Abstract
Animal models have been used extensively to investigate neuropsychiatric disorders, such as depression, and their treatment. However, the etiology and pathophysiology of many such disorders are largely unknown, which makes validation of animal models particularly challenging. Further, many diagnostic symptoms are difficult to define, operationalize and quantify, especially in experimental animals such as rats. Thus, rather than attempting to model such complex human syndromes as depression in their entirety, it can be more productive instead to define and model components of the illness that may account for clusters of co-varying symptoms, and that may share common underlying neurobiological mechanisms.
In our preclinical investigations of the neural regulatory mechanisms linking stress to depression and anxiety disorders, as well as the mechanisms by which chronic treatment with antidepressant drugs may exert their beneficial effects in these conditions, we have employed a number of behavioral tests in rats to model specific cognitive and anxiety-like components of depression and anxiety disorders. In this paper, we review the procedures for conducting four such behavioral assays: the attentional set-shifting test, the elevated-plus maze, the social interaction test and the shock-probe defensive burying test. The purpose is to serve as a guide to the utility and limitations of these tools, and as an aid in optimizing their use and productivity.
Keywords: anxiety, attentional set-shifting, defensive burying, depression, elevated-plus maze, social interaction
Introduction
Preclinical investigation of neuropsychiatric disorders and their treatment relies heavily on the use of animal models. However, for many neuropsychiatric disorders, knowledge of the neurobiology underlying the etiology and pathophysiology of the disorder, as well as the mechanisms underlying effective clinical response to treatement, are largely unknown, leaving gaps in our ability to evaluate fully the construct validity, relevance and predictive validity of many of the animal models most commonly used to investigate such disorders (see 1, 2–5). An additional challenge to investigators trying to model human neuropsychiatric disease is that many of the symptoms are difficult to define and operationalize, much less quantify, in animals such as rats (for example, suicidal ideation, or feelings of guilt and worthlessness in depression).
As elaborated in a recent review (6), an emerging trend in both psychobiology and neuropsychiatry is to attempt to define a limited number of behavioral dimensions that might account for clusters of symptoms that frequently co-vary within and even across different psychiatric illnesses. It is thought that such symptom clusters might be attributable to dysregulation of a common underlying neurobiological process or system. Thus, rather than attempting to develop an animal model that emulates all aspects of a complex human neuropsychiatric syndrome such as depression, a more practical focus of preclinical studies adopting such a “dimensional” approach is to model components of an illness, representing a specific dimension, and to investigate the neural basis for subsets of related symptoms. This approach can make such models more amenable to testing and validation, and to experimental manipulation by relevant environmental, developmental and pharmacological factors.
There is extensive co-morbidity between depression and anxiety disorders. Neuro-psychological, genetic and psychometric approaches have suggested that dysregulation of a limited number of behavioral dimensions can account for many of their shared symptoms (see, for example, 7) (reviewed in 6). Anxiety is a prominent component of both depression and anxiety disorders. In addition to anxiety, cognitive dysfunction is also a major component of depression and anxiety disorders, and it co-varies with anxiety (7–11). Anxious and depressed patients exhibit cognitive biases for emotionally salient material, particularly related to stressful life events (8, 9). A perseverative focus on perceived threats, loss, personal deficiency or worthlessness may contribute to vulnerability for affective disorders (12). Neuropsychological studies of depressed patients have revealed cognitive impairments associated with frontal lobe dysfunction (13–15), including deficits in cognitive flexibility, perseveration, problem solving, motivation, and response inhibition (14–18). Depressed patients show abnormal responses to performance feedback, a narrowing of attentional focus to depression–relevant thoughts (15), and difficulty shifting cognitive set from one affective dimension to another (15). This is consistent with the perseverative attention to themes of loss and worthlessness, and the persistent ruminations that are prevalent in depression (15, 18). Neuroimaging studies have further revealed an association between cognitive dysfunction and hypoactivity in regions of prefrontal cortex (PFC) in depression (19–21). Deficits in cognitive flexibility are associated with a number of neuropsychiatric disorders involving reduced PFC function (14, 22).
Depression and anxiety disorders not only share several symptoms, they also have common risk factors, including life stress. Thus, in our preclinical research, we investigate the neural regulatory mechanisms that may link stress to increased vulnerability for depression and anxiety disorders, as well as mechanisms of therapeutic drug action, using a variety of behavioral tests to assess alterations in prefrontal cortical cognitive function and anxiety-like behaviors in rats. In these studies, the behavioral assays are not used to model depression per se, but rather to model specific dimensions of depression and anxiety disorders. This, then, is not intended as a comprehensive review of animal models of depression, but as a guide to the utilization of these specific tools, and as an aid, based on our experience, in optimizing their utility for investigating the neurobiological bases of anxiety and depression, and factors involved in their development.
The first assay presented in this review is the attentional set-shifting test (AST), a model of cognitive flexibility dependent on prefrontal cortical function in rats. As this is the newest test discussed, and therefore the least well-known and least well-documented, we devote the most space and detail to this assay. We then present a number of tests we have used to assess different types of anxiety-like behavior in rats, including the elevated plus maze, the social interaction test, and the shock probe defensive burying test.
The Attentional Set-Shifting Test (AST): A Model for Cognitive Flexibility Dependent Upon Function of the Prefrontal Cortex in Rats
The attentional set-shifting task (AST) is a relatively new animal model developed to assess prefrontal cortical function in rats (23). The prefrontal cortex has been the focus of considerable scientific investigations in recent years, owing in part to the growing recognition that dysfunction of this region and associated circuitry may underlie the cognitive and behavioral disturbances associated with many major neuropsychiatric disorders, including depression. Neuropsychological studies of depressed patients have revealed impairments in executive functions related to frontal lobe activity, for example, verbal fluency, cognitive set-shifting, behavioral flexibility, and perserveration (see 14). Recent findings in non-human primates suggest that divergent cognitive processes may be mediated by closely related but anatomically distinct sub-regions of prefrontal cortex. In primates, the medial prefrontal cortex (mPFC) mediates the ability to shift attention between perceptual features of complex stimuli (24–26). In rats, the same region has been implicated specifically in attentional set-shifting, or the ability to “unlearn” an established contingency in order to learn a new one by shifting attention from a previously salient stimulus dimension to a previously irrelevant one (23). Lesions of rat mPFC resulted in a state of “emotional perseveration”, or a failure to unlearn old contingencies in a fear-conditioning extinction paradigm (27). On the other hand, lesions in the orbitofrontal cortex (OFC) specifically impair reversal learning, but not extradimensional set shifting (23–25, 28, 29).
The AST represents the rodent version of a diagnostic test used in the clinic, the Wisconsin Card Sorting Test (WCST). In humans, the WCST is used to assess strategy-switching deficits in patients with frontal lobe damage or dysfunction (see 30). Briefly, in the WCST, subjects are asked to match cards from a deck to a set of exemplars. The cards are defined by pictures that differ along three dimensions: the shapes pictured on the card, the color of the shapes, and the number of shapes pictured. The subject must learn the rule for sorting (shape, color or number) based only on feedback indicating whether the response on each trial is correct or incorrect. Once a given contingency is mastered, the rules are changed unbeknownst to the subject. They must then react to the error, suppress their previously successful but now incorrect strategy, and learn the new sorting rule by switching attention from the previously salient dimension to a previously irrelevant dimension. This procedure was later adapted and refined by Roberts et al. (31) to tease apart the many complex cognitive processes involved in the WCST, and specifically to investigate the ability of both humans and non-human primates to develop and then switch attentional set. They developed a series of visual discriminations on which performance was compared on transfer tests requiring new contingencies to be learned using stimuli that varied along the same dimension as a previously salient cue (an intradimensional shift, ID) or along a different, previously irrelevant dimension (an extradimensional shift, ED) (31). Birrell and Brown (23) subsequently developed a test to assess the same attentional set shifting capability in rats. In this test, rats are taken through a series of tasks in which they must dig in small flower pots to locate a food reward. After they master the contingency required at each stage, typically to a criterion of 6 consecutive correct responses, the rules are changed and they progress to the next stage. In the first stage, the rats must learn to locate the reward based only on a single salient cue, either the odors applied to the rims of the two pots, or the texture of the digging material in each pot (simple discrimination, SD). Then, the second distracting dimension is introduced, but the salient cue remains the same. For instance, if odor was the cue that signaled the reward in the SD stage, then two different digging media are introduced into the pots, but odor is still the salient cue. This is called a compound discrimination (CD). The third stage is a reversal (R1), in which the negative cue from the previous stage becomes positive, and the positive cue from the previous stage is negative, but the informative dimension remains the same (e.g., odor). The fourth stage is the first dimensional shift, in that all new stimuli (odors and media) are used, but in this case odor would still be relevant and media would still be irrelevant, making it an intradimensional shift (ID). After the rats master this new discrimination, a second reversal (R2) occurs. In all stages up to this point, the rats will have learned repeatedly that one stimulus dimension is informative when the rules are changed and an error is encountered, i.e., they will have formed a cognitive set. However, the sixth stage is then an extradimensional set shift (ED), in which all new stimuli are again introduced, but this time the previous irrelevant dimension (e.g., medium) becomes the relevant dimension, and the previously informative dimension becomes irrelevant. The last stage is a final reversal (R3). The dependent measure in this test is the number of trials required to reach criterion at each stage.
Validity
Validity of the AST as a model of prefrontal cortical cognitive function in rats has been demonstrated primarily using electrolytic or neurotoxic lesions that have localized specific components of cognitive performance on the test to specific sub-regions of prefrontal cortex. Most notably, lesions of mPFC selectively disrupted ED set-shifting, while orbitofrontal lesions disrupted reversal learning (see 23, 29). These results are consistent with the regional selectivity of effects seen using similar tests of reversal learning and ED set-shifting in primates (24, 25).
Because so little is known about the neural substrates underlying prefrontal cortical cognitive function, its dysregulation in psychiatric illness, and the mechanisms by which it is affected by pharmacological treatments, subsequent studies of the neural substrates and physiological contexts that influence performance on the AST do not comprise tests of validity per se. Rather, examples of such studies, some of which we offer from our own published work, illustrate how this tool might be used experimentally to test hypotheses regarding the etiologic factors and mechanisms involved in the treatment of depression and anxiety disorders.
The monoaminergic neurotransmitter norepinephrine (NE) has been shown to enhance vigilance, attention and other executive processes associated with prefrontal cortical function (e.g., 32). Thus, we recently investigated the neuromodulatory effects of NE in mPFC on performance on the AST. We found that elevating noradrenergic tone by acute systemic administration of the α2-adrenergic autoreceptor antagonist, atipamezole, improved ED set-shifting performance, and this facilitation was blocked by local administration of the α1-adrenergic receptor antagonist, benoxathian, directly into mPFC (33). Likewise, increasing central noradrenergic activity by chronic administration of the antidepressant, desipramine (a selective norepinephrine reuptake inhibitor, NRI), improved performance on the ED set-shifting task (34). With respect to depression and antidepressants, the dependence of cognitive performance on the AST on frontal lobe function is relevant to the dysfunction in mPFC seen in depressed patients (14). Also, chronic stress is a known risk factor for depression, and we have shown recently that chronic stress induces replicable disruptions of cognitive performance on the AST (34, 35). Rats previously exposed to chronic unpredictable stress (CUS) had impairments in ED set-shift performance, and less consistent but still significant deficits in reversal learning (35). The CUS-induced deficits were prevented by concurrent chronic treatment with desipramine, or with the selective serotonin reuptake inhibitor (SSRI), escitalopram (35). On the other hand, exposure of rats to a metabolic stressor, chronic intermittent cold stress, selectively impaired reversal learning; this effect was mimicked by serotonin depletion with the neurotoxin PCPA, and was reversed by the SSRI, citalopram (36). Others have shown that early post-natal stress, another risk factor for depression, produced by artificially rearing pups in the absence of maternal care, induced deficits in reversal learning, ID and ED set-shifting (37).
Method
Our method, as described in Lapiz and Morilak (33), was adapted from Birrell and Brown (23). Adult Sprague-Dawley rats, weighing 200–225 g on arrival, are initially group-housed (3 rats/cage) in 25 x 45 x 15 cm plastic cages, maintained on a 12/12-hr light/dark cycle (lights on at 07:00 hr), and given access to food and water ad libitum. Rats are allowed to acclimatize for a minimum of 4–7 days before beginning any behavioral procedures. For one week prior to the testing day, the rats are individually housed and maintained on a restricted diet of 14 g of food pellets per day, given in the morning, with water freely available. All experiments are conducted during the light portion of the cycle, between 09:00–17:00h.
The testing apparatus is a rectangular wooden arena (inner dimensions, length x width x height: 71 x 40 x 20 cm) painted white on all surfaces (see Figure 1). A removable, white plexiglas divider separates one-third of the length of the arena from the rest, forming a start box. This also serves as a holding area following each trial, allowing the experimenter to clean the arena and change pots. To begin each trial, a rat is placed in the start box, and given access to the rest of the arena by lifting this divider. A plexiglas panel divides the opposite third of the arena into two sections. At testing, one digging bowl is placed in each section. This separation enables the experimenter to quickly remove the rat following an incorrect response, thus preventing it from moving to the other bowl to retrieve the bait. The digging bowls are small terracotta pots (internal rim diameter 7 cm; depth 6 cm). Each pot is defined by a pair of cues along two stimulus dimensions, the digging medium with which the pot is filled and an odor (see Table 1).
Figure 1.

Schematic diagram of the attentional set shifting test arena (dimensions: 40 x 71 x 20 cm, l x w x h). A fixed Plexiglas panel divides the distal third of the arena into two sections, and a digging pot is placed in each section. A removable divider separates the proximal third of the arena from the rest, forming a start box. To begin a trial, a rat is placed in the start box, and the divider lifted. Reprinted, with permission from Elsevier, from (33).
Table 1.
Behavioral protocol for attentional set-shifting testing
| Discrimination Stage | Dimensions | Example Combinations | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove/Sawdust | Nutmeg/Sawdust | |
| Compound (CD) | Odor | Medium |
Clove/Raffia
Clove/Metallic Filler |
Nutmeg/Metallic Filler
Nutmeg/Raffia |
| Reversal 1 (R1) | Odor | Medium |
Nutmeg/Raffia
Nutmeg/Metallic Filler |
Clove/Metallic Filler
Clove/Raffia |
| Intradimensional Shift (ID) | Odor | Medium |
Rosemary/Wood balls
Rosemary/Plastic beads |
Cinnamon/Plastic beads
Cinnamon/Wood balls |
| Reversal 2 (R2) | Odor | Medium |
Cinnamon/Plastic beads
Cinnamon/Wood balls |
Rosemary/Wood balls
Rosemary/Plastic beads |
| Extradimensional shift (ED) | Medium | Odor |
Velvet/Citronella
Velvet/Thyme |
Crepe/Thyme
Crepe/Citronella |
| Reversal 3 (R3) | Medium | Odor |
Crepe/Thyme
Crepe/Citronella |
Velvet/Citronella
Velvet/Thyme |
Representative example of stimulus pairs and the progression through the stages of the attentional set shifting protocol. In this example, odor was the initial discriminative stimulus dimension, shifting to digging medium in the ED stage. For each stage, the positive stimulus is in bold, and is paired randomly across trials with the two stimuli from the irrelevant dimension. Reprinted, with permission from Elsevier, from (33).
To mark each pot with a distinct odor, two drops (10 μl/drop) of scented aromatic oil (Frontier Natural Brands, Boulder, CO, USA) are applied to the inner rim at least 5 days prior to use. Then, 3–5 μl of the same oil are reapplied on the day before testing. A different pot is used for each combination of digging medium and odor, and only one odor is ever applied to a given pot. The bait, which is buried under the digging medium in the “positive” pot, is a 1/4 Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA). In all discrimination trials, a small quantity of powdered Cheerio is sprinkled onto the digging medium of both pots at the start of the task to obviate the possibility that the rat may locate the bait by smell rather than by learning the discrimination.
Digging is defined as a vigorous displacement of the medium to retrieve the reward buried within the pot. Simply investigating the rim of the pot or the surface of the digging medium with paws or snout without displacing material is not scored as a “dig”, and the trial continued until a “dig” response is executed. Thus, rats are able to access tactile, visual and olfactory characteristics of the pots to make their choices based on these stimulus dimensions.
The behavioral procedure entails three days for each rat:
Day 1, Habituation
Before receiving their daily food ration, on the habituation day (i.e., the 6th day of food restriction), the rats are trained to dig reliably in the pots to obtain a food reward. Two unscented pots, both baited, are placed in the home cage for a series of three exposures of 5 min each. With each exposure, the bait is covered with an increasing amount of sawdust (see below). Once the rat is digging reliably, it is introduced into the test arena, and given 3 trials to retrieve reward from both of the sawdust-filled baited pots.
Day 2, Training
The following day, again prior to receiving their daily food ration, the rats are trained on a series of simple discriminations (SD), to a criterion of six consecutive correct trials. For these trials, the rats first learn to associate the food reward with an odor cue (lemon vs. rosewood, both pots filled with sawdust). After criterion is reached for the odor discrimination, the pots are changed, and they learn to discriminate the digging media (felt strips vs. shredded paper, no odor present). All rats are initially trained using these same stimuli, and in the same order. The positive and negative cues for each rat are determined randomly and represented equally. These training stimuli are not used again in later testing trials.
Day 3, Testing
On this day, before receiving the daily food ration, the rats are tested on a series of increasingly difficult discriminations (see Table 1). Testing continues at each stage to a criterion of 6 consecutive correct responses before proceeding to the next stage.
The first stage is a simple discrimination (SD), involving only one stimulus dimension. Half the rats in any group are required at this stage to discriminate between 2 odors, only one of which is associated with reward, with both pots filled with sawdust. For the other half, this first discrimination involved digging media, with no odors applied to the pots (for the sake of clarity, the remainder of this description will only consider the example beginning with the odor discrimination). The second stage is then a compound discrimination (CD), wherein the second, irrelevant stimulus (in this case medium) is introduced. Only one odor is associated with reward, as in the SD stage, but two different digging media are now paired randomly with the odors. The third stage is a reversal of this discrimination (R1), in which the same odors and media are used, and odor is still the relevant dimension, but the negative odor from the previous stage is now positive (i.e., associated with the reward), and the positive odor from the previous stage is now negative (no reward). The fourth stage is an intradimensional (ID) shift. This stage represents a new acquisition, in that all new stimuli are used (i.e., new odors and new media), and it is “intradimensional” because odor is still the relevant dimension and medium is still irrelevant. The fifth stage is a reversal of this discrimination (R2), in which the previously negative odor is now positive, as in R1. Up to this point, even as the rules have been changed at each successive stage, the same stimulus dimension has always provided the salient cue associated with reward, leading to formation of a cognitive set. The sixth stage, however, requires an extradimensional (ED) set shift, in that all new stimuli are again introduced, but this time the relevant dimension is also changed, e.g., the digging medium became the relevant dimension and odor is now irrelevant. Finally, the seventh stage is another reversal (R3), wherein the previously negative medium is now positive. The assignment of each exemplar in a pair as being positive or negative in a given stage, and the left-right positioning of the pots in the arena on each trial, are randomized in advance. For half the rats, the discrimination series begins with odor as the relevant cue, as described above, and for half the discrimination begins with medium as the salient cue. Table 2 outlines the progression through these stages, and provides examples of the cue combinations used, beginning in this case with an odor discrimination, shifting to medium in the ED shift stage. The primary dependent measure in this procedure is the number of trials required to reach the criterion of 6 consecutive correct responses at each test stage (Trials to Criterion, TTC). Figure 2 shows a representative pattern of TTC obtained on the AST. We have also analyzed “set loss” errors, which we defined as the number of times an incorrect response occurred after 3 or more consecutive correct responses had been made (34). Such reversion to a previous contingency is one form of perseverative behavior, based on previous analyses of errors using the Wisconsin Card Sorting Test (38).
Figure 2.

Data from a representative study showing trials to criterion of six consecutive correct responses (expressed as mean ± SEM) for each stage of the AST. Significantly more trials were required to reach criterion during the ED set-shift compared to other tasks (n=6; *p < 0.05 compared to SD, CD, R1, R2 and ID). Abbreviations: SD, simple discrimination; CD, compound discrimination; R1, reversal 1; ID, intradimensional set-shift; R2, reversal 2; ED, extradimensional set-shift; R3, reversal 3. Adapted and reprinted, with permission from Elsevier, from (33).
Discussion and Recommendations
Both the construct validity and predictive validity of the AST, particularly with respect to cognitive components of depressive and anxiety-related symptomatology, are still under active investigation. However, lesion studies showing that specific components of the test are dependent upon the integrity of specific subregions of prefrontal cortex in rats have demonstrated an impressive functional homology with results obtained from studies in non-human primates (see 23, 29). We have utilized this assay to investigate the effects of various stressors on cognitive function, and the modulatory influence of central monoaminergic neurotransmitters in prefrontal cortex (33–35). For example, we have shown that rats exposed previously to chronic unpredictable stress exhibited impairments on the AST that were prevented by concurrent chronic treatment with antidepressants, either a norepinephrine reuptake inhibitor, desipramine, or an SSRI, escitalopram (35). In these studies, the model has proven adaptable and robust, in that we have employed a variety of challenging and invasive manipulations, including chronic stress treatment; acute and/or chronic drug treatment, including systemic as well as local microinjections into prefrontal cortex immediately prior to specific test stages; and we have conducted microdialysis, involving chronic stereotaxic implantation of intracerebral guide cannulae into prefrontal cortex, and tethering of the animal to a syringe pump during testing. The same basic behavioral procedure has been employed with these different manipulations, and the results have remained reliable, in that similar response patterns are seen in control animals across experiments. Moreover, although variability has sometimes been observed in the total number of trials across all tasks, the general pattern of responding (i.e., the relative number of trials in each task compared to the others) has been remarkably consistent despite any variability in “baseline”.
A major practical drawback of the AST as compared to other behavioral models that have been used to assess “depressive-like” behaviors (for example, the forced swim test) is that the AST protocol is extremely labor intensive. It requires considerable hands-on time to train and test each animal. A team of two investigators, working full time, are capable of testing at most 2–4 rats per day, and this level of productivity requires multiple test apparatuses. Considerable thought must be devoted to scheduling the apparatus, and to “spacing” of animal treatments, especially chronic treatments. On a given day in a given apparatus, only one rat can be habituated, one rat trained, and one rat tested. However, compared to several other tests of cognitive function, the fact that training and testing can be completed within a few days, rather than after several weeks of intensive training, is an advantage.
Testing typically takes about 2–3 hours per rat, although this can vary considerably. As in most behavioral studies, performance can be affected by emotionality, motivation and other non-selective factors. A change in locomotion per se is not necessarily a confound, as the dependent measure is not a function of speed or overall time on task, but depends on the rat making a correct or incorrect choice. However, if it takes an inordinate amount of time to test an animal, behavior is more likely to be affected by changes in hunger, motivation, fatigue or circadian factors during testing, and an increasing number of rats are likely to simply stop performing. Recognizing and planning for this is critical, as attrition can approach 20% for subjects exposed to chronic stress, surgical preparation, etc. before testing. It is not always possible to eliminate such factors, and it serves well to pay attention to the behavioral tendencies and overall level of activity of each rat from the start of the experiment, and then to respond and handle them gently and patiently at all times. Moreover, the investigator must also remain alert and vigilant throughout the test period, as scoring can only be done manually. The subjective determination of whether an animal made a “dig” response requires constant attention, and investigator fatigue can occur. Below are some in-house strategies we have evolved to optimize the number of animals that will complete the experiment and contribute meaningful data. These practices also serve to limit variability across experiments and between experimenters.
Preparation and materials
The AST paradigm in rats usually employs two stimulus cue dimensions: odor and the digging medium that fills a vessel. The vessel may be a pot (33, 34) or a bowl (23, 29, 39–41). The type of vessel used is a matter of preference, depending on availability of materials and the size of the testing arena. Factors to be taken into consideration are the composition, depth, size and stability of the vessel. We use terracotta flower pots with an internal rim diameter of 7 cm and depth of 6 cm (see 33, 34, 35). Unglazed terracota pots are preferable to ceramic. The odor is applied to the inner rim of the pot, and the clay allows for absorption and retention of the odor for several days without contaminating the digging medium placed in the pot, and does not risk contaminating the animal’s fur or whiskers with scented liquid when they contact the pot. The depth of the pots should allow for the food reward to be well-hidden within the digging medium, otherwise, the rat may simply locate it by smell. The pot should also be physically stable so it is not upended or knocked over when the rat investigates and digs. We increase the stability with tape attached to the bottom of the pot. The opening of the vessel should be wide enough for the rat to dig freely into the medium. If experiments require surgical procedures that increase the bulk or size of the rats head, for instance with surgical implants, the opening of the vessel must still allow the rat to dig unimpeded.
The rat AST literature shows that different laboratories use a variety of odors as cues for the discriminations, although most of these are oil-based. Scent selection is again a matter of preference and availability. However, there are some factors to take into consideration. Choose scents that are not aversive to rats, nor so pungent as to overpower the other odors (e.g., “patchouli” dominates a room even in minute amounts). When pairing odors, select scents that are dissimilar enough that they can be discriminated. For example, do not pair lime and lemon. Only one odor is applied to each pot, so extreme care must be exercised when scenting the pots so as not to cross-contaminate. Gloves should be worn throughout the procedure, and the gloves, syringe needles and pipet tips should be changed after each application. To scent the pots, the scented oil is drawn into a 1 ml syringe fitted with a 26-gauge needle (1/2 inch long). We prime the pots two weeks prior to first use by applying about 50 μl of the scented oil onto the inner rim of the pot. For subsequent scent applications, 5–10 μl is applied 2–3 days prior to using that pot in a test session. Apply less (3–5 μl) if the pots are scented only one day prior to use. Scenting should of course be done only after all experiments for the day are completed and all animals are out of the room. After scent application, air the room overnight. This allows excess odor that may permeate the room to dissipate, leaving the signal odor concentrated only on the pot. This also minimizes the potential commingling of odors in the testing environment. Hence, on test day, the scents should not be detectable by the investigator throughout the testing room. Rather, they should be detectable only by holding a pot close to the nose. Moreover, too much scent can be aversive to the rats, deterring exploration and digging, so with scent application, it is better to err on the lesser side. Pot scent should be checked regularly, preferably the day prior to use, as scent retention can be affected by humidity, temperature, airflow, etc.
The selection of digging medium is also dependent on preference and availability. However, there are a few common sense guidelines, such as size, weight and texture. The medium should allow the reward to be hidden, and allow the rat to dig for it inside the vessel. Hence, material that is too bulky or heavy should not be used. When pairing media, they should have comparable, yet distinguishing, features to avoid “bias”. For example, we pair wood balls with plastic beads, and shredded velvet with strips of crepe paper.
For the food reward, most laboratories have adopted the use of cereal products, as they are easily broken into small standardized pieces. Some have used Honey Nut Cheerios (General Mills Cereals, MN, USA)(33–35, 39, 42, 43) while others have used Honey Loops (Kellogg Company, UK)(23, 29, 41, 44). The rounded shape of these cereals allows for division into relatively uniform segments. However, the sizes of these cereals are not consistent, and this has to be considered when deciding on the size of the reward. We use a quarter of a small cheerio, which is adjusted to 1/6th section for larger pieces. Depth of placement below the surface of the digging medium should also be constant, about 2 cm in our procedure. If the depth is too great, they may not persist in digging to reach the reward, and if too shallow they may locate it by smell rather than learning the discrimination. It will become evident that this is occurring if a rats digs immediately in the correct pot without exploring the surface, focuses its digging activity directly and immediately on the location of the reward, and makes no errors, including on the first trial of a new test stage (which, by definition, should be an error). To further minimize this possibility, we sprinkle a very small quantity of cheerio dust onto the medium in both pots, so that a small amount of food scent is present in both. This is only done at the start of each task, unless a pot is tipped over and the medium must be replaced. During the AST procedure, bait is handled with a pair of forceps to allow for easier insertion of the pieces of cereal into the digging medium at a constant depth, and to limit the possibility of contamination. The cereal should of course be stored in a sealed container to keep it fresh. While some rats will eat any food reward, others can be finicky and will not work for stale cereal.
The order of stimulus pairings (each odor with each medium within a task) and the left-right positioning of the correct pot within the arena are pseudo-randomized, but with no more than three sequential trials having the same pairing and positioning of the exemplars. It is very helpful to construct a guide sheet in advance specifying the pairings and positioning for every trial in every task. This can then serve also as the scoring sheet, where the correct or incorrect responses, as well as the time taken for each trial, can be recorded.
In every experiment, half the animals are begun with odor as the discriminative stimulus, and half with digging medium. Anecdotal evidence from some labs suggests that there may be differences in the ability to shift cognitive set in the ED stage when the discrimination begins with odor as opposed to medium. Thus, we always test for any such differences (2-way ANOVA, with initial Stimulus Dimension as one factor, and Task as the second factor with repeated measures). No consistent effect of initial stimulus dimension on subsequent performance, nor on subsequent treatment effects, has been observed in our studies.
Our test arena is constructed of wood covered with a water-resistant white laminate. Acrylic paint would work equally well, and other groups have used plastic or plexiglas (23, 37, 42). The key criterion for selection of material for the arena is water resistance, to prevent absorption of urine or other odors, and to allow for rapid and thorough cleaning using 70% ethanol followed by water. If the rat urinates or defecates during testing, the arena is cleaned with moistened paper towels and dried. Between rats, the arena is cleaned with 70% ethanol followed by water and then dried. The arena is located on a small table, at a height that facilitates cleaning and changing pots while avoiding undue stress on the experimenter’s back.
The testing room should ideally be situated in an area with little traffic, as loud noise may startle or distract the rat and affect subsequent behavior. The habituation and training sessions prior to testing accustom the rats to performing the task under normal ambient noise and lighting conditions (our test rooms have standard overhead fluorescent lighting with diffuser panels). We do not typically use background white noise, but that might be advantageous if extraneous noise is unavoidable. The room should obviously be free from extraneous odors, including any emanating from the experimenter (good hygiene, no perfume, no scented soap, deodorant or lotion!) We routinely conduct AST in at least two different rooms, with varying light intensities (300–425 lux to 600–800 lux). Results have been comparable. Studies that dictate reversed light cycles may require testing to be conducted under red lighting. We have not done this, and are unaware of published studies comparing performance under such conditions.
Published studies have used various strains of rats, including Hooded Lister (23, 29, 40, 41, 44), Sprague Dawley (33–35, 37) and Long Evans (39), with comparable performance in control groups, indicating that strain differences, at least using standard outbred strains, may not be a factor. However, there may be exceptions. For example, we found that the inbred Wistar-Kyoto (WKY) rat, which is characterized by exaggerated hypothalamic-pituitary-adrenal responses to stress (45), was less active on the AST, less likely to explore the arena, and less likely to complete the full sequence of tasks. This is perhaps consistent with reports that WKY rats tend to exhibit behavioral immobility, reduced activity in an open field, and reduced baseline exploratory behavior in several contexts compared to Sprague-Dawley rats (46, 47). Difficulty in even habituating and training WKY rats to perform the AST task meant that this strain could not be tested reliably (unpublished data). Adaptations of the AST have been reported for mice. Some of these employed procedural modifications, such as extending the training over several days or repeating a number of the test stages (e.g., 48, 49). This appears to have been necessary in part to overcome the greater difficulty exhibited by mice in forming a cognitive set (evident as an ID/ED difference smaller than that seen in rats), although this was not observed in all studies (e.g., 50).
Pre-habituation procedures
Two days prior to habituation, pieces of the food reward are offered to the rats in their home cage to familiarize them and reduce neophobia. This also offers an opportunity for verbal and manual interaction between the experimenter and the rats so they will be comfortable with the degree of handling during the test procedure. Rats previously exposed to such handling are easier to habituate and train, and are generally less reactive to extraneous noise and other stimuli on succeeding days. This session also allows the experimenter to gauge the “emotionality” of the rat. “Timid” rats might stay in a corner far away from the experimenter, while more “comfortable” rats will even rear up in the cage near the experimenter waiting for the next food reward. Signs of timidity alert the investigator that the rat may need more time to habituate to the food reward and the experimenter before the behavioral procedure is initiated. The day before habituation, the pots are introduced to the animals in their home cage. The food reward is initially offered, as on the previous day, followed by the introduction of empty pots (i.e., no digging medium) baited with a piece of food reward. One or two pots can be introduced to the rat at this time; the number of pots is not important, as the objective is to familiarize the rat to the pot and to the process of pot removal and re-baiting. Three such introduction trials normally suffice. We found this procedure to be helpful, because some rats may react aversively to the pots when they are first introduced on the habituation day. Some rats may even bury the pots or avoid them. If such defensive or neophobic behavior is exhibited, the pots are left in the cage overnight to allow familiarization and exploration.
Habituation day procedural hints
There are two aims on this day: for the rat to learn to dig for the reward, and to habituate them to the test arena. Prior to receiving their daily amount of food, rats are transported into the testing room in the morning, and allowed at least 1 hr to acclimatize to the room after transporting. Still in their home cage, they are then exposed to two pots with a piece of cereal in the bottom of each. After retrieving the bait from each pot, they are re-baited and the rat allowed to retrieve the bait for a total of three trials. Then for subsequent trials, an increasing amount of sawdust is introduced into the pots. First, one piece of cereal in each pot is covered by sawdust to 1/3 the depth of the pot, and a second, smaller piece of cereal is placed on the surface of the sawdust to entice them to dig. The rat is again allowed to retrieve the bait from both pots, and then the pots are re-baited and the procedure repeated for a total of three trials. This procedure is repeated with the pots filled to 1/2 their depth, and the reward buried 2 cm below the surface, followed by three trials with the pots filled completely with sawdust. If a rat fails at any stage to retrieve the bait from both pots within 5 min, that trial is repeated until they do so. When the rat is thus digging reliably and retrieving the bait consistently from both pots within 5 min, it is transferred to the test arena.
In the arena, the rat is initially placed in the holding area. The barrier is lifted to give them access to two baited pots, one located in each of the divided sections at the far end of the arena (see Figure 1). As in the preceding home cage trials, a small piece of cereal is placed initially on the surface, and one is buried to 2 cm under the surface of the sawdust of each pot until the rat digs reliably. If a rat fails to dig at all, it is ‘assisted’ by the experimenter indicating where the reward is hidden with a forceps. The sawdust is then replaced, and the rat is allowed another opportunity to dig for the bait. Habituation is complete when the rats have retrieved the reward from both pots on three consecutive trials. Most rats, after exhibiting a slight aversive reaction when the procedure first begins, e.g., defecation and/or urination, eventually dig reliably in both pots. However, rats occasionally exhibit excessive aversive reactions and fail to participate, e.g., they stay in a corner. In such cases, steps that we have used to prompt the target behavior include offering a piece of food reward directly to the rat, then “leading” them to the pot with it; or instituting a “time out” period of 30 min, during which the rat is returned to their home cage with the baited pots, and the experimenter leaves the room before re-introducing them to the arena. The full habituation procedure usually takes about 90–120 min, after which the rat is handled for 2 min, then returned to the home cage and given the 14 g food ration for the day.
Training day procedural hints
In the morning, prior to the daily feeding, the rat to be trained is transported from the housing facility to the testing room and given a 1 hr acclimatization period. It is then transferred to the arena and allowed to dig for reward buried in two sawdust-filled pots, a reminder of the last task in the preceding habituation session. They are then trained in the two successive discrimination tasks, first between two odors and then two digging media, as outlined in the Methods section. We begin each trial by first diverting the rat’s attention away from the movable gate by tapping on the back wall of the arena, as some rats may demonstrate a side-bias if they begin a trial while facing the barrier.
From this point on, the criteria for judging a “dig response”, as described in the Methods, are applied rigorously. Digging involves the displacement of medium to locate and retrieve the reward. The rats are allowed to investigate the rim or surface of the medium with paws or snout without being scored as a dig, as long as no displacement occurs. The latency to dig in any of the pots is recorded, up to a maximum of 10 min. Pilot experiments have shown that for most control animals, a dig usually occurs within a few seconds up to a minute after release from holding area. Hence, a “non-dig” after 10 min is scored as an error. If a rat remains inactive without investigating either of the pots after 5 min, it is gently led back to the holding area, which normally stimulates activity, and the trial continues from that point up to 10 min.
We routinely analyze and compare the number of trials to criterion measured on these training tasks, to determine if there may be differences between the treatment groups in the ability to acquire the simple discrimination, which could confound any apparent differences seen in subsequent tasks on the testing day. In addition, we note the latency to dig for each trial, and the total time for the animals to finish each task and the entire training session. These data may also be useful in revealing potential non-specific treatment effects. At the end of the training session, the rats are again handled briefly (2 min), replaced in their cage, given their daily food ration, and returned to the housing facility. At this point, it is useful to check the pots to be used for the testing day, to assure that there is no accumulation of cheerio dust in the bottom and that they are adequately scented, and re-scent if necessary.
Testing day procedural hints
Unfed rats are brought to the testing room in the morning and acclimatized for 1 hr. The testing procedure is as outlined in the Methods section. Scoring is similar to the training session. The most critical aspect is to be able to reliably score a “dig”, which comes with experience, and to maintain vigilance and alertness through the entire test period so as not to miss a response. Pilot studies should be used to train new investigators and provide experience at scoring. Other important practices to learn and employ consistently throughout the procedure include: draw the rat’s attention away from the barrier before opening for each trial to avoid any side bias, as in the training session; be sure the pots are completely filled with digging medium before each trial, as material can be scattered when the rat digs; and clean the arena after every trial. If the digging medium is displaced from a pot during a trial, it can be returned to the appropriate pot, but not if contaminated with feces or urine. Finally, clean the arena with 70% ethanol followed by water and then towel dry after every animal is tested.
As in the training session, the rats are allowed to investigate the rim or surface of the medium with paws or snout without being scored as a dig, as long as no displacement of the medium occurs. Latency to dig is recorded, up to a maximum of 10 min, after which the failure to dig is scored as an error. If a rat has not approached either pot after 5 min, it is led back to the holding area, which normally stimulates activity, and the trial continues up to 10 min. If 6 consecutive non-dig trials occur (i.e., one hour elapses with no attempt to dig for a reward) the test is terminated and that rat is eliminated from the experiment. This rarely occurs in un-manipulated control rats, but we have encountered this on occasion after certain treatments.
AST on operated animals
We have utilized this assay to investigate the effects of various stressors on cognitive function, and the involvement of central neurotransmitters (33–36). Pharmacological manipulations have included systemic drug administration, as well as local microinjections directly into brain targets (33–35). In other studies, our test system was adapted to allow for simultaneous collection of microdialysate samples during behavioral testing to assess possible changes in extracellular neurotransmitter levels in the prefrontal cortex during performance of specific tasks. Surgeries to implant intracranial guide cannulae and probes have been well tolerated and rats prepared thusly can be tested (see 33, 34, 35). As for most behavioral experiments, sufficient time must be allowed for recovery after surgery to allow reliable testing. In our studies, the rats are operated at least two weeks before AST testing. This allows one full week of un-manipulated recovery, and the second week comprises the time required for the 7-day food restriction before testing. Similar timing factors must be considered when planning any experiment requiring chronic or invasive treatments. Of course, any treatment that interfered with olfaction or tactile sensation would be incompatible with the AST.
In summary, as a rodent test of cognitive executive function modeled after the WCST, the AST has proven useful to assess prefrontal cortical function. More recently, used in conjunction with stress manipulations, we have used the AST as a model to assess cognitive aspects of depressive-like symptomatology. Although labor intensive, the AST has proven to be a reliable, adaptable and informative assay. Further, it has tremendous potential as a novel tool to study heretofore inaccessible constructs, such as dysregulation of prefrontal cortex in disease states.
The Elevated-Plus Maze (EPM)
A behavioral assay that we have used extensively in our studies of acute behavioral stress reactivity is the elevated plus maze (EPM) (46, 51–54). Montgomery (55) first reported that when animals were placed in a home cage and given access to either an open maze alley or a closed maze alley, they spent more time exploring the closed arms. This was characterized as an approach-avoidance conflict (55). Based on this, Handley and Mithani (56) later devised an adaptation using an elevated maze in the shape of a plus, having two open arms and two arms enclosed by walls, with the like arms situated opposite each other (Figure 3).
Figure 3.
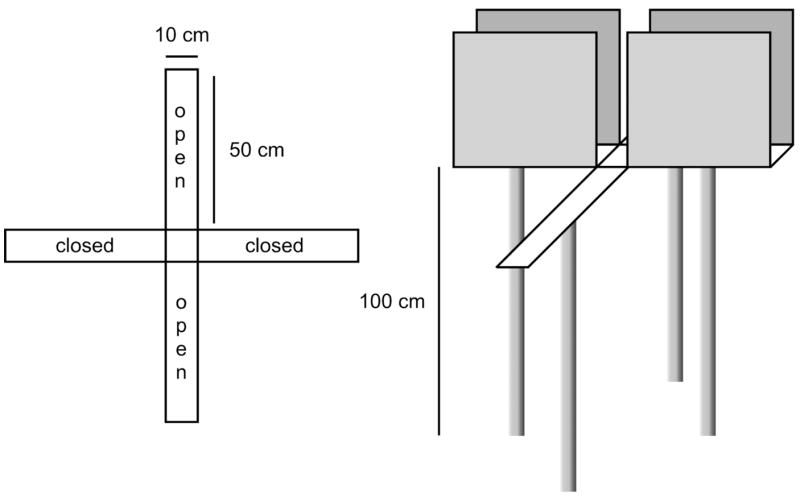
Schematic diagram of a typical elevated plus-maze apparatus.
The EPM test has several characteristics that make it particularly useful. It reliably detects anxiolytic and anxiogenic activity of a variety of therapeutic and experimental drugs of different classes (57, 59). Unlike models that require extensive conditioning, it relies on an innate conflict between competing “drives”, the balance of which is affected by the level of anxiety. Thus, it requires no training, deprivation, pain or aversive stimuli. The response involves the redirection of an ongoing activity (i.e., exploration) rather than the suppression of behavior, which could be confounded by sedation or ataxia. Both the conduct of the test and the theoretical concepts behind it are fairly simple. A motivational conflict is established in which the innate tendency of rats to explore a novel environment is opposed by their innate fear of open spaces (59), and the behavior of the rat on the apparatus represents a balance between these opposing drives. Manipulations that increase anxiety bias behavior away from exploration of the novel open space, reducing the drive to explore the open areas and at the same time increasing the drive to avoid them. Anxiolytic manipulations reduce the drive to avoid the open spaces, and increase the proportion of time spent exploring the open arms. Thus, a change in anxiety is inferred from the extent to which the animals explore the open arms relative to their overall exploration of all arms, typically expressed as a percentage or ratio (the Open-to-Total Ratio, or OTR). These ratios are calculated by dividing the amount of time spent in the open arms by the amount of time spent in the open + closed arms (OTR for Time), or by dividing the number of entries made into the open arms by the total number of entries into all four arms (OTR for Entries). Time spent in the center platform, i.e. not exploring any of the arms, is not considered in these calculations. These proportional measures thus normalize for different levels of overall exploratory behavior, and obviate effects attributable solely to differences in locomotion, due either to individual variation or to non-specific treatment effects. In addition to rats, mice have also been tested on the EPM (61–64), but the dimensions of the maze are scaled accordingly, and baseline OTR tends to be much lower in mice than in rats. However, as our experience is only with rats, this description of methodology and procedural hints will be limited to rats. Modern EPM systems have not deviated significantly from the original design of Handley and Mithani (56) (see Figure 3), but automated scoring methods now allow greater objectivity and standardization between laboratories (see below). It is worth noting, however, that a variation of the EPM has been devised, the “elevated-zero maze”, comprising a circular maze with opposing closed and open quadrants, to allow continuous exploration and also to obviate any potential ambiguity in interpreting time spent in the central area of the EPM (65).
Validity of the EPM
As for most tests of anxiety-like behavior, validation of the EPM has relied primarily on pharmacological demonstration of sensitivity to known anxiolytic and anxiogenic drugs. A number of benzodiazepine and non-benzodiazepine anxiolytics (e.g., chlordiazepoxide, diazepam, phenobarbitone, tracazolate, CL 218 872), along with several anxiogenic compounds (e.g., pentylenetetrazole, yohimbine, caffeine, FG 7142, CGS 8216, Ro 5-4864), have been used to establish the phamacological validity of the EPM (57, 66). Anxiolytic drugs increase the proportion of open arm exploration relative to total exploration. By contrast, anxiogenic agents reduce open arm exploration.
In a study addressing construct validity, rats confined to an open arm of the EPM had elevated plasma corticosterone levels compared to controls (57), confirming the aversive quality of the open arms. However, it is important to remember that exploration of the open arms in the normal context of EPM testing is most likely not as aversive as this observation alone might suggest. The rats in that study were confined to the open arms and could not “escape”, whereas during EPM testing, the rats freely choose to enter, exit and explore the open arms. Thus, while there is certainly an aversive quality to the open areas that balances the drive to explore (67), it is debatable whether the test procedure itself provokes anxiety in order to assess it, or if the relative degree of exploratory behavior in the open areas is instead a more subtle reflection of the state or degree of anxiety that exists in the rat at the time of testing.
Using data from several independent studies, our lab and others have conducted principal components and factor analyses confirming that OTR for Time and OTR for Entries co-vary, and that they are representative of a single anxiety-related dimension that is different from that measured on other tests (e.g., social interaction test). By contrast, total arm entries and closed arm entries load onto a different, locomotion-related factor that covaries with other independent measures of locomotor activity (46, 51, 56–59, 68).
An important consideration in designing experiments, especially those that may require chronic or sequential treatment and testing, is that rats can be tested only once using the EPM. More precisely, the dependent measures of anxiety-like behavior have been validated only for the first test exposure. Open arm avoidance on the first test appears to be determined primarily by the open nature of the arms (69). By contrast, if animals are tested a second time, their behavior is thought to reflect a different construct than that measured on the first test (70), although OTR values may be similar. It has been suggested that elevation rather than openness may influence open arm avoidance in a second test exposure (71), but this has yet to be established definitively (see 60). Moreover, open arm exploration on the second test is insensitive to benzodiazepine treatment (69, 71, 72).
One issue that has been problematic for many investigators using the EPM has been the variability in baseline behavior. Certainly, many factors contribute to determining baseline exploratory behavior, not all of which can always be recognized or controlled by the investigator. However, we have found that using the EPM to assess acute stress reactivity, for instance by measuring the change in behavior evoked by exposure to a mild acute stressor, such as a brief immobilization stress, can be more informative and reliable than simply measuring differences in baseline exploration between treatment groups (Figure 4). In other words, an acutely evoked change in open arm exploration may be replicable and consistent, even if a given treatment causes a change in baseline exploration. In essence, this means using two “control” groups, an unstressed “baseline” control, and a group that is untreated but exposed to the acute stimulus prior to testing. The difference between these two groups is then indicative of the acute anxiety-like response to stress, regardless of differences in baseline across experiments. The difference between these two groups can then be compared after chronic drug treatment, chronic stress, surgical intervention, local drug microinjections, etc. For example, we showed that exposure to 5 min of acute immobilization stress, administered 20 min before EPM testing, elicited a replicable and robust reduction in OTR on the EPM, and that NE release in the lateral bed nucleus of the stria terminalis facilitated this behavioral response to acute stress, but had no effect on unstressed baseline exploratory behavior (51). We have since used this acute stress response to investigate changes in the modulatory role of brain NE in a number of physiological and pharmacological contexts (35, 46, 51–54).
Figure 4.
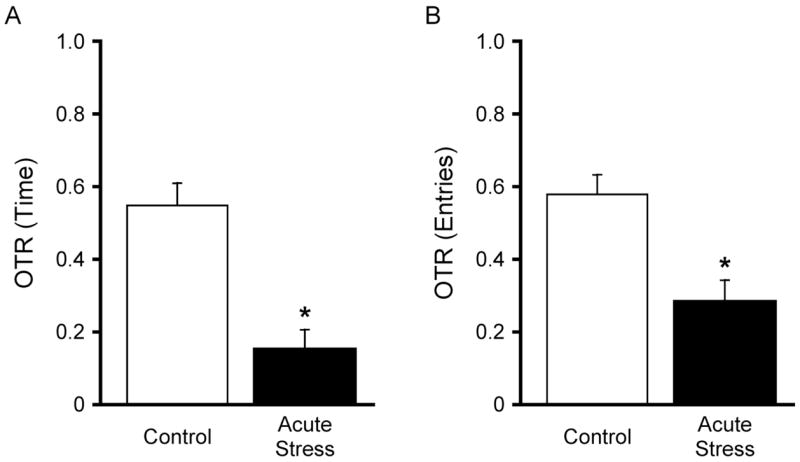
Results of a representative experiment showing the anxiogenic effect of acute stress on exploratory behavior in the open arms of the EPM. Acute immobilization stress (5 min), administered 20 min before testing, significantly decreased both OTR for Time (A) and OTR for Entries (B) compared to unstressed controls (*p<0.01, n=5–8). In this study, all rats had been previously implanted bilaterally with guide cannulae aimed at the central nucleus of the amygdala, and all received bilateral vehicle microinjections (0.25 μl/side) 3 min before acute stress exposure, or at an equivalent time (23 min prior to testing) in the unstressed controls. Adapted and reprinted, with permission from Elsevier, from (52).
Method and Procedural Hints
Apparatus
For testing rats, the typical EPM consists of four arms, 10 x 50 cm, intersecting at a central platform, 10 x 10 cm (Figure 3). The two “open arms”, situated opposite each other, are essentially flat planks extending into space, and the other two opposing arms are enclosed by walls. In our apparatus, these walls are 48 cm in height, but the key feature is that they should be high enough that the rat cannot explore “above” the walls by rearing, and therefore has the sense of being enclosed. Mazes can be constructed of wood, metal or plastic. Wood offers the advantage of giving the animals more secure footing so they don’t fall off the maze in the open arms, but it has the disadvantage of being more difficult to clean thoroughly. By contrast, acrylic or other plastics are easy to clean, but can be slippery (see 60). Our maze is made of white acrylic, and we found that affixing a small clear 0.5 cm plastic rim to the edges of the open arms prevented animals from slipping and falling off when they turned around in the open arms. Addition of such a rim has been reported to alter the nature of avoidance behavior in the open arms, and to reduce sensitivity to anxiolytic drugs (71), but this has not been examined extensively (see 60). After addition of the rim to our apparatus, we observed no obvious differences in baseline behavior or in the OTR of control rats, except that fewer animals had to be eliminated from our experiments for falling off the maze.
The color of the maze may be a factor, although that also has not been studied at length. However, a practical consideration we have encountered when video-recording is that it is more difficult to establish video contrast when recording the behavior of a white animal on a white maze. For a different reason, it is also important to minimize the visual contrast between the maze floor and the laboratory floor below the maze. When we first set up our EPM, this contrast was high (white maze on a tan tile floor). Rats frequently jumped from the open arms of the maze, resulting in elimination from the experiment. Placing a white acrylic sheet on the floor below the maze to minimize this contrast eliminated the problem entirely. A similar consideration applies when establishing the elevation of the maze, typically 50–100 cm above the laboratory floor. The elevation should be sufficient to prevent the animals from perceiving the presence of the floor below the maze (see 60). Our maze is elevated 100 cm above the laboratory floor, and experiments are conducted during the light portion of the cycle, with normal fluorescent overhead ambient lighting (~300–450 lux in closed and open arms). Although lighting and time of day have not been shown to influence behavior on the EPM (57, see 60, 73), we test all rats within the same range of time each day (~09:00–13:00), which is sufficiently separated from light-dark transitions (lights on at 07:00, lights of at 19:00) to avoid any potential diurnal variations in exploratory behavior. Background white noise (60 dB) is used to dampen extraneous noises that may distract, interrupt or arrest exploratory behavior.
Procedure
The EPM testing procedure requires no pre-training or pre-conditioning of the animals. However, handling the animals prior to the testing day can influence baseline behavior and even subsequent drug effects (74). For most experiments, it is desirable to generate a baseline level of open-arm exploration in control rats that will allow both increases and decreases to be detected in treatment groups. We have found that handling the rats for 1 min on each of 3 days prior to the testing day generates an optimal level of baseline exploration in controls, with OTR typically between 0.40–0.50 (46, 51). In addition to handling, factors such as gender, strain, and single- vs. group-housing may also influence baseline behavior (see 60). Manipulations specific to a given experiment, such as cannula implantation into specific brain areas, can also alter baseline behavior, so it is always useful, when initiating a new experiment or procedure, to compare appropriately sham-treated controls to a group of un-manipulated controls to assess any such non-specific effects.
After rats are delivered to our housing facility, at least one week is allowed before beginning any experimental procedures, including handling. On the test day, rats are transported in their home cages to the testing room, and allowed 20–40 min to acclimatize prior to testing. If too little time is allotted for this purpose, behavior on the EPM can be erratic, most likely due to the disruption of transporting. However, if the rats are left too long, especially when testing during the light portion of the cycle, we have observed that the rats will often sleep during the latter part of this acclimatization period. When awakened for testing, they can exhibit a startle response, resulting in an artificially low OTR unrelated to their treatment condition.
For testing, the rat is removed gently from its home cage and placed in the center platform of the EPM, facing the junction of an open and closed arm. The experimenter backs quietly away from the arena, and observes the rats behavior remotely, either on a video feed, or directly from behind a screen to shield them from the rats view. The rat freely explores the maze for 5 min, during which behavior is recorded for offline analysis using a camera mounted on the ceiling, or on a frame above the apparatus.
After testing each rat, debris (bedding, feces, etc.) is removed from the maze, and all interior surfaces, walls and floors, are cleaned thoroughly, first with 70% ethanol, then water, and then wiped dry. Allowing at least 5 min between tests ensures complete drying and dissipation of any residual odor of alcohol before the next rat is introduced into the maze.
The primary dependent measures collected on the EPM are the time spent in each area of the maze (central platform, open arms, closed arms), and the number of entries made into each area. Some investigators also record other variables to enhance analysis and interpretation, including distance traveled and number of end-arm entries, indicators of risk assessment such as stretch attends and head-dips (exploration under the edges of the maze arms), and rearing frequency (68, 71). The primary data for time and entries are then used to calculate the proportional measures of open arm exploration relative to total exploration: the OTR for Time (time in open arms divided by time in open + closed arms), and OTR for Entries (number of entries into open arms divided by entries into open + closed arms). These variables are then analyzed to assess changes in anxiety-like behavior. Closed-arm entries and total arm entries are analyzed as measures of locomotion independent of anxiety (51, 67, 68).
Thus, to obtain reliable data, it is essential to establish a consistent, objective definition of what constitutes an entry, and to apply that criterion strictly to each transition from the center into an arm. Entries have been defined by various criteria, usually by all four paws crossing into an area. However, assessing this can be difficult, especially when rats linger at a boundary between the center area and an arm, or when the rats exhibit a form of risk-assessment behavior called a “stretch-attend” at the boundaries between areas. In this case, the rat extends the front portion of its body far into one area, while keeping one or both hind paws planted just inside the other area, and often executes several rapid back and forth excursions from this position.
Automated video tracking software can standardize the determination of an entry to some extent, and there are many options available. We use the ANYmaze system (Stoelting Co., Wood Dale, IL, USA). With this system, a single contiguous area comprising the rats body is defined by contrast with the background. In our current application, an entry is then scored when 80% of the body has crossed from the center area into a maze arm. To avoid “flickering”, the spurious scoring of rapid repetitive entries when a rat remains on or near the boundary, an exit is scored only if 55% of the rats body has left the maze arm, after which 80% must again enter to be scored as a new entry. When a rat is determined to be in an arm, the measure of time spent in that arm accumulates until an exit occurs. If a rat is not in an arm, it is by definition in the center, and the total time spent in all five areas (4 arms + center area) must equal 5 min.
We have also used a system employing dual infrared sensor beams positioned at the entry of each arm of the maze (AccuScan Instruments, Inc., Columbus, OH, USA). The definition for an entry was more stringent using this system, as the rat had to break the first beam, then break the second beam situated further in the arm, then release the first beam in order to be defined as being “in” the arm. Then the reverse sequence had to occur to define an exit out of the arm and back into the center. Nonetheless, when we directly compared these two systems, measuring the behavior of the same rats, and also compared these data to manual scoring in the same tests, the results were remarkably similar, especially for the proportional OTR values. Thus, the specific method for scoring is probably not important, as long as the criteria established to define when an animal has entered and exited an arm are applied consistently, stringently and objectively.
However, even with automated scoring methods and objective criteria in place, it is still important for an experimenter, blinded to the treatment condition, to monitor the behavior of the rats throughout the EPM test, and to record any notable or unusual behavioral observations, such as excessive running from arm to arm, stereotyped behavior at the end of an arm or at a boundary, excessive immobility, etc. The proportional OTR measures normalize for non-specific differences in overall exploratory behavior or locomotor activity. However, we have found that extreme levels of locomotor activity, especially extreme inactivity, can confound the OTR measures. This creates a situation wherein the presence of the rat in an arm may be dissociated from anxiety and exploration, for instance if an animal freezes immediately upon entering an arm, irrespective of whether it is open or closed. Likewise, if an animal is sedated, such that exploratory behavior slows or stops during the test, OTR may not be a reliable indicator of exploration and anxiety-like behavior. We encountered such a situation while studying stress-induced behavioral responses in Wistar-Kyoto rats, a strain that exhibits a high degree of behavioral inhibition and passive immobility in a number of test environments (46). In cases such as this, analysis of the independent measures of locomotion (closed-arm entries and total entries) can provide important clues that there may be a confound, allowing an appropriate degree of caution to be applied in interpreting the OTR data. In our experience, such extreme reductions in locomotion, independent of exploration in specific areas of the maze, will sometimes generate a somewhat bimodal distribution of high and low OTR scores within a given experimental treatment group. In such cases, any interpretations of differences in anxiety-like behavior relevant to specific treatment effects are likely to be difficult if not impossible.
Social Interaction (SI) test
Similar to the EPM, the SI test is an assay for rodent anxiety-like behavior developed to meet several explicit criteria: 1) sensitivity to both anxiogenic and anxiolytic stimuli; 2) sensitivity to pharmacologic, physiologic and environmental manipulations; and 3) measurement of ethologically relevant behaviors that do not require potentially confounding manipulations like food deprivation, pain, or conditioned fear (75–77) (see 78). Based on the premise that anxiety is incompatible with social behavior, the SI test has been widely used and extensively validated as a rat model of anxiety-like behavior fitting these criteria (75, 76, 79). The dependent measure in this assay is the amount of time a test animal spends engaged in active social behavior (e.g., sniffing, approaching, following, communal grooming, climbing on or under, etc.) with an unfamiliar “stimulus” rat. An increase in anxiety results in a decrease in social interaction time (75, 76). More specifically, as with the EPM, we have used the SI test as an indicator of anxiety-like behavioral reactivity provoked in response to a mild acute stressor (46, 51, 52, 54). Several excellent resources are available for more in depth review of the SI protocol and validation (60, 76, 78).
Validity of the SI test
Like the EPM and other models of anxiety, the SI test has been validated by demonstrating sensitivity to known anxiogenic and anxiolytic compounds (see 78). In particular, benzodiazepines exert anxiolytic effects on the SI test, increasing social interaction in anxiogenic contexts. However, caution must be exerted when testing benzodiazepines or other drugs that can induce sedation, especially administered acutely, as that can decrease social interaction non-specifically by reducing locomotor activity (see 78). Sub-chronic 5-day treatment with benzodiazepines has been shown to increase social interaction with no sedation (75, 76, 80), whereas chronic treatment for 21 days or longer can lead to tolerance (81), and even anxiogenic effects (82). Benzodiazepine antagonists induce anxiogenic responses acutely (83), but also prevent the anxiogenic effects of prolonged benzodiazepine treatment (84).
Non-benzodiazepine reagents have also been investigated. For instance, barbiturates were found to have anxiolytic effects in the SI test (see 78). Ethanol, on the other hand, has been shown to have a biphasic effect, with low doses facilitating and high doses inhibiting social interaction (85). The SI test is sensitive to anxiolytic and anxiogenic effects of alpha-2-adrenoceptor agonists and antagonists (e.g. clonidine and yohimbine, respectively), and also to serotonergic compounds (5-HT1A, 5-HT2 and 5-HT3), neuropeptides (e.g., corticotrophin-releasing factor, ACTH, cholecystokinin, substance P, neuropeptide Y, galanin), and antidepressant drugs (for review, see 78).
File and Hyde (75) showed that decreases in SI behavior correlated with other validated measures of anxiety-like behavior, such as freezing, defecation and displacement activity (e.g., extensive self-grooming, impulsive eating of inedible objects by rats that are not food deprived, etc.). Two important factors involving the testing environment have been shown to reliably influence behavior on the SI test: lighting and familiarity/novelty of the testing arena (75, 76). Testing in a novel, brightly lit arena induces low levels of baseline SI behavior, whereas a familiar, dimly lit testing environment enables the highest baseline SI (75). These environmental factors can be varied systematically to induce baseline levels of SI behavior that are more conducive to detecting anxiolytic treatment effects (i.e., starting from a low SI baseline) or anxiogenic effects (i.e., starting from a high SI baseline). In our studies, to avoid potentially confounding effects induced by novelty of the testing apparatus, experimental subjects are habituated by pre-exposing them individually to the testing arena for 5 min on each of 3 days immediately preceding the test day. Thus, at testing, the environment itself is not novel, but the presence of an unfamiliar conspecific is, thereby comprising the approach-avoidance conflict that determines social behavior. Subsequent studies have shown that acutely stressful or fear-provoking stimuli, e.g., immobilization stress (46, 51, 52, 54) or predator odor stress (86, 87), reduce social interaction relative to unstressed control levels.
Other pre-testing factors capable of influencing social behavior on the SI test include housing conditions (75, 88, 89), handling (76), gender (90, 91), and strain (46, 91, 92). Noisy housing conditions have been found to significantly reduce social interaction, regardless of the testing context (88). Perhaps surprisingly, isolation housing has been reported to increase social interaction, with maximal effect seen after 4–7 days (89). Thus, it has become common practice to house animals individually for at least this period of time prior to SI testing (78). Results obtained with the SI test appear to be less consistent using female rats (90, 91), and familiarizing females with the test arena does not increase SI to the same extent as it does in male rats (90). Inbred Wistar-Kyoto rats have been found to express very low levels of baseline SI behavior (46), whereas hooded Lister and Sprague-Dawley rats exhibit higher baseline SI (46, 60, 78).
Versions of the SI test have been adapted for mice (see 78, 93). The SI test has been useful in behavioral phenotypic characterization of genetically modified and inbred strains of mice (e.g., 93). However, some anxiogenic and anxiolytic drug treatments that exert the expected effects on SI in rats have not always produced comparable effects in mice (78). Also, factors such as lighting and familiarity with the test environment have not been found to have the same influence on SI behavior in mice as they do in rats (78). Thus, the SI test has perhaps not been as extensively or convincingly validated as a measure of anxiety-like behavior in mice.
Method and Procedural Hints
Our general protocol is as described in Cecchi et al. (51, 52) and Pardon et al. (46). Briefly, male rats, typically weighing 200–250 g upon arrival in the animal facility, are initially group housed (2–3 animals per cage) and allowed one week to acclimate before any procedures or treatments are initiated. Animals are maintained on a 12-h light cycle under regular fluorescent lighting and given food and water ad libitum. Following the acclimatization period and/or any subsequent surgical procedures, animals are individually housed for 7 days prior to SI testing. During this period, the experimental rats are gently handled for 2 min on each of 5 days preceding SI testing. In addition, they are individually familiarized with the testing room and SI arena for 5 min on each of 3 days immediately before testing (on these days, the animals can be handled first, then immediately given their 5 min habituation exposure to the SI testing arena).
If lighting is to be manipulated as a variable, for instance to establish a high-anxiety context, it is important that the rats are not habituated to this stimulus by housing in a brightly lit room (76). Our standard test conditions include a relatively brightly lit testing room (~360 lux). However, our rats are housed and tested in similar daytime lighting conditions, and they are familiarized with the SI arena prior to testing, both of which serve to reduce baseline anxiety-like behavior and generate higher baseline SI (see 78).
Apparatus
The SI arena is a wooden box, 60 x 60 cm, with 40 cm walls. It is painted with white enamel, and has grid lines marked every 10 cm on the floor with permanent marker to allow measurement of locomotion by the number of grid lines crossed during the test. To prevent the gridlines from fading or washing off, a thin plexiglas sheet is fitted over the floor of the arena, and the edges are sealed to the walls using a latex sealant. This greatly facilitates cleaning the arena between each test. The interior of the arena is somewhat reflective, which can make it difficult to videotape behavior due to glare. Thus, it is helpful if the room lights can be fitted with suitable diffuser panels. Alternatively, sheets of thin, flexible, semi-transluscent material (e.g. a bench-top diaper pad or a thin white shower curtain) can be affixed over the lights to minimize glare. In our studies, testing is conducted during the light portion of the cycle, between 09:00–14:00 hr, in an isolated room with 60 dB background white noise and normal overhead ambient lighting, ~360 lux measured in the center of the SI test arena. We have not explored differences in SI behavior when tested in the dark phase of the light/dark cycle, but others have reported that baseline SI behavior is elevated (94).
In many published studies, two experimental animals from the same treatment group have been tested together and scored as a single unit (e.g., 75, 95, 96–98). This may offer an economy of time, resources, and rat numbers. However, in our view, this also runs a potential risk of exaggerating effects somewhat artificially (e.g., a treatment that only slightly impacts social behavior may have a seemingly larger and more significant effect if the social behavior of both animals is affected). Thus, we test only one experimental subject at a time, and pair them with a “stimulus” rat matched to the test subjects for age, gender and body weight (within ± 10g). Practically speaking, to achieve this matching, a group of stimulus rats are purchased from the same lot and shipment as the experimental animals, usually 6–8 stimulus rats for every cohort of 24–30 test subjects. These stimulus rats essentially comprise a component of the SI test apparatus, and as such, it is important that their behavior be as constant as possible. Thus, prior to SI testing, the stimulus rats are group housed (2–3 rats per cage) and familiarized with the test arena (5 min per day for 3 days prior to testing). However, unlike the experimental subjects, stimulus rats are pre-exposed to the arena together in pairs, taken from different home cages, and with a different partner on each pre-exposure day. The purpose of this is to familiarize the stimulus rats not only with the testing environment, but also with the experience of encountering novel conspecific rats in that environment, to ensure that their behavior is constant during testing. However, we have also observed that the stimulus rats can become territorial with repeated testing in the arena, exhibiting aggressive behavior toward the test rats and inhibiting social interaction. Thus, each stimulus rat is used for no more than 2 test sessions per day, and for no more than 4 test days. Any signs of aggression or territorial dominance upon introducing a test subject into the arena is a signal to withdraw that stimulus rat from further participation.
Procedure
To begin testing, the experimental and stimulus rats are placed at opposite corners of the arena facing away from each other. It is of course important to differentiate the two rats, as they will be age-, gender- and weight-matched. We typically mark the backs or tails of the stimulus rats with an indelible marker, with a color reserved solely for stimulus rats, especially if the experimental test rat is also marked for identification purposes. The 5-min test is recorded by a video camera for offline analysis by an experimenter who is blinded to the treatment condition of the test animal. Only the time spent in active social behavior that is initiated by the experimental animal (e.g., sniffing, climbing on, crawling under, following, grooming, wrestling) is scored. Time spent simply in contact, for instance if the two rats lay down next to each other, but with the attention of the test rat clearly oriented away from the stimulus rat, is not counted. In addition to SI time, the number of gridlines crossed by the test rat is scored as an indicator of changes in locomotor activity. However, factor analyses have shown that locomotion is a component of social behavior on this test, and these measures can covary to an extent, so the number of line crossings in this test is not entirely independent of anxiety (46, 51, 75, see 78).
After each test, any debris (bedding, feces, etc.) is removed from the arena and all interior surfaces, walls and floor are cleaned thoroughly, first with 70% ethanol, then with water, and then dried. Allowing at least 5 min between tests ensures that any residual odor of alcohol will have dissipated before the next rats are introduced into the test apparatus.
With the procedures and conditions described above, using adult male Sprague-Dawley rats as both test subjects and stimulus rats, we typically observe baseline SI scores of about 60–120 sec in unmanipulated control groups (46, 51, 52, 54). Further, as with the EPM, we have demonstrated a replicable reduction in SI time after exposure to a mild acute immobilization stress (Figure 5) (46, 51, 52, 54).
Figure 5.

Results of a representative experiment showing the anxiogenic effect of acute stress on social behavior in the SI test. Acute immobilization stress (5 min), administered 20 min before testing, significantly decreased SI Time compared to unstressed controls (*p<0.01, n=6–8). In this study, all rats were implanted bilaterally with guide cannulae aimed at the central nucleus of the amygdala, and all received bilateral vehicle microinjections (0.25 μl/side) 3 min before acute stress, or at an equivalent time (23 min prior to testing) in the unstressed controls. Adapted and reprinted, with permission from Elsevier, from (52).
The Shock Probe Defensive Burying Test (SPDB)
The behavioral measures of anxiety discussed in the preceding sections each represent an inhibition or redirection of ongoing exploratory behavior, either open-arm exploration or social interaction. However, acute stress can invoke a wide variety of species-specific defensive behaviors which, depending on the context, comprise the “fight, flight or freeze” response. Thus, in investigating the neural substrates of anxiety-like behavior, it may be necessary or desirable to also examine a response that represents the de novo elicitation of an active behavior, provoked specifically by a definable stimulus and directed specifically toward that stimulus, rather than an inhibition of ongoing behavior. One such active behavior is defensive burying (99, 100), a species-specific, innate response exhibited by rodents in the field to an intrusion of predators or other potential threats into their burrows (e.g., scorpions, snakes, etc.) (reviewed in 100). Based on this observation, the shock-probe defensive burying (SPDB) test has now been established as a well-validated and ethologically relevant laboratory model of rat defensive behavior (101, 102).
Indeed, the SPDB test affords a unique opportunity to investigate two qualitatively different types of anxiety-like behavioral responses. After receiving a shock from an electrified probe, rats typically exhibit both an active burying response and a passive immobility response in varying proportions (100, 103). The active burying that is exhibited is directed specifically to the shock-probe stimulus, and is not observed in the absence of the shock (101, 102, 104). This obviates the potential problem of non-specific treatment effects that may alter ongoing baseline behavior, as could occur in the EPM or social interaction tests.
Validity of SPDB
A variety of anxiolytic agents have been found to attenuate shock-probe burying behavior in a clinically relevant dose-dependent manner, including benzodiazepines (diazepam, midazolam, chlordiazepoxide) and serotonergic drugs (buspirone, 8-OH-DPAT, indorenate, alnespirone). The decrease in defensive burying induced by these agents was blocked by benzodiazepine/GABA-receptor antagonists and serotonin (5-HT)1A-receptor antagonists, respectively. The anxiolytic-induced suppression of defensive burying was not a result of behavioral sedation, analgesia or general motor impairment. By contrast, burying was increased by anxiogenic agents, including the α2–adrenergic receptor antagonist, yohimbine, corticotrophin-releasing hormone (CRH), and benzodiazepine receptor inverse-agonists (see 100). Non-anxiolytic agents, such as morphine, barbiturates, or antipsychotics, had no consistent or specific effect on defensive burying behavior.
Burying time increases proportionally with shock intensity, over a range of 0.5 to 10 mA DC, although extremely high shock levels can mask anxiolytic drug effects (102). Consistent and reliable findings have been obtained using 1–2 mA DC shocks (103, 105–108). Shock-probe exposure increases autonomic and endocrine indicators of fear/anxiety, such as heart rate, blood pressure, plasma norepinephrine, epinephrine, prolactin, ACTH and corticosterone (103, 109), although the magnitude of physiological response reflected in these measures is less pronounced in comparison with other laboratory stressors (e.g., foot shock, restraint, etc) (100). Shock-probe induced elevations in plasma catecholamines and corticosterone were attenuated by benzodiazepines, despite a seemingly paradoxical increase in immobility (110), whereas 5-HT1A receptor agonists did not affect these stress-related hormonal measures (111). In rats that were prevented from exhibiting the active behavioral burying response and were forced instead to adopt only the passive immobile response, by providing them with just enough bedding to cover the bottom of the test cage, activation of the HPA axis during exposure to the shock-probe was significantly higher than in rats given the normal amount of bedding and allowed to bury the probe (103, 110, 112). This suggests that the active behavioral response is more adaptive and reduces stress more effectively than the passive response.
Neuroanatomical circuitry shown to be involved in mediating the defensive burying response in the SPDB test includes the lateral septal region, the dorsal hippocampus, and the dorsal raphe nucleus (100). The lateral septum (LS) is particularly important in the active burying response, as electrolytic or excitotoxic lesions of the LS in rats decreased defensive burying behavior (113). Microinjections of the GABAA agonist, muscimol, directly into LS also decreased defensive burying (114).
In our investigations of modulatory neurotransmitters that may influence behavior on the defensive burying test, we found that NE release was elevated in LS by exposure to the shock-probe, and that blockade of both α1- and β-adrenergic receptors in LS reduced active burying but increased immobility (103) (see Figure 6). Likewise, bilateral microinjections of the galanin antagonist M40 into the LS attenuated defensive burying behavior, but had no affect on immobility (106). Thus, it appears that the active, adaptive burying response and the more passive immobility response may be mediated and modulated independently, by different brain circuitry and/or by different neurotransmitter systems.
Figure 6.
Bilateral microinjections, administered into the lateral septum (LS), of either the α1-adrenergic receptor antagonist benoxathian (2.0 nmole/side), or a cocktail of β1β2-receptor antagonists betaxolol + ICI 118,551 (1.0 nmole each/side), attenuated defensive burying behavior and increased immobility on the shock-probe defensive burying test (n=8–18/group; *p<0.05 compared to vehicle control group). Note that in this experiment, because a number of LS-implanted rats did not exhibit any burying behavior, burying time was analyzed non-parametrically; nonetheless, only the reduction caused by benoxathian achieved significance in comparison to vehicle-treated rats in this analysis. By contrast, immobility was normally distributed, and both of the drug treatment effects were significant for this measure. Adapted and reprinted, with permission from Elsevier, from (35).
Method and Procedural Hints
Apparatus
The SPDB is conducted in a clean polystyrene cage, 26 x 48 x 21 cm (w x l x h). In our studies, this chamber is identical to the rats home cage, except that a small hole is drilled in one end of the test cage, 7 cm above the floor, and the lid is modified by cutting out the center to allow for videotaping of behavior from above. For each test, the test chamber is filled with clean fresh bedding to a depth of 5 cm (Teklad Sani-Chips, Harlan, Indianapolis, IN). The shock probe is a glass rod, 1.0 cm diameter, wrapped with two alternating, non-touching 18 ga copper wires, spaced 5 loops/cm. In addition, we wrap loops of polyethylene tubing (PE-50) between the wires to maintain separation and spacing, and the PE tubing is affixed to the glass rod with a suitable adhesive. PE-50 tubing is thick enough to maintain spacing between the wires, but thin enough to not interfere with contact when the probe is touched. The probe is mounted on a ring stand outside the cage, so that it protrudes 6 cm into the cage through the hole, 2 cm above the surface of the bedding. The wires are attached to a shock generator (model H13-15, Coulbourn Instruments, Allentown, PA) set to deliver 2 mA DC current when the probe is touched.
As described above for the other behavioral tests, we generally conduct our studies early in the light portion of the light cycle, under normal overhead ambient lighting conditions (175–225 lux measured in the center of the test chamber). A video camera is mounted on a tripod above the test cage to record behavior for offline scoring and analysis. Position the camera and cage relative to the light source such that there are no shadows cast in the test cage. This is important not only to be able to observe and score the rats behavior accurately, but also because we have found that rats will often tend to remain immobile in any shadowed areas during testing. Background white noise (60 dB) is used to dampen extraneous noises.
We have found it important to keep a lid on the cage during testing, hence the modification to allow videotaping. Without at least a partial lid in place, animals exhibit frequent rearing behavior, focus their attention toward the outside of the test chamber, and eventually attempt to climb out of the cage and escape. Thus, the lid we use has a few cm intact just at the edges to keep the animals from exploring above the walls of the cage, but the center of the lid is cut out so that the animal is visible to the camera in all areas of the cage.
After each animal is tested, the bedding is discarded and the cage cleaned with 70% ethanol, then with water and then dried before it is filled again with fresh bedding to a depth of 5 cm. The shock-probe must also be cleaned to remove any dust or debris that might have accumulated during the test. In addition, the wires of the probe must be cleaned regularly with a light brush or steel wool to remove any protein film that might coat the surface with repeated use, and which can act as an insulator to reduce the intensity of the shock. For this same reason, the probe itself should never be cleaned with ethanol, as ethanol can “fix” proteins that may coat the wires and effectively reduce the shock. It is recommended to regularly inspect the probe, to confirm that the wires are not touching, and to periodically test the output with a multimeter.
Procedure
The SPDB requires no pre-training or pre-conditioning of the animals. In initial pilot studies, we found that more burying behavior was elicited if the rats were not handled prior to testing. However, if other procedures are incorporated into the experimental design that are facilitated by handling (e.g., microinjections, blood sampling, or any pre-treatment of experimental groups that dictate handling be instituted as a control procedure), then animals are handled as described above (e.g., 1–2 min per day for three days prior to testing).
On the test day, rats are transported to the testing room in their home cage and allowed 20 min to habituate. The rat is placed gently into the test chamber at the end opposite the shock-probe, facing away from the probe. The experimenter may remain in the testing room during the test to monitor behavior, as long as a suitable distance can be maintained to avoid distracting the animal. In fact, we have found it can be useful, although not necessary, for two experimenters to participate; one to place the rat in the test chamber and the other to operate the switch on the shock generator to shut the current off after the rat contacts the probe and receives a shock.
After a rat is placed in the test chamber, it will typically approach and contact the probe with a paw or snout within about 10–15 sec. The 15 min test period, during which behavior is scored, begins once the rat makes contact with the probe and exhibits a clear reaction to the shock (i.e., an obvious recoil). Some investigators choose to keep the probe electrified after the initial contact, so that any subsequent contact with the probe results in additional shocks. The rationale for this is to prevent extinction from occurring if the probe is contacted but no shock is received. However, we have elected in our studies to limit the number of shocks received to the one experienced upon initial contact. Thus, after the initial contact and an obvious reaction by the rat, the current is shut off. In our opinion, this ensures that a consistent stimulus is experienced by all animals, although we recognize that extinction can be a potential confound if there are any differences in the number of subsequent, non-shocked probe contacts experienced. To monitor this, we count the number of non-shocked probe contacts made during testing, and have generally found this measure not to differ between groups. Nonetheless, it is important to at least be aware of this possibility.
A number of behavioral parameters can be scored during the 15 min test, as adapted from De Boer and Koolhaas (100), with burying time and immobility time being the primary measures of anxiety-like behavior. After initially recoiling and withdrawing from the probe, rats typically showed a variable period of inactivity before beginning to bury, usually within 5–8 min. “Burying” is a cumulative time measure, consisting of burrowing into the bedding with the snout and upper body, then “plowing”, pushing or shoveling the bedding toward the probe, and also flicking or spraying bedding material toward the probe using the dorsal surface of the forepaws. This behavior is not exhibited in the absence of the probe, nor is it exhibited even in the presence of the probe if no shock has been received (101). “Immobility” is also a cumulative time measure, defined as a lack of movement other than that required for breathing. However, this can in practice be quite difficult to assess. We include in “immobility” sniffing and slight lateral scanning movements of the head, but with no movement whatsoever of the feet, and no rotation of the head that requires upper body movement. Obviously, this has a degree of subjectivity to it, and whatever the definition, some consensus must be reached within a lab, and all scorers must apply the same criteria stringently and consistently.
Other behavioral parameters that can be informative, primarily as control measures, include the latency to contact the probe (an indicator of any potential non-specific alterations in locomotion or exploratory activity), number of rearings, number of non-shocked probe returns (an indicator of potential differences in extinction), and behavioral reactivity to the initial shock, scored on a subjective, categorical 4-point scale as an indicator of potential analgesia or sub-sensitivity to the shock stimulus itself (see 115).
Because of the sporadic nature of the behavior exhibited in the SPDB test, scoring from videotape is ideal, and it is often useful to watch the same session two or more times, scoring a different component of behavior each time (e.g., burying vs immobility). Initially, it can be informative for scoring to be performed by two independent observers, both blinded to the treatment conditions of the animals. This is especially helpful to attain uniformity in scoring immobility. With practice, careful and consistent training, and continuing evaluation and discussion among lab members, inter-rater reliability measures > 0.98 can be achieved.
The degree of defensive burying observed may be sensitive to a number of environmental, procedural or organismic variables (see 100). For instance, strain differences have been reported, with Fisher and Long Evans rats exhibiting more burying behavior than Wistar rats, whereas Wistar-Kyoto rats bury less than the Wistar strain (47, 116). Burying time can be increased by testing in a familiar versus an unfamiliar environment, by group-housing, use of clean bedding in the test cage compared to stress odor-contaminated bedding, and testing in the dark rather than the light period (117–119). Older rats exhibit more burying behavior than younger rats (116). Food deprivation was found to decrease burying (120). Some studies have shown higher levels of baseline burying when testing during the dark phase of the light cycle compared to the light phase (see 100). Testing during the dark phase also increased airjet-induced burying behavior, but not burying of an unfamiliar object (e.g., a steel ball) (117). Thus, if required by the experimental design, or if it might be advantageous either to elevate baseline burying or to enhance evoked burying responses, it is feasible to test in the dark using dim red lighting (e.g., see 121). In general, regardless of testing conditions, although there is considerable individual variability in the amount and proportion of time spent burying after contact with the shock-probe, reports indicate a strong inverse relationship between the two different coping styles animals can display in this test, e.g., the predominantly active behavioral response strategy (high defensive burying) or the predominantly passive/reactive strategy (high immobility) (110, 118, 122).
A similar procedure for shock-probe defensive burying has been developed for mice (100, 123). The marble-burying test is a related paradigm also used for mice, involving the spontaneous burying of novel but harmless objects (see 100, 124). Although sensitive to benzodiazepine anxiolytics, this test may not be as sensitive as others to mildly stressful or anxiogenic treatments (124, 125). The marble-burying test has not been validated as a reliable measure of anxiety in mice (100).
As for many behavioral tests, treatments that decrease locomotion, induce ataxia, or provoke excessive freezing independent of the test stimulus (i.e., in the absence of contact with the shock-probe) can render the SPDB test unsuitable as a measure of anxiety-like behavioral reactivity. Also, treatments that induce analgesia can potentially reduce sensitivity to the shock, decreasing its effectiveness as a stimulus, and result in a non-specific decrease in burying behavior. This possibility can be assessed by testing reactivity to a noxious cutaneous stimulus using a measure (e.g., paw withdrawal) that is independent of anxiety (see 106).
An important limitation we have encountered with the SPDB test is that a proportion of animals tested do not exhibit active burying behavior, about 20% in our hands. Moreover, this behavior can be highly sensitive to surgical implantation or other manipulations of key brain regions. In our studies, for instance, cannulae implantation into the lateral septum reduced both the mean burying time and the number of animals that exhibited any burying behavior at all, even in vehicle-treated control groups (103, 106). Similar observations have been reported by others, and for a number of other brain regions (e.g., see 126). As in any such studies, where a subset of subjects across groups do not exhibit the target behavior, the resulting data may violate assumptions of normality and homogeneity of variance, thus requiring an appropriate use of non-parametric statistical analyses (e.g., see Figure 6). In the worst case, the SPDB test may simply not be a viable option.
Conclusion
Modeling complex human psychiatric conditions such as depression and anxiety disorders presents a unique challenge to the preclinical scientist, due in part to a significant lack of knowledge regarding the etiology and neural mechanisms involved, as well as diagnostic schemata that can sometimes define broad or ambiguous symptoms, many of which cut across diagnostic categories. This makes it difficult to model all aspects of a given syndrome. However, a more “dimensional” approach, identifying symptom clusters or “endophenotypes” that can be attributed to definable neural substrates and mechanisms, is amenable to modeling distinct components of a psychiatric illness, rather than the entire syndrome.
In this review, we present four well-validated rat behavioral tests that have proven useful in modeling specific dimensions of depression and anxiety disorders. The methodological details outlined herein may be useful in optimizing the utility, validity and replicability of these tests. The rigorous application of such models can contribute to elucidating the neural substrates underlying specific cognitive and affective dimensions of psychiatric illnesses, and the mechanisms by which environmental, genetic or pharmacological factors may influence their development and treatment. The continuing refinement of such models, incorporating data from neuroimaging, neuropsychological and human clinical studies, may further contribute to the development of novel or more effective treatment strategies for these disorders.
Questions from the Bench
1) Can one animal be tested in more than one test? If so, is there a preferable order, or combination of tests that can be used?
Rats tested in the AST can be used on subsequent tests. Because they are food restricted, the rats are given food ad libitum immediately after completing the AST. Testing then begins with other paradigms the next day. Indeed, the most conservative approach for any sequential testing would be to conduct all tests on different days. However, this is not always possible. In studies where an acute treatment could only be used once for a given animal, we used the SI test followed immediately by the EPM (e.g., 51, 52), also as described by File and colleagues (127, 128). In these studies, we observed no differences in either baseline behavior or in stimulus-evoked changes in behavior on the EPM in rats that had SI testing immediately preceding the EPM compared to those who had not. File et al. have reported various combinations and sequences of testing on the EPM, SI test and hole-board test (71, 86). Nonetheless, it is always advisable to establish the consistency of results obtained with any planned combination or sequence of tests relative to single testing in a pilot study before proceeding.
By contrast with the EPM and SI tests, in which the animals voluntarily explore the open arms or engage in social behavior, the SPDB test involves the application of an explicitly aversive stimulus beyond the rats’ control. Thus, we have been hesitant to test rats on any other assays of anxiety-like behavior immediately after SPDB testing. Likewise, testing SI immediately before SPDB has been reported to alter burying behavior, but the direction of effect depended on the length of SI exposure and the time between tests (see 129). Thus, as a general practice, we do not conduct other behavioral tests on the same day as SPDB. However, the single shock delivered in the SPDB test is relatively mild, and the test is of short duration, so there seems no reason not to conduct subsequent behavioral testing on days following SPDB.
2) All the tests appear to be done during the day when rodents are normally sleeping. Would results differ for animals tested in the dark? Under dim red illumination? Is it feasible, with reverse light/dark conditions, to test during the dark phase, or would that be less reliable?
We have not conducted the AST under reversed light/dark conditions, nor are we aware of a literature by which to compare results obtained in the light phase to those obtained in the dark phase under dim red illumination. Although there is nothing inherent in the nature of the behavior, nor in the test conditions, that would preclude testing in the dark phase, doing so could present logistic challenges to the investigator, including behavioral scoring, changing pots, etc. Activity level per se does not influence the animal’s ability to choose a pot correctly, although higher activity and arousal could alter total test time. For the tests of anxiety-like behavior, lighting and time of day have not been shown to influence behavior on the EPM. Nonetheless, we still prefer always to test at about the same time of day, usually late morning to early afternoon (09:00–13:00). This is sufficiently separated from light-dark transitions to obviate any diurnal variations in exploratory behavior. By contrast, testing in a brightly lit arena reduces baseline SI behavior, and a dimly lit testing environment increases SI (75). Further, it has been shown that baseline SI is elevated when rats are tested under dim-light conditions in the dark phase of the circadian cycle (94). Higher levels of defensive burying have also been reported when testing for SPDB in the dark portion of the light cycle (see 100). Thus, for any of these tests, although it is important to be aware that baselines may differ, it would seem feasible to test in the dark phase using dim red lighting if that is required by the experimental design.
3) What is the best way to ensure and test inter-investigator reliability?
For all tests, every new investigator goes through a period of training. First, they observe the procedures and scoring of as many experienced investigators as possible to obtain the “consensus” definition of the behaviors of interest. Then, they score the same tests that an experienced rater will have already scored, to compare results and discuss any differences or difficulties that arise. When they then begin to conduct and score tests on their own, an experienced rater will also score the same results for comparison and further refinement. The goal is to achieve and maintain consistency between investigators and across experiments.
For most tests, a quantitative index of inter-rater reliability is the correlation coefficient calculated from a set of scores generated by two independent investigators for the same tests. Because we videotape or electronically record all test sessions on the EPM, SI and SPDB, which allows offline analysis and scoring, it is a simple matter to have two or more people watch and score the same sessions. These sample tests can and should include multiple treatment groups, even from different experiments, to elicit a wide range of scores. While there is no “minimum acceptable” coefficient, the higher it is the more reliable the results from the lab as a whole. Our informal standard is to obtain and maintain correlation coefficients >0.95 between investigators. This does not mean they generate exactly the same numbers, only that any differences between them are consistent, and any differences between animals were detected comparably. There may be slight individual differences between investigators in scoring certain behaviors, for example, immobility. But these differences should be consistent over time and across experiments. For this reason, the same individual or individuals should score all animals in a given study.
For the AST, it is more difficult to quantify inter-rater reliability (or more accurately, inter-rater correlation). The behavior cannot be scored post hoc, but must be scored as the test proceeds. At specified points in the process, determined by the animal’s pattern of responses, the stimulus contingencies must be changed by the investigator when the animal reaches criterion and a new test stage is initiated. Thus, there is no way to generate a set of independent scores by two investigators on the same test. Nonetheless, we take all new investigators through an extensive period of training, which includes sessions where they score the behavior in parallel with an experienced investigator, and then discuss instances where they disagree on whether the rat executed a “dig” or made an error, or where there was any uncertainty as to the response. Further, we regularly compare both the absolute number of trials to criterion and the overall pattern of errors across tasks in control groups in different experiments, both between and within investigators, to ensure consistency. Finally, of course, in all behavioral experiments, the investigator scoring the behavior should be blind to the treatment conditions of the animals.
4) Are there other behavioral models that a reader should consider for assessing anxiety-like or depression-like behavior?
The 5-choice serial reaction time task has been used widely to assess prefrontal cortical cognitive and executive functions in rats (130, 131). As for other aspects of depressive-like symptomatology, the forced swim test (FST) is a widely-used screen for antidepressant drug efficacy (132–134). Because antidepressants reduce immobility in this test, experimentally-induced increases in immobility have often been interpreted as representing depressive-like behavior, frequently referred to somewhat anthropomorphically as “despair”. Another model of depressive-like “behavioral despair” is learned helplessness, in which repeated exposure to an inescapable and uncontrollable stressor, usually a shock, leads to a subsequent deficit of shuttle-escape behavior (135–137). An additional affective component of depression, distinct from anxiety and cognitive dysfunction, is anhedonia, an inability to experience pleasure from normally enjoyable events or activities. This is often modeled in rats using the sucrose preference test. In this paradigm, a decrease in proportional consumption of a highly preferred sucrose solution induced by an experimental test manipulation is interpreted to represent anhedonia. This has been validated as a model of depressive-like behavior by demonstrating a restoration of sucrose preference with chronic antidepressant drug treatment (138).
There are numerous tests of anxiety-like behavior in rodents, with many variations on their design, conduct and application, and several excellent reviews of their validation and use to which the interested reader can refer (e.g., see 58, 60, 139, 140, 141). Some tests require food or water deprivation, and/or an extended period of operant training, to establish a conflict by punishing deprivation-motivated consummatory behavior with an aversive stimulus, e.g., shock. The degree to which the behavior is then suppressed is taken as an index of anxiety, and the degree to which the suppression of punished behavior is released is indicative of an anxiolytic effect. Such tests include the Geller-Seifter test of punished operant responding for food, and the Vogel test of punished licking for water (see 60, 142). Other tests, such as the light/dark test (also see 60), are similar to the EPM in establishing a conflict between the drive to explore a novel environment and fear induced by some aversive characteristic of that environment. Advantages to these kinds of tests are that no deprivation, pain or extended training are required, and the animal chooses to explore the aversive environment or not, suggesting that the test procedure itself may not necessarily be an especially stressful experience. Fear-potentiated startle is used specifically to assess anxiety-like enhancement of the reflexive acoustic startle response by aversively-conditioned stimuli, including explicitly conditioned cues (e.g., a tone paired with shock) or environmental contexts (the room in which shock is delivered) (139, 143). Other parameters that have been used as indirect physiologic indices of anxiety include autonomic measures of sympathetic activation (e.g., 144) and ultrasonic vocalizations (145–147). There are many other tests of anxiety-like behavior in rats. In selecting the most appropriate tests to use, it is important to keep in mind that different tests may lend themselves to different experimental limitations and requirements, but they may also model and assay different forms of anxiety related to different neurobiological mechanisms, and all of these tests possess varying degrees of face, construct and predictive validity.
Acknowledgments
Original research conducted in the authors’ laboratory was supported by research grants from the National Institute of Mental Health (MH072672 and MH053851), and by a Young Investigator Award from NARSAD: The Mental Health Association (to MDSL-B). All experiments and procedures described in the context of the authors’ original research were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and were consistent with NIH guidelines for the care and use of laboratory animals. The authors would like to acknowledge the contributions made to original research described in this paper by Dr. Marco Cecchi, Mr. Gabe Barrera, Dr. David Echevarria, Dr. Habibeh Khoshbouei, Dr. Shuaike Ma and Dr. Marie-Christine Pardon. The authors have no conflicts of interest or financial involvements to disclose, financial or otherwise, that may bias the conduct, interpretation or presentation of this work.
References
- 1.Duyk G. Attrition and translation. Science. 2003;302:603–605. doi: 10.1126/science.1090521. [DOI] [PubMed] [Google Scholar]
- 2.Enserink M. Can the placebo be the cure? Science. 1999;284:238–240. doi: 10.1126/science.284.5412.238. [DOI] [PubMed] [Google Scholar]
- 3.Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: Navigating through the clinical fog. Neurosci Biobehav Revs. 2005;29:503–513. doi: 10.1016/j.neubiorev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.McArthur R, Borsini F. Animal models of depression in drug discovery: A historical perspective. Pharm Biochem Behav. 2006;84:436–452. doi: 10.1016/j.pbb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Winsky L, Brady L. Perspective on the status of preclinical models for psychiatric disorders. Drug Discovery Today: Disease Models. 2005;2:279–283. [Google Scholar]
- 6.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioral effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 7.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Ann Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT. Cognitive therapy and the emotional disorders. New York: Int. Univ. Press; 1976. [Google Scholar]
- 9.Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: A test of the cognitive content-specificity hypothesis. J Abnormal Psychol. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- 10.Coles ME, Heimberg RG. Memory biases in the anxiety disorders: Current status. Clin Psychol Rev. 2002;22:587–627. doi: 10.1016/s0272-7358(01)00113-1. [DOI] [PubMed] [Google Scholar]
- 11.Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Ther Res. 1998;22:539–560. [Google Scholar]
- 12.MacLeod C, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behav Res Ther. 1992;30:151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- 13.Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Revs. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 15.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 16.Austin M-P, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. Brit J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 17.Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- 18.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairments in patients with major depressive disorder: The effects of feedback on task performance. Psychol Med. 2003;33:455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RJ, Pizzagallli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Ann Rev Psychol. 2002;53:545–5574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 20.Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia: A comparison of an early and late onset neurodevelopmental disorder. Arch Clin Neuropsychol. 2002;17:461–475. [PubMed] [Google Scholar]
- 23.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex of the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 25.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalohippocampectomy. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 27.Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: The memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 29.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Tollefson GD. Cognitive function in schizophrenic patients. J Clin Psychiatry. 1996;57:31–39. [PubMed] [Google Scholar]
- 31.Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 32.Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- 33.Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- 35.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 36.Morilak DA, Lapiz-Bluhm MD. Chronic intermittent cold stress induces impairment in reversal learning in the rat on an attentional set-shifting task. Soc Neurosci Abstr. 2007;33 Online: Program number 643.14. [Google Scholar]
- 37.Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task: Reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 38.Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: Effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 39.Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004;20:1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- 40.Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Beh Brain Res. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Annals New York Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- 42.Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learning & Memory. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci. 2003;23:676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DNC. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- 45.Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R353–R360. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- 46.Pardon M-C, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 47.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 48.Colacicco G, Welzl H, Lipp H-P, Wurbel H. Attentional set-shifting in mice: Modification of a rat paradigm, and evidence for strain-dependent variation. Beh Brain Res. 2002;132:95–102. doi: 10.1016/s0166-4328(01)00391-6. [DOI] [PubMed] [Google Scholar]
- 49.Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA. Animal neuropsychology: Validation of the intra-dimensional extra-dimensional set shifting task for mice. Beh Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 50.DeSteno DA, Schmauss C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience. 2008;152:417–428. doi: 10.1016/j.neuroscience.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 52.Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on a1-receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 53.Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: Amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol, Biochem & Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- 54.Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002;27:25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery KC. The relation between fear induced by novel-stimulation and exploratory behavior. J Comp Physiol Psychol. 1958;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 56.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn-Schmiedeberg’s Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 57.Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 58.Lister RG. Ethologically-based animal models of anxiety disorders. Pharmac Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 59.Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Meth. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- 60.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. New York: John Wiley & Sons; 2000. pp. 8.3.1–8.3.19. [Google Scholar]
- 61.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Beh Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 62.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 63.Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH. Ethopharmacological analysis of the effects of putative ‘anxiogenic’ agents in the mouse elevated plus-maze. Pharm Biochem Behav. 1995;52:805–813. doi: 10.1016/0091-3057(95)00190-8. [DOI] [PubMed] [Google Scholar]
- 64.Rodgers RJ, Johnson NJT. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- 65.Shepard JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterization of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 66.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharm Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 67.File SE, Zangrossi H, Sanders FL, Mabbutt PS. Raised corticosterone in the rat after exposure to the elevated plus-maze. Psychopharmacology. 1994;113:543–6. doi: 10.1007/BF02245237. [DOI] [PubMed] [Google Scholar]
- 68.Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 69.Treit D, Menard J, Royen C. Anxiogenic stimuli in the elevated plus-maze. Pharm Biochem Behav. 1993;44:463–469. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- 70.File SE, Zangrossi H, Viana M, Graeff FG. Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology. 1993;111:491–494. doi: 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- 71.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 72.File SE. One-trial tolerance to the anxiolytic effects of chlordiazepoxide in the plus-maze. Psychopharmacology. 1990;100:281–282. doi: 10.1007/BF02244419. [DOI] [PubMed] [Google Scholar]
- 73.Becker A, Grecksch G. Illumination has no effect on rats’ behavior in the elevated plus-maze. Physiol Behav. 1996;59:1175–1177. doi: 10.1016/0031-9384(95)02224-4. [DOI] [PubMed] [Google Scholar]
- 74.Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;235:109–112. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- 75.File SE, Hyde JRG. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meths. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 77.File SE. Animal models for predicting clinical efficacy of anxiolytic drugs: Social behaviour. Neuropsychobiology. 1985;13:55–62. doi: 10.1159/000118163. [DOI] [PubMed] [Google Scholar]
- 78.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 79.File SE. How good is social interaction as a test of anxiety? In: Simon P, Soubrie P, Widlocher D, editors. Selected Models of Anxiety, Depression and Psychosis. Basel: Karger; 1988. pp. 151–166. [Google Scholar]
- 80.File SE, Hyde JRG. A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquillizers and of stimulants. Pharm Biochem Behav. 1979;11:65–69. doi: 10.1016/0091-3057(79)90298-3. [DOI] [PubMed] [Google Scholar]
- 81.Vellucci SV, File SE. Chlordiazepoxide loses its anxiolytic action with long-term treatment. Psychopharmacology. 1979;62:61–65. doi: 10.1007/BF00426036. [DOI] [PubMed] [Google Scholar]
- 82.Fernandes C, Arnot MI, Irvine EE, Bateson AN, Martin IL, File SE. The effect of treatment regimen on the development of tolerance to the sedative and anxiolytic effects of diazepam. Psychopharmacology. 1999;145:251–259. doi: 10.1007/s002130051056. [DOI] [PubMed] [Google Scholar]
- 83.File SE, Pellow S. The anxiogenic action of Ro 15-1788 is reversed by chronic, but not by acute, treatment with chlordiazepoxide. Brain Res. 1984;310:154–156. doi: 10.1016/0006-8993(84)90020-9. [DOI] [PubMed] [Google Scholar]
- 84.Baldwin HA, Johnston AL, File SE. Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. Eur J Pharmacol. 1989;159:211–215. doi: 10.1016/0014-2999(89)90709-7. [DOI] [PubMed] [Google Scholar]
- 85.Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcoholism: Clin & Exper Res. 2001;25:377–385. [PubMed] [Google Scholar]
- 86.Zangrossi H, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res Bull. 1992;29:381–388. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 87.Zangrossi H, File SE. Chloriazepoxide reduces the generalized anxiety, but not the direct responses, of rats exposed to cat odor. Pharm Biochem Behav. 1992;43:1195–1200. doi: 10.1016/0091-3057(92)90502-7. [DOI] [PubMed] [Google Scholar]
- 88.File SE. Chronic exposure to noise modifies the anxiogenic response, but not the hypoactivity, detected on withdrawal from chronic ethanol treatment. Psychopharmacology. 1994;116:369–372. doi: 10.1007/BF02245342. [DOI] [PubMed] [Google Scholar]
- 89.Niesink RJM, Van Ree JM. Short-term isolation increases social interaction of male rats: A parametric analysis. Physiol Behav. 1982;29:819–825. doi: 10.1016/0031-9384(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 90.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 91.Ramos A, Berton O, Mormede P, Chaoloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Beh Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 92.Kantor S, Anheuer ZE, Bagdy G. High social anxiety and low aggression in fawn-hooded rats. Physiol Behav. 2000;71:551–557. doi: 10.1016/s0031-9384(00)00374-7. [DOI] [PubMed] [Google Scholar]
- 93.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kask A, Nguyen HP, Pabst R, von Hörsten S. Factors influencing behavior of group-housed male rats in the social interaction test: Focus on cohort removal. Physiol Behav. 2001;74:277–282. doi: 10.1016/s0031-9384(01)00587-x. [DOI] [PubMed] [Google Scholar]
- 95.Baldwin HA, File SE. Caffeine-induced anxiogenesis: The role of adenosine, benzodiazepine, and noradrenergic receptors. Pharm Biochem Behav. 1988;32:181–186. doi: 10.1016/0091-3057(89)90230-x. [DOI] [PubMed] [Google Scholar]
- 96.Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm & Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 97.File SE, Andrews N, Al-Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: Effects of tianeptine. Alcohol & Alcoholism. 1993;28:281–286. [PubMed] [Google Scholar]
- 98.File SE, Clark A. Intraventricular ACTH reduces social interaction in male rats. Pharm Biochem Behav. 1979;12:711–715. doi: 10.1016/0091-3057(80)90154-9. [DOI] [PubMed] [Google Scholar]
- 99.Bolles RC. Learning, motivation, and cognition. In: Estes WK, editor. Handbook of learning and cognitive processes. Hillsdale, NJ: Erlbaum; 1975. [Google Scholar]
- 100.De Boer SF, Koolhaas JM. Defensive burying in rodents: Ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 101.Pinel JPJ, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978;92:708–712. [Google Scholar]
- 102.Treit D, Pinel JPJ, Fibiger HC. Conditioned defensive burying: A new paradigm for the study of anxiolytic agents. Pharm Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- 103.Bondi CO, Barrera G, Lapiz MDS, Bédard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Treit D, Pesold C, Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci. 1993;107:770–785. doi: 10.1037//0735-7044.107.5.770. [DOI] [PubMed] [Google Scholar]
- 105.Basso AM, Spina M, Rivier JWV, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 106.Echevarria DJ, Hernandez A, Diogenes A, Morilak DA. Administration of the galanin antagonist M40 into lateral septum attenuates shock probe defensive burying behavior in rats. Neuropeptides. 2005;39:445–451. doi: 10.1016/j.npep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 107.Matuszewich L, Karney JJ, Carter SR, Janasik SP, O’Brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav. 2007;90:674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- 109.Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: Effects of ipsapirone. Physiol Behav. 1992;52:355–361. doi: 10.1016/0031-9384(92)90284-9. [DOI] [PubMed] [Google Scholar]
- 110.De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: Effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–1098. doi: 10.1016/0031-9384(90)90357-a. [DOI] [PubMed] [Google Scholar]
- 111.Groenink L, Van der Gugten J, Verdouw PM, Maes RAA, Olivier B. The anxiolytic effects of flesinoxan, a 5-HT1A receptor agonist, are not related to its neuroendocrine effects. Eur J Pharmacol. 1995;280:185–193. doi: 10.1016/0014-2999(95)00209-4. [DOI] [PubMed] [Google Scholar]
- 112.Korte SM, Buwalda B, Bouws GA, Koolhaas JM, Maes FW, Bohus B. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and young rats. Physiol Behav. 1992;51:815–822. doi: 10.1016/0031-9384(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 113.Menard J, Treit D. Lateral and medial septal lesions reduce anxiety in the plus-maze and probe-burying tests. Physiol Behav. 1996;60:845–853. doi: 10.1016/0031-9384(96)00138-2. [DOI] [PubMed] [Google Scholar]
- 114.Degroot A, Kashluba S, Treit D. Septal GABAergic and hippocampal cholinergic systems modulate anxiety in the plus-maze and shock probe tests. Pharm Biochem Behav. 2001;69:391–399. doi: 10.1016/s0091-3057(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 115.Treit D, Pesold C. Septal lesions inhibit fear reactions in two animal models of anxiolytic drug action. Physiol Behav. 1990;47:365–371. doi: 10.1016/0031-9384(90)90155-w. [DOI] [PubMed] [Google Scholar]
- 116.Treit D, Terlecki L, Pinel JPJ. Conditioned defensive burying in rodents: Organismic variables. Bull Psychosom Soc. 1980;16:451–454. [Google Scholar]
- 117.Pinel JP, Mumby DG, Dastur FN, Pinel JG. Rat (Rattus Norvegicus) defensive behavior in total darkness: risk-assessment function of defensive burying. J Comp Psychol. 1994;108:140–147. doi: 10.1037/0735-7036.108.2.140. [DOI] [PubMed] [Google Scholar]
- 118.Treit D, Lolordo VM, Armstrong DE. The effects of diazepam on “fear” reactions in rats are modulated by environmental constraints on the rat’s defensive repertoire. Pharm Biochem Behav. 1986;25:561–565. doi: 10.1016/0091-3057(86)90141-3. [DOI] [PubMed] [Google Scholar]
- 119.Williams JL. Influence of conspecific stress odors and shock controllability on conditioned defensive burying. Animal Learn Behav. 1987;15:333–341. [Google Scholar]
- 120.Davis SF, Hazelrigg M, Moore SA, Petty-Zirnstein MK. Defensive burying as a function of food and water deprivation. Bull Psychosom Soc. 1981;18:323–327. [Google Scholar]
- 121.Roy V, Chapillon P. The positive effects of postnatal handling on defensive burying are more obvious in a situation that enlarges the potential coping responses. Beh Brain Res. 2002;136:67–73. doi: 10.1016/s0166-4328(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 122.Tsuda A, Tanaka M, Georgiev V, Emoto H. Effects of angiotensin II on behavioral responses of defensive burying paradigm in rats. Pharm Biochem Behav. 1992;43:729–732. doi: 10.1016/0091-3057(92)90401-z. [DOI] [PubMed] [Google Scholar]
- 123.Gasparotto OC, Carobrez SG, Bohus BGJ. Effects of LPS on the behavioural stress response of genetically selected aggressive and nonaggressive wild house mice. Beh Brain Res. 2007;183:52–59. doi: 10.1016/j.bbr.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 124.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharm Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 125.Chotiwat C, Harris RBS. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm & Behav. 2006;50:489–495. doi: 10.1016/j.yhbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 126.Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: The amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- 127.Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- 128.File SE, Ouagazzal AM, Gonzalez LE, Overstreet DH. Chronic fluoxetine in tests of anxiety in rat lines selectively bred for differential 5-HT1A receptor function. Pharmacol Biochem Behav. 1999;62:695–701. doi: 10.1016/s0091-3057(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 129.Saldívar-González A, Gómez C, Martínez-Lomelí I, Arias C. Effect of flumanezil and diazepam on transient actions on defensive burying elicited by the social intercation experience in rats. Pharm Biochem Behav. 2000;66:265–273. doi: 10.1016/s0091-3057(00)00177-5. [DOI] [PubMed] [Google Scholar]
- 130.Dalley JW, Cardinal RN, Robbins TW. Prefrontal cognitive and executive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Revs. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Robbins TW. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 132.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 133.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: Forced swimming and tail suspension behavioral despair tests in rats and mice. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. New York: John Wiley & Sons; 2001. pp. 8.10A.1–8.10A.10. [DOI] [PubMed] [Google Scholar]
- 134.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Revs. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Drugan RC. Rodent models of depression: Learned helplessness using a triadic design in rats. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. New York: John Wiley & Sons; 2001. pp. 8.10B.1–8.10B.12. [DOI] [PubMed] [Google Scholar]
- 136.Maier SF. Learned helplessness and animal models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 137.Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- 138.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 139.Davis M. Fear-potentiated startle in rats. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. New York: John Wiley & Sons; 2001. pp. 8.11A.1–8.11A.11. [DOI] [PubMed] [Google Scholar]
- 140.File SE. Animal models of different anxiety states. Adv Biochem Psychopharmacol. 1995;48:93–113. [PubMed] [Google Scholar]
- 141.Handley SL, McBlane JW. 5HT drugs in animal models of anxiety. Psychopharmacology. 1993;112:13–20. doi: 10.1007/BF02247358. [DOI] [PubMed] [Google Scholar]
- 142.Millan MJ, Brocco M. The Vogel conflict test: Procedural aspects, -aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- 143.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Beh Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 144.Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res. 1995;699:250–259. doi: 10.1016/0006-8993(95)00915-d. [DOI] [PubMed] [Google Scholar]
- 145.Brudzynski SM. Principles of rat communication: Quantitative parameters of ultrasonic calls in rats. Behav Genetics. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 146.Jelen P, Soltysik S, Zagrodska J. 22-kHz Ultrasonic vocalization in rats as an index of anxiety but not fear: Behavioral and pharmacological modulation of affective state. Beh Brain Res. 2003;141:63–72. doi: 10.1016/s0166-4328(02)00321-2. [DOI] [PubMed] [Google Scholar]
- 147.Litvin Y, Blanchard DC, Blanchard RJ. Rat 22 kHz ultrasonic vocalizations as alarm cries. Beh Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]



