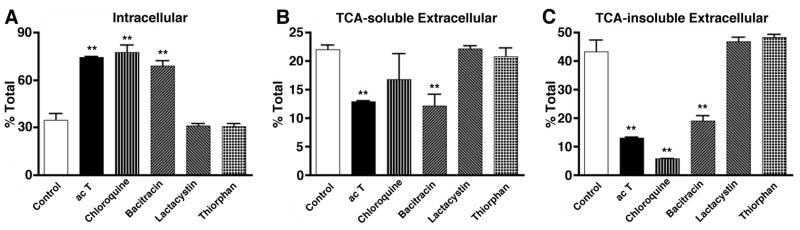
Lysosomal and IDE inhibitors block Aβ degradation
To address which protein degradation pathway is involved in Aβ degradation in MDM, we tested a panel of inhibitors for lysosomal enzymes (chloroquine), IDE (bacitracin), proteasomal enzymes (lactacystin), and neprilysin (thiorphan) (Fig. 5 A–C). IDE inhibitor closely mimics the effect of pro-inflammatory cytokines on fibrillar Aβ40 degradation (increased intracellular retention, decreased TCA-soluble and insoluble extracellular fractions, Fig. 5 A–C). Lysosomal inhibitor strongly increased intracellular retention; however, the effect seems to be predominantly due to suppression of the TCA-insoluble extracellular fraction (Fig. 5C), which resulted in increased intracellular retention (Fig. 5A) without significant changes in Aβ40 digestion (TCA-soluble extracellular fraction, Fig. 5B). Other inhibitors had no effect on Aβ40 degradation in any of the fractions. Thus, IDE and lysosomal pathways are involved in Aβ40 degradation, with IDE more specific to the degradation of Aβ in human MDM.
FIGURE 5. Lysosomal and IDE inhibitors suppress Aβ degradation.
MDM were pulse-labeled with fibrillar 125I-Aβ40, and chased with fresh tissue culture media for 120 hrs in the presence or absence of activated T-cells (500,000 cells/well), chloroquine (50 μM), bacitracin (1 mg/ml), lactacystin (5 μM), or thiorphan (10 μM). Intracellular 125I-Aβ retention (A), extracellular TCA-soluble 125I-Aβ (B) and insoluble 125I-Aβ fractions (C) were collected and counted by the γ–counter. Each fraction was presented as % total 125I-Aβ (a sum of each fraction for each group). ** denotes p < 0.01 vs. control MDM group (open column) as determined by ANOVA and Newman-Keuls post hoc.
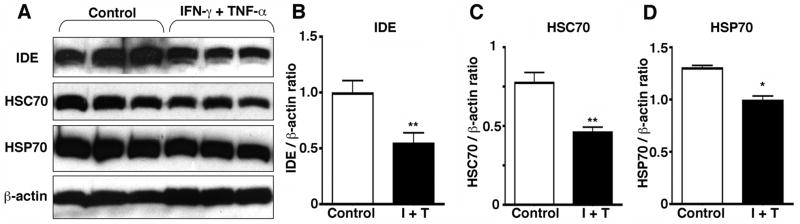
IFN-γ and TNF-α downregulate IDE and proteasomal enzymes
To address if pro-inflammatory cytokines alter the expression levels of Aβ degrading enzymes, we treated MDM with IFN-γ and TNF-α, followed by SDS-PAGE and immunoblotting analysis (Fig. 6 A–D). Co-stimulation of MDM with two cytokines significantly suppressed expression of IDE (45% reduction) as well as chaperone molecules (HSC70 and HSP70, 41 and 24% reduction, respectively). Co-stimulation with the two cytokines did not alter lysosomal cysteine proteases (cathepsin B and D, data not shown), and individual stimulation of MDM with IFN-γ or TNF-α had no effect on the expression levels of the aforementioned molecules (data not shown). These data suggest that IFN-γ and TNF-α suppress Aβ degradation via suppression of IDE activity and reduction of chaperone molecules, which may inhibit refolding and clearance of aggregated Aβ in MDM.
FIGURE 6. IFN-γ and TNF-α co-stimulation reduces IDE and chaperone molecules.
A, Cell lysates (20 μg/lane) from human MDM (2,00,000 cells/well) stimulated by murine IFN-γ (10 ng/ml) and TNF-α (10 ng/ml) for 120 hrs, were subjected to SDS-PAGE and immunoblotting with anti-IDE (1: 6,000), anti-HSC70 (1: 3,000), anti-HSP70 (1: 100,000), or anti-β-actin (1: 5,000,000) antibodies. B–D, Quantification of immunoreactive bands in A for IDE (B), HSC70 (C), and HSP70 (D). I+T; treatment group with IFN-γ and TNF-α. The band intensity was quantified by Typhoon imaging system (Amersham Pharmacia Biotech), normalized by that of β-actin band, and presented as β-actin ratio. * denotes p < 0.01 vs. control group by Student’s t-test.
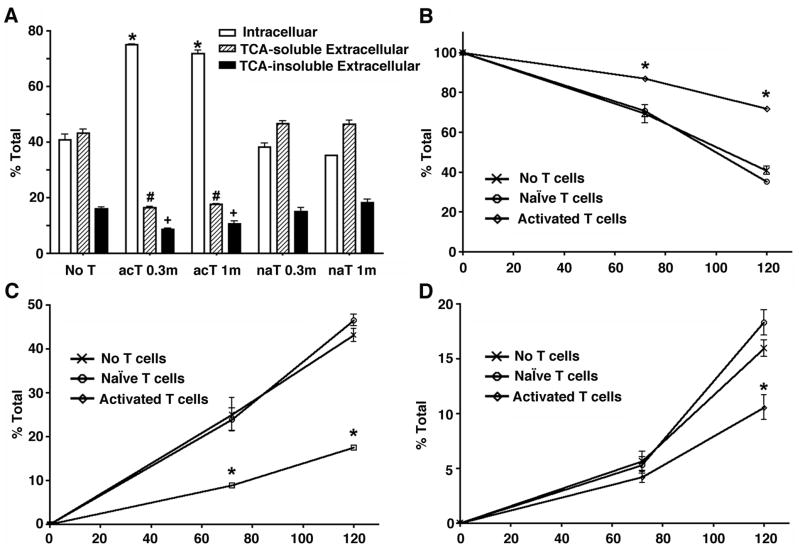
Co-cultured activated T-cells enhance intracellular retention of Aβ
To address the effect of T-cells on Aβ degradation, human MDM (0.5 million cells/well) were incubated with fibrillar 125I-Aβ40 for 1 hr at 37°C, followed by washing and co-culture with naïve (na T) or activated T-cells (ac T) in Transwell for 72 and 120 hrs in MDM tissue culture media (Fig. 7A–D). Activated T-cells predominantly produced more pro-inflammatory cytokines, such as IFN-γ, TNF-α, CD40L, IL-6, and less anti-inflammatory cytokines, such as TGF-β1, IL-4, and IL-10 as determined by multiplex ELISA (Human Cytokine Multiplex kit, Biosource International, data not shown), which is consistent with previously reported observations of activated T-cells (26). In addition, secreted pro- and anti-inflammatory cytokines from naïve T-cells were either undetectable or significantly lower than those from activated T cells (data not shown). There is no direct contact of T-cells with MDM in this experimental design, therefore, haplotype differences due to using different donor sources was not a concern. Co-culture with either 0.3 or 1.0 million (0.3m or 1m) activated T-cells significantly enhanced intracellular retention of aggregated Aβ, and reduced both TCA-soluble and insoluble fractions of extracellular Aβ (Fig. 6A). Naïve T-cells (na T) had no effect on any of the fractions at any time points. This observation is consistent at both 72 and 120 hr time points (Fig. 7B–D), demonstrating the increased intracellular retention and reduced extracellular secretion of both TCA-soluble and insoluble fractions of Aβ, which were dependent on T-cell activation. The difference in degradation kinetics between Fig. 1 and 6 on non-co-cultured control is due to donor variation of primary cultured MDM from leukopheresis. Prior incubation of aggregated Aβ with anti-Aβ monoclonal antibody (6E10) did not enhance Aβ degradation in this system (data not shown), suggesting that the post-endocytic degradation pathway is indifferent to antibody dependent or independent phagocytosis. These data indicate that pan activated T-cells prone to pro-inflammatory cytokine production significantly suppress degradation of aggregated Aβ. At both time points, the most constant indicator of Aβ degradation was the fractions of intracellular retention and extracellular TCA-soluble Aβ (Fig. 7B–C).
FIGURE 7. Co-cultured activated T cells suppressed the Aβ degradation in macrophage.
A, Primary cultured human MDM (500,000 cells/well) were pulse-labeled with fibrillar 125I-Aβ40 (200,000 cpm/well) for 1 hr, and chased with fresh tissue culture media for 120 hrs in the presence or absence of naïve (na T) or activated T cells (ac T) at 0.3 × 106 (0.3m) or 1 × 106 cells (1m) in Transwell insert (A). After chasing, the total cell lysate was collected and subjected to γ-counting, which represents intracellular 125I-Aβ retention (open columns). The tissue culture media was subjected to 10% TCA precipitation to separate extracellular TCA-soluble 125I-Aβ (dashed columns) and insoluble 125I-Aβ (closed columns). Each fraction was presented as % total 125I-Aβ (a sum of each fraction for each group). *, #, or + denotes p < 0.05 vs. control MDM group of the same fraction as determined by ANOVA and Newman-Keuls post hoc. B–D, time course study of Aβ degradation. 125I-Aβ40 pulse-labeled MDM were co-cultured with 1 × 106 cells of naïve or activated T cells in Transwell insert for 72 and 120 hr time points. B, Intracellular retention. C, TCA-soluble extracellular fraction. D, TCA-insoluble extracellular fraction. * denotes p < 0.001 vs. control or naïve T-cell co-cultured MDM group at the same time point as determined by two-way ANOVA and Bonferroni posttests.
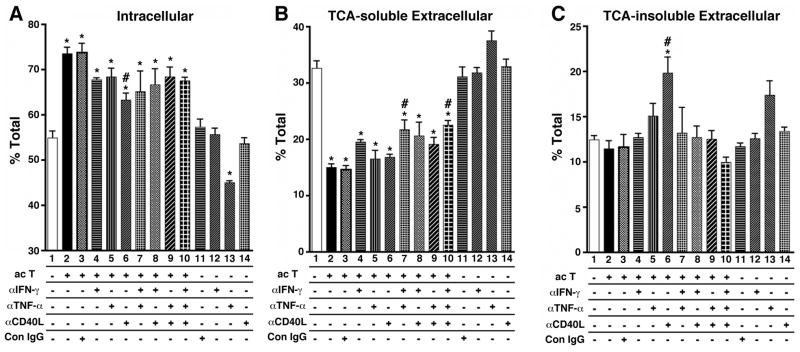
Specific pro-inflammatory cytokine inhibition neutralizes T-cell effect
Since it is classically known that T-cells activate MDM via pro-inflammatory cytokine production, we tested for which cytokines are involved in the T-cell effect using the Transwell co-culture system. We focused on a panel of known T-cell-mediated pro-inflammatory cytokines produced upon T-cell activation and regulated after Aβ deposition in transgenic APP mouse models: IFN-γ, TNF-α, and CD40L (11, 12). We inhibited the effect of each cytokine using specific neutralizing antibodies during the co-culture chasing period (Fig. 8 A–C). Co-culture with activated T-cells enhanced intracellular retention of aggregated Aβ (Fig. 8A, Column 2), which was reduced by individual treatment with anti-CD40L antibody or combination treatment against IFN-γ, TNF-α, and CD40L (Fig. 8A, Column 3–10). Anti-TNF-α antibody significantly suppressed intracellular retention of Aβ in the absence of activated T-cells (Fig. 8A, Column 13) as compared to non-co-cultured untreated group (Fig. 8A, Column 1) or control IgG treated group (Fig. 8A, Column 11), suggesting an inhibitory role for autocrine TNF-α on Aβ degradation.
FIGURE 8. The effect of neutralizing antibodies against pro-inflammatory cytokines on Aβ degradation.
Human MDM were pulse-labeled with fibrillar 125I-Aβ40 for 1 hr, and chased with fresh tissue culture media with (Columns 2–10) or without (Columns 1 and 11–14) activated T cells (ac T) for 120 hrs in the presence, absence, or combination of anti-IFN-γ (αIFN-γ), anti-TNF-α (αTNF-α), anti-CD40L (αCD40L), and control mouse IgG (Con IgG, all from R&D Systems at final 1μg/ml). Intracellular 125I-Aβ retention (A), extracellular TCA-soluble 125I-Aβ (B) and insoluble 125I-Aβ fractions (C) were collected and counted by the γ–counter. Each fraction was presented as % total 125I-Aβ (a sum of each fraction for each group). * or # denotes p < 0.05 vs. control MDM group (Column 1) or MDM with activated T cells (Column 2) as determined by ANOVA and Newman-Keuls post hoc.
Degradation of Aβ was inhibited by co-culture with activated T-cells (Fig. 8B, Column 2–10). However, combinations of neutralizing antibodies against IFN-γ and TNF-α (Fig. 8B, Column 7), or IFN-γ, TNF-α, and CD40L (Fig. 8B, Column 10) significantly increased Aβ degradation as compared to T-cell-co-cultured group (Fig. 8B, Column 2), suggesting that these cytokines are primarily involved in the suppression of Aβ degradation. Secretion of TCA-insoluble Aβ from MDM was enhanced by inhibition of CD40L (Fig. 8C, Column 6), although the effect of activated T-cells was not significant (Fig. 8C, Column 2). These data suggest that abortive secretion of undigested Aβ is the smallest portion of Aβ clearance mechanism, and may not be a good indicator of Aβ degradation, as suggested in the time course study (Fig. 7D). Neutralizing antibodies against interleukin-12 (IL-12) and IFN-α had no effect in this experimental design (data not shown). Taken together, these data indicate that a combination of T-cell-mediated pro-inflammatory cytokines synergistically suppress Aβ degradation and enhance its intracellular retention.
Discussion
The current study on Aβ degradation part shows 1) distinct differences in initial uptake and time course of degradation between oligomeric and fibrillar Aβ40 and Aβ42 in MDM, 2) similar time course and degradation pattern of fibrillar Aβ40 and Aβ42 in both MDM and primary cultured microglia, 3) suppression of Aβ degradation in MDM and microglia by pro-inflammatory cytokines (mainly IFN-γ), although the cytokine response was small in microglia, 4) enhanced degradation of both fibrillar Aβ40 and Aβ42 by select anti-inflammatory and regulatory cytokines (IL-4, IL-10, and TGF-β1), although cytokine response was negligible in microglia. We also showed that Aβ degradation in MDM was sensitive to inhibitors for IDE and lysosomal enzymes, although IDE, but not cathepsin, was downregulated by IFN-γ and TNF-α co-stimulation. In addition to IDE, the expression levels of chaperone molecules (HSC70 and HSP70) were also downregulated by pro-inflammatory cytokine stimulation. When MDM were co-cultured with T-cells using Transwell system, activated, but not naïve, T-cells suppress Aβ40 degradation in MDM through reduced Aβ40 digestion and increased intracellular retention after pulse-incubation with fibrillar 125I-Aβ40. This T-cell effect was partially blocked by a combination of neutralizing antibodies against pro-inflammatory cytokines (IFN-γ, TNF-α, and CD40L). Since inhibitors for IDE or lysosomal enzymes show very similar patterns of change in Aβ degradation in MDM compared to those induced by activated T-cells, our data indicate that activation of MDM by T-cells inhibits Aβ degradation.
Interestingly, anti-inflammatory and regulatory cytokines, IL-4, IL-10 and TGF-β1, exhibited positive effects on Aβ degradation in MDM. We also tested IL-13, which induces FoxP3-expressing regulatory T cells (27) and also secreted from IL-10 producing regulatory T cells (28), and IL-27, which is involved in Th2-type immune responses (29) and induction of T regulatory type 1 cells (30). We found that IL-13 had no effect on fibrillar Aβ40 degradation, and IL-27 rather suppressed Aβ degradation, suggesting that select anti-inflammatory or regulatory cytokines can enhance Aβ degradation in MDM. IL-4 was also suggested as a mediator of copolymer-1-induced Aβ clearance in a transgenic mouse model of AD in vivo (31). The effect of TGF-β1 is also consistent with the previous report that transgene-expression of TGF-β1 reduced parenchymal Aβ deposition in APP mice in vivo and TGF-β1 treated BV-2 cells in vitro (32). TGF-β1 can also enhance T cell infiltration in brain after Aβ42 immunization (33), suggesting the active role of TGF-β1 in T cell chemotaxis, which may also be involved in enhanced Aβ clearance in APP/TGF-β1 bigenic mice. Taken together, IL-4 and TGF-β1 have therapeutic potentials for Aβ clearance by peripheral macrophages. The action of IL-10 is distinct from other cytokines, since although it reduced intracellular retention of fibrillar Aβ40 or Aβ42, it also significantly enhanced secretion of TCA-insoluble Aβ40 or Aβ42. Thus, IL-10 may not be beneficial for overall clearance of Aβ by MDM unless it is employed in combination with IL-4 and TGF-β1.
IDE was one of the first Aβ degrading enzymes to be biochemically isolated from tissue culture media of immortalized murine microglia cell line (BV-2) (34, 35). Although neprilysin is reported to degrade aggregated Aβ more efficiently than IDE (36, 37), our results indicate that IDE plays a more significant role in the degradation of aggregated Aβ in MDM. This is further supported by our data that IFN-γ and TNF-α significantly suppressed IDE expression in MDM, suggesting that IDE is one of the targets of cytokine-mediated inhibition of Aβ degradation.
Although the accumulation of aggregated Aβ in late endosomes and lysosomal compartments was previously reported in primary cultured murine microglia (23), Aβ is accumulated in HSP70-positive aggresome or IDE in MDM, which is consistent with the co-localization of Aβ aggregates in HSC70-positive aggresomes in bone marrow-derived macrophages (24). These data suggest two pathways for intracellular Aβ degradation. Internalized aggregated Aβ in phagosome or endosome may be subsequently transferred to lysosome for degradation, or shuttled to aggresome via retrograde transport machinery. In the aggresome, aggregated Aβ will be unfolded by chaperone molecule, then transferred to cytoplasm for degradation by IDE. IFN-γ and TNF-α significantly suppressed the expression levels of HSC70 and HSP70, suggesting their inhibitory role on Aβ refolding, which lead to its accumulation at refolding steps and containment in aggresome. Since 99% of intracellular Aβ is TCA-insoluble form, most remain as filamentous form in MDM with a minor fraction subjected to extracellular transport machinery. Oligomeric Aβ40, on the other hand, is very efficient in degradation after phagocytosis, suggesting that oligomeric structure is more susceptible to lysosomal or IDE-mediated degradation in MDM.
The mechanism of extracellular transport of undigested Aβ is not well understood, but one potential mechanism is via exosome release. Exosomes are membrane vesicles secreted by hematopoietic cells upon fusion of late multivesicular endosomes with the plasma membrane. Since aggregated Aβ is localized in late endosome, this fraction is a likely target for exosome release. Mononuclear cells, especially dendritic cells efficiently secrete exosomes, which are highly enriched in major histocompatibility complex II (MHC-II) and considered one of the mechanisms of transcellular antigen presentation. Overall, this is the first study to address the role of pro- and anti-inflammatory and regulatory cytokines on fibrillar and oligomeric Aβ degradation in human MDM or microglia. These findings will be relevant to understand the role of identified cytokines on T-cell mediated Aβ degradation and refolding of aggregated proteins in human disease.
Acknowledgments
We would like to thank Dr. Larisa Poluektova for critical reading of the manuscript, Lindsey Martinez and Michael Jacobsen for editorial assistance, James Buescher for radioactivity usage, Dr. Luda Shlyakhtenko for AFM service, and Dr. Howard Gendelman and Li Wu for Tissue Culture Core Facility service.
Footnotes
This work was supported by Vada Kinman Alzheimer’s Research Awards (TI and JK), NIH P01 NS043985 (TI), and Nebraska Tobacco Settlement Biomedical Research Development Fund (JK).
References
- 1.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 2.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 3.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 5.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, Quervain DJ de, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 6.Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 8.Abbas N, Bednar I, Mix E, Marie S, Paterson D, Ljungberg A, Morris C, Winblad B, Nordberg A, Zhu J. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J Neuroimmunol. 2002;126:50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 9.Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 10.Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti- inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-{gamma} and Tumor Necrosis Factor-{alpha} Regulate Amyloid-{beta} Plaque Deposition and {beta}-Secretase Expression in Swedish Mutant APP Transgenic Mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 14.Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- 15.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl L, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikezu T, Luo X, Weber GA, Zhao J, McCabe L, Buescher JL, Ghorpade A, Zheng J, Xiong H. Amyloid precursor protein-processing products affect mononuclear phagocyte activation: pathways for sAPP- and Abeta-mediated neurotoxicity. J Neurochem. 2003;85:925–934. doi: 10.1046/j.1471-4159.2003.01739.x. [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Weber GA, Zheng J, Gendelman HE, Ikezu T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer’s disease. J Neuroimmunol. 2003;135:62–71. doi: 10.1016/s0165-5728(02)00444-7. [DOI] [PubMed] [Google Scholar]
- 18.Borgmann K, Gendelman HE, Ghorpade A. Isolation and HIV-1 infection of primary human microglia from fetal and adult tissue. Methods Mol Biol. 2005;304:49–70. doi: 10.1385/1-59259-907-9:049. [DOI] [PubMed] [Google Scholar]
- 19.Chao CC, Gekker G, Hu S, Peterson PK. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 20.Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL. Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Yuan B, Emadi S, Zameer A, Schulz P, McAllister C, Lyubchenko Y, Goud G, Sierks MR. Single chain variable fragments against beta-amyloid (Abeta) can inhibit Abeta aggregation and prevent abeta-induced neurotoxicity. Biochemistry. 2004;43:6959–6967. doi: 10.1021/bi049933o. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, McAllister C, Lyubchenko Y, Sierks MR. Residues 17–20 and 30–35 of beta-amyloid play critical roles in aggregation. J Neurosci Res. 2004;75:162–171. doi: 10.1002/jnr.10859. [DOI] [PubMed] [Google Scholar]
- 23.Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Kiyota T, Walsh SM, Ikezu T. Kinetic analysis of aggregated amyloid-b peptide clearance in adult bone-marrow-derived macrophages from APP and CCL2 transgenic mice. J Neuroimmune Pharmacol. 2007;2:213–221. doi: 10.1007/s11481-006-9049-8. [DOI] [PubMed] [Google Scholar]
- 25.Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 26.Chabot S, Yong FP, Le DM, Metz LM, Myles T, Yong VW. Cytokine production in T lymphocyte-microglia interaction is attenuated by glatiramer acetate: a mechanism for therapeutic efficacy in multiple sclerosis. Mult Scler. 2002;8:299–306. doi: 10.1191/1352458502ms810oa. [DOI] [PubMed] [Google Scholar]
- 27.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 28.Stern JN, Keskin DB, Zhang H, Lv H, Kato Z, Strominger JL. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc Natl Acad Sci U S A. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci U S A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 31.Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 33.Buckwalter MS, Coleman BS, Buttini M, Barbour R, Schenk D, Games D, Seubert P, Wyss-Coray T. Increased T cell recruitment to the CNS after amyloid beta 1–42 immunization in Alzheimer’s mice overproducing transforming growth factor-beta 1. J Neurosci. 2006;26:11437–11441. doi: 10.1523/JNEUROSCI.2436-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 35.Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ. Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J Biol Chem. 1997;272:6641–6646. doi: 10.1074/jbc.272.10.6641. [DOI] [PubMed] [Google Scholar]
- 36.Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 37.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]