Abstract
Expression quantitative trait loci (eQTLs) represent genetic control points of gene expression, and can be categorized as cis- and trans-acting, reflecting local and distant regulation of gene expression respectively. Although there is evidence of co-regulation within clusters of trans-eQTLs, the extent of co-expression patterns and their relationship with the genotypes at eQTLs are not fully understood. We have mapped thousands of cis- and trans-eQTLs in four tissues (fat, kidney, adrenal and left ventricle) in a large panel of rat recombinant inbred (RI) strains. Here we investigate the genome-wide correlation structure in expression levels of eQTL transcripts and underlying genotypes to elucidate the nature of co-regulation within cis- and trans-eQTL datasets. Across the four tissues, we consistently found statistically significant correlations of cis-regulated gene expression to be rare (<0.9% of all pairs tested). Most (>80%) of the observed significant correlations of cis-regulated gene expression are explained by correlation of the underlying genotypes. In comparison, co-expression of trans-regulated gene expression is more common, with significant correlation ranging from 2.9%–14.9% of all pairs of trans-eQTL transcripts. We observed a total of 81 trans-eQTL clusters (hot-spots), defined as consisting of ≥10 eQTLs linked to a common region, with very high levels of correlation between trans-regulated transcripts (77.2–90.2%). Moreover, functional analysis of large trans-eQTL clusters (≥30 eQTLs) revealed significant functional enrichment among genes comprising 80% of the large clusters. The results of this genome-wide co-expression study show the effects of the eQTL genotypes on the observed patterns of correlation, and suggest that functional relatedness between genes underlying trans-eQTLs is reflected in the degree of co-expression observed in trans-eQTL clusters. Our results demonstrate the power of an integrative, systematic approach to the analysis of a large gene expression dataset to uncover underlying structure, and inform future eQTL studies.
Introduction
The use of linkage analysis in combination with genome-wide expression profiling by microarray, also known as ‘genetical genomics’ [1], enables the genetic control points of gene expression to be mapped to the genome. These have come to be referred to as expression quantitative trait loci (eQTLs) [2]. This study design has in the past five years been applied to the investigation of regulatory processes in, among others, yeast, rodents, plants and humans [3], [4], [5], [6], [7].
A major strength of the eQTL approach is the inherent ability to distinguish between local and distant regulation [8] of gene expression, by classifying eQTLs as cis and trans. Cis-eQTLs are those in which the eQTL maps to the physical location of the transcript [5]. Cis-eQTLs have been shown generally to be highly heritable and to have a larger genetic effect than trans-eQTLs [9]. Of particular interest are those cis-eQTLs that co-localise with mapped physiological quantitative trait loci (pQTLs) [5], [10], [11], [12], as these can be considered to be strong candidates for the genetic regulation underlying variation in the physiological trait. To prioritize cis-eQTLs, Schadt et al applied data resulting from the correlation of expression profiles with phenotypic measurements to identify candidate genes underlying physiological QTLs in mice [10]. Similar procedures have been carried out in other model organisms [7], [11], [13], [14], [15]. Using these approaches in humans, Deutsch et al [16] and Goring et al [17] identified candidate genes for Down's syndrome phenotypes and plasma HDL cholesterol concentration respectively.
Trans-eQTLs are those in which the expression of a given gene maps to a location remote from the physical location of the gene itself. Trans-eQTLs are considered indicative of gene expression levels that are under polygenic control [9]. Because trans-eQTLs generally have small genetic effects and often a correspondingly high False Discovery Rate (FDR), they are relatively difficult to detect [9], [17]. They are however of interest as potential indirect regulators of gene expression [18]. It has been observed that trans-eQTLs form clusters, or ‘hot-spots’ [19], where mRNA levels of transcripts across the genome show linkage to the same genetic locus. Such trans-eQTL clusters have also been detected through the application of quite straightforward correlation-based methods [19], [20] or functionally-informed correlation analysis [7]. The possibility of functional enrichment within trans-eQTL clusters has been explored in other datasets [20], [21], with outcomes suggesting that the presence of clusters is predictive of biological relationships between the underlying genes.
Co-expressed, functionally enriched trans-eQTL clusters are suggestive of co-ordinated regulation, perhaps by a ‘master’ transcriptional regulator located within the linkage region [3], [22]. Previous attempts to prioritise candidate regulators in silico have involved the correlation of the expression levels of the genes comprising the trans-eQTL cluster with those of candidate regulators co-localising with the cluster locus [18].
Correlation-based methods have been applied to prioritise candidate genes or to infer co-expression networks, but in many cases genotype information is not utilised in the analysis [23], [24]. Others have taken into account the effect of correlation due to linkage disequilibrium [12], [25] but not long-range allelic association [26].
Here, we extend these analyses, taking into account the correlation of the genotypes underlying cis- and trans-eQTLs in multiple tissues. We carried out a full-scale integrative co-expression analysis of gene expression in a large panel of rat recombinant inbred (RI) strains, the BXH/HXB panel [27]. Genotypic and genetic map information was fully integrated into the analysis. We show that taking into account genetic distance data is requisite in the prediction of candidate regulators of trans-eQTL clusters. The outcomes of this study provide new insight into the relationship between gene expression and underlying genotype in cis- and trans-eQTLs across multiple tissues.
Results
Correlation of gene expression levels of cis- and trans-acting eQTLs
Pairwise correlation analysis was performed in all eQTLs detected with genome-wide significance p<0.05 in fat, kidney, adrenal and left ventricle tissues (Table 1). The correlation structure in genes that form cis-eQTLs were consistently found to differ markedly from that in genes that form trans-eQTLs in all tissues. Gene expression levels of cis-eQTLs were found to be significantly (FDR<0.05) correlated to one another in 0.6 to 0.9% of all pairs (Table 2). 80.4 to 91.5% of these were found to have correlated genotypes across the RI strains (i.e., Strain Distribution Patterns (SDPs)), most of which (>67.9% (Table S1)) could be explained by linkage disequilibrium (LD) (Figure 1). Of the significantly correlated pairs of cis-eQTLs that can not be explained by LD, 39.6 to 55.6% have correlated SDPs (Table S1), even though the eQTLs are often located on different chromosomes. The remainder of the correlated pairs of cis-eQTL genes cannot be explained by similarity of the underlying genotypes.
Table 1. Numbers of cis- and trans- expression QTLs identified in each of the four rat tissues at genome-wide corrected p≤0.05.
| Tissue | No. of cis-eQTLs* | No. of trans-eQTLs* |
| Fat | 558 | 923 |
| Kidney | 718 | 1,033 |
| Adrenal | 602 | 933 |
| Left Ventricle (LV) | 1,362 | 2,140 |
| Total | 3,240 | 5,029 |
cis- and trans-eQTLs are defined as follows: A cis-eQTL is one in which the peak of linkage is within 10 Mb of the transcript. Any other eQTL is defined as a trans-eQTL.
Table 2. Outcomes of correlation analysis of cis-eQTL genes in each of the four tissues.
| Tissue | Total Number of Pairs of cis-eQTLs | No. Significantly Correlated Pairs of cis-eQTL genesa (q<0.05) | % Significantly Correlated Pairs of cis-eQTL genesa | No. (%) Correlated Pairsa with Significantly Correlated Genotypesb at Peaks of Linkage |
| Fat | 155,403 | 1,386 | 0.9 | 1,114 (80.4%) |
| Kidney | 257,403 | 2,284 | 0.9 | 1,979 (86.6%) |
| Adrenal | 180,901 | 1,443 | 0.8 | 1,294 (89.7%) |
| LV | 926,841 | 5,371 | 0.6 | 4,912 (91.5%) |
Significantly correlated pairs are defined as those in which the absolute Pearson correlation of the gene expression levels was empirically found to be statistically significant (FDR<0.05), following control for multiple testing.
Correlation of genotypes at a pair of eQTLs across the RI strain panel was assessed by calculating the matching coefficient. Genotypes are described as significantly correlated if the total number of matched pairs is found to be significant by a two-tailed test of probability (p<0.05).
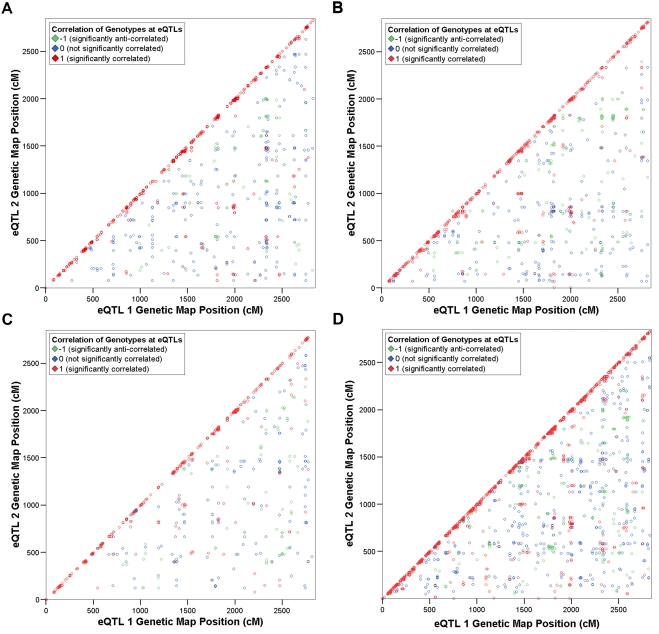
Figure 1. Scatter plots showing pairs of cis-eQTL genes with significantly correlated expression profiles by genetic map location.
Significantly correlated pairs of cis-eQTL genes identified in a) fat, b) kidney, c) adrenal and d) left ventricle are plotted according to genetic map location (cM), indicating significance or otherwise of correlation of strain distribution patterns (SDPs) at the peak of linkage. Colour coding indicates significance of correlation of SDPs (p<0.05; see Methods). Pairs consisting of probesets located close to one another, which correspondingly have nearby peaks of linkage, are disproportionately represented among significantly correlated pairs as indicated by the red diagonal.
Consistently across all tissues, we found a greater proportion of pairs of trans-eQTL genes (2.9 to 14.9%) that showed significant correlation of expression levels (Table 3), compared with cis-eQTLs. Unlike cis-eQTLs, a substantial overrepresentation of pairs of trans-eQTLs whose underlying genes are both located on the same chromosome was not observed (Figure 2). However, when the SDPs at the significantly correlated trans-eQTLs were tested for correlation (Figure 3), we detected a high proportion (47.5 to 63.7%) of significantly (p<0.05) similar SDPs (Table 3). This was investigated more closely by analysing the distances between the map locations of these trans-eQTLs. We found that 23.1 to 39.1% of significantly correlated trans-eQTL genes form eQTLs that are located within 1 cM of one another (Table S2). This suggests the presence of groups, or clusters, of co-regulated trans-eQTLs.
Table 3. Outcomes of correlation analysis of trans-eQTL genes in each of the four tissues.
| Tissue | Total Number of Pairs of trans-eQTLs | No. Significantly Correlated Pairs of trans-eQTL genesa (q<0.05) | % Significantly Correlated Pairs of trans-eQTL genesa | No. (%) Correlated Pairsa with Significantly Correlated Genotypesb at Peaks of Linkage | No. (%) Correlated Pairsa with Significantly Correlated Genotypesb at Probeset Locations |
| Fat | 425,503 | 63,477 | 14.9 | 31,288 (49.3%) | 6,375 (10.0%) |
| Kidney | 533,028 | 39,257 | 7.4 | 18,628 (47.5%) | 4,601 (11.7%) |
| Adrenal | 434,778 | 12,482 | 2.9 | 7,945 (63.7%) | 1,370 (11.0%) |
| LV | 2,288,730 | 78,826 | 3.4 | 44,110 (56.0%) | 8,450 (10.7%) |
As described in Table 2.
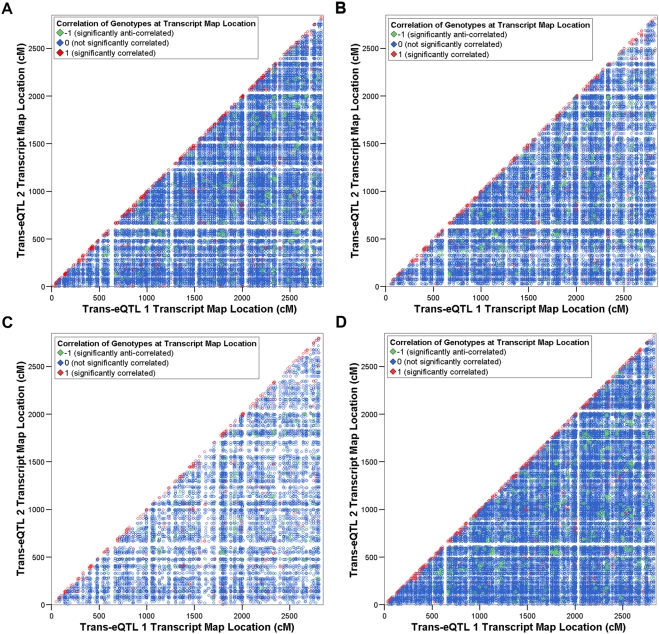
Figure 2. Scatter plots showing pairs of trans-eQTL genes with significantly correlated expression profiles by transcript genetic map location.
Significantly correlated pairs of trans-eQTL genes identified in a) fat, b) kidney, c) adrenal and d) left ventricle are plotted according to genetic map location of the transcripts. Colour coding is as in Figure 1.
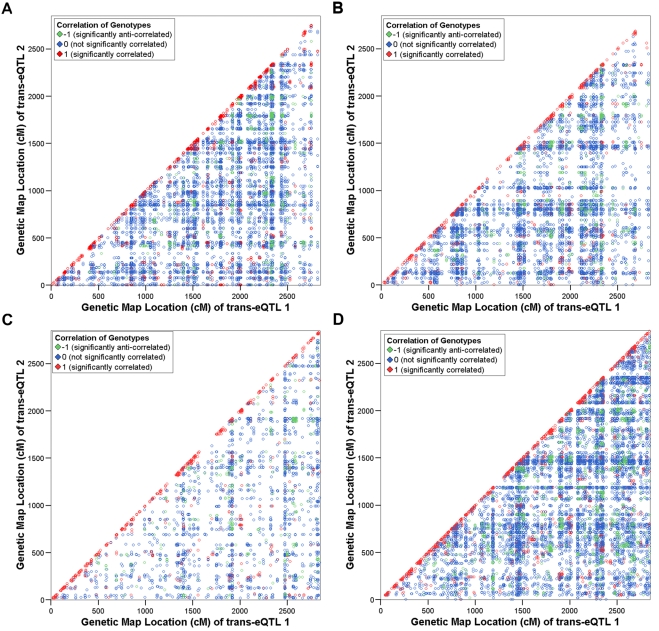
Figure 3. Scatter plots showing pairs of trans-eQTL genes with significantly correlated expression profiles by genetic map location of peak of linkage.
Significantly correlated pairs of trans-eQTLs identified in a) fat, b) kidney, c) adrenal and d) left ventricle are plotted according to genetic map position of the peak of linkage. Colour coding is as in Figure 1. Over-representation of pairs of trans-eQTLs with nearby peaks of linkage among the significantly correlated set can be observed (in the red diagonal) in all four tissues.
Co-regulation analysis of trans-eQTL clusters
A total of 81 trans-eQTL clusters, defined as 10 or more co-localising trans-eQTLs, were identified across all four tissues (Table S3). Levels of significant correlation within trans-eQTL clusters (Figure 4) were found to be much greater than in the rest of the trans-eQTL dataset. Significant correlations ranged from 77.2 to 90.2% of all pairs (Table 4) whereas in the trans-eQTL dataset as a whole they ranged from 2.9 to 14.0% of all pairs (Table 3).
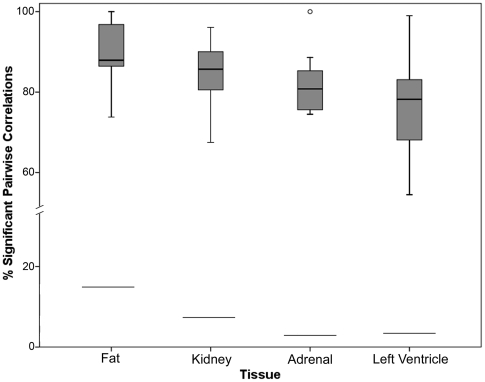
Figure 4. Boxplots showing percentage within-cluster correlation of genes underlying trans-eQTLs forming trans-eQTL clusters.
For each of the four tissues, the percentages of significantly correlated pairs of trans-eQTL genes within trans-eQTL clusters are displayed. The boxplots indicate the median, interquartile range, and range of within-cluster correlation in each tissue. The percentage significant correlation among all trans-eQTLs is shown for each of the four tissues as horizontal lines, for purpose of comparison with the trans-eQTL clusters. One outlier is shown, a cluster in adrenal found to have 100% significant pairwise correlation of gene expression levels (see Table S3).
Table 4. Summary of outcomes of pairwise correlation of trans-eQTL cluster genes.
| Tissue | No. of Trans-eQTL Clusters (≥10 trans-eQTLs) | Mean Trans-eQTL Cluster Size (no. of trans-eQTLs) | Trans-eQTL Cluster Ave. % Significantly Correlated Pairsa |
| Fat | 11 | 30.8 | 90.2 |
| Kidney | 23 | 19.5 | 84.2 |
| Adrenal | 9 | 19.5 | 82.4 |
| LV | 38 | 25.1 | 77.2 |
| Overall | 81 | 23.7 | 83.5 |
As described in Table 2.
We tested for functional enrichment within trans-eQTL clusters through Gene Ontology (GO) annotations using DAVID, a web-based functional annotation tool [28]. This analysis was performed on 15 large trans-eQTL clusters consisting of 30 or more transcripts, because smaller clusters were considered unlikely to provide sufficient statistical power for such an analysis (see Methods). Of these, 12 (80%) were found to have at least one significant biological process or metabolic function GO term at an uncorrected significance level of p<0.01 and almost half have at least one significant term at uncorrected p<0.001 (Table 5). To assess the significance of these findings, simulation studies were carried out using DAVID on 200 random sets of 47 unique trans-eQTL genes. We found a significantly increased functional enrichment at all three GO p-value thresholds (p<0.001, p<0.01, p<0.05) in the trans-eQTL datasets relative to the random sets (Kolmogorov-Smirnov Exact test p-values: p = 0.001, p = 0.002, p = 0.004).
Table 5. Outcome of GO analysis of trans-eQTL clusters consisting of 30 or more eQTLs using DAVID, a functional annotation software tool.
| Tissue | Marker at Cluster Peak of Linkage | No. Cluster trans-eQTLs | Significant GO terms at p<0.001 | Significant GO terms at p<0.01 | Significant GO terms at p<0.05 |
| LV | D15Rat98 | 30 | 0 | 0 | 1 |
| Adrenal | D11Rat16 | 31 | 0 | 2 | 7 |
| Fat | D4Rat240 | 31 | 2 | 4 | 8 |
| Fat | Cacna1s | 33 | 0 | 1 | 4 |
| LV | Cyp45c | 35 | 0 | 0 | 0 |
| LV | Ckb | 43 | 3 | 4 | 12 |
| LV | D15Ucsf1 | 46 | 0 | 2 | 12 |
| Adrenal | D17Rat144 | 47 | 0 | 1 | 8 |
| Kidney | Igk | 49 | 1 | 3 | 14 |
| LV | D15Utr2 | 51 | 2 | 13 | 20 |
| LV | D15Rat29 | 54 | 8 | 15 | 28 |
| Kidney | D15Rat69 | 57 | 9 | 13 | 20 |
| LV | D8Mit12 | 77 | 0 | 0 | 3 |
| Fat | D17Rat1 | 146 | 3 | 20 | 53 |
| LV | Crabp1 | 165 | 0 | 4 | 14 |
Prediction of candidate ‘master regulators’
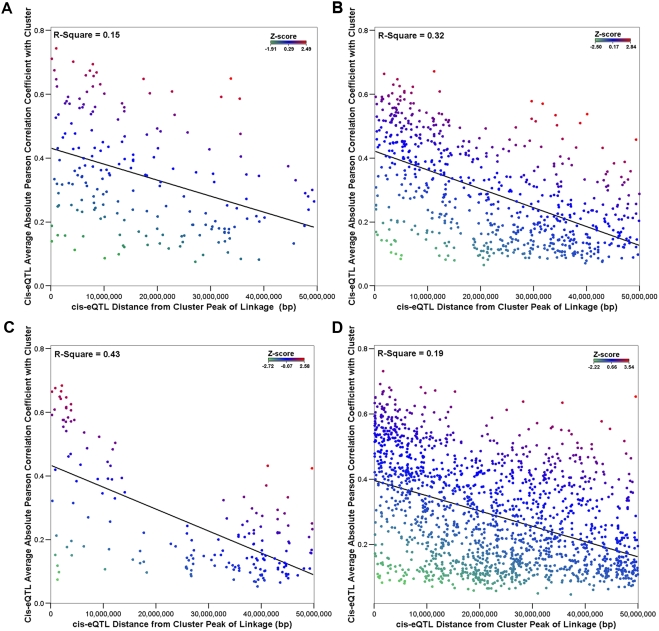
In order to prioritize cis-eQTLs as candidate ‘master regulators’ [19] of the trans-eQTL clusters, expression profiles of all cis-eQTL genes located within 50 Mb either side of the cluster locus were tested for co-expression with those of the trans-eQTL cluster genes. We found levels of correlation to be dependent on the distance of the cis-eQTL from the cluster peak of linkage. Upon linear regression of correlation against distance between gene and peak of linkage, R2 in all four tissues was found across clusters to show a negative relationship (Figure 5) with distance from the peak; ranging from 0.15 to 0.43.
Figure 5. Scatter plots showing correlation of genes underlying cluster trans-eQTLs and cis-eQTLs located in the window region, plotted against their distance from the peak of linkage.
Each point on the scatter plot represents a cis-eQTL within the defined window region of a trans-eQTL cluster. The average Pearson coefficient of correlation of the underlying transcript expression levels of the cis-eQTL with those of the cluster trans-eQTLs is shown to be strongly negatively correlated with distance in a) fat, b) kidney, c) adrenal, d) LV, R2 for this relationship ranges from 0.15 to 0.43. The Z-score, calculated from the vertical distance of each cis-eQTL from the regression line, is indicated by the colour of each point, indicated by the colour legend in the top right of each plot.
The analysis was subsequently carried out on all transcripts located in the same region, regardless of whether they form eQTLs or not. Regression analysis between distance and average correlation with the trans-eQTL cluster genes showed no relationship (R2: 0–0.01) (Figure S1), indicating that the relationship observed in cis-eQTLs is explained by the linkage of those probesets to the genetic region concerned. In order to investigate the extent of genetic control of gene expression in the cis-eQTLs in the region, we also calculated transcript heritability as previously described [9]. There was found to be no relationship between distance of the cis-eQTL from the cluster linkage region and heritability of the cis-eQTL (Figure S2).
These findings suggest that correlation of expression levels of cis-eQTLs in the vicinity of the peak of linkage of a trans-eQTL cluster with those of the trans-eQTLs making up said cluster, without taking into account factors relating to genetic linkage is inappropriate for the purpose of identifying putative regulators. Analysis of outliers of the relationship between distance and correlation may hold more promise to identify candidates than simply looking at highly correlated eQTLs. For this purpose, Z-scores (based on the outcome of the regression analysis) were calculated for each tissue. Cis-eQTLs with significant Z-scores (defined as Z>2) in each tissue are shown in Table S5. These cis-eQTLs may represent candidates for the regulation of the trans-eQTL clusters.
Discussion
Our genome-wide studies of expression levels of genes underlying cis- and trans-eQTLs provide strong support for the hypotheses that the categorisation of eQTLs has a genuine biological basis that can be detected in transcript expression levels, and that trans-eQTL clusters consist of functionally related and co-ordinately regulated transcripts.
We observed that patterns of correlation of expression profiles and of SDPs at the genetic location of the transcript whose expression was measured are strikingly different between the sets of cis- and trans-eQTLs in all four tissues (Figures 1– 3). Significant correlation between pairs of cis-eQTLs was found to be rare (<1%, Table 2). A significant relationship has previously been shown between the absolute correlation coefficient of pairs of eQTLs derived from genes located on the same chromosome and the distance apart of those genes [10], [24]. Hence it was hypothesised that correlation of cis-eQTL genes can be explained in terms of linkage disequilibrium. We found that most of the observed correlations between cis-eQTL genes' expression profiles for co-localised genes can be explained by similarity of underlying genotypes. We also found that most significant correlations between cis-eQTLs located on different chromosomes can be explained by long-range allelic association. Such patterns of association have previously been observed in a SNP map of a mouse inbred strain panel [26], and hypothesised to have the potential to produce spurious associations between transcripts' expression profiles. Our findings suggest that correlation of expression profiles, in isolation, is not a suitable basis for the analysis of relationships between cis-eQTLs.
Much higher levels of significant correlation, encompassing 2.9–14.9% of pairs, were observed between trans-eQTL genes. This was not found to be explained by correlation of the genotypes at the regions to which the transcripts map. However, when genotypes at the trans-eQTL are similar or the same, the gene pairs were disproportionately correlated (47.5 to 63.7% of significantly correlated pairs). This observation provides strong support for the hypothesis, formulated in studies of genetic regulation of gene expression in S. cerevisiae [19], that trans-eQTL clusters are indicative of co-regulation of the transcripts. In our dataset, the level of significant correlation within trans-eQTL clusters was found to be far higher than across the trans-eQTL dataset as a whole, averaging 83.5% across the 81 clusters (Table 4). Additionally, we observed that much of the variability in the levels of trans-eQTL correlation between tissues can be explained by differences in the distribution and size of the trans-eQTL clusters (data not shown).
Functional investigation of the large trans-eQTL clusters showed significant over-representation of Gene Ontology (GO) terms in 80% of these clusters. While GO analysis may have limitations in scope and accuracy, owing to the annotation challenges posed by genes which often have complex and imprecisely defined roles and functions [29], the findings presented here are in agreement with those of Ghazalpour et al [25], who observed that genes in functionally related ‘pathway sets’ are typically highly correlated. KEGG pathway analysis of the trans-eQTL clusters was carried out through the DAVID interface, and pathways of potential interest were identified in a minority of the clusters (Table S6). It has previously been shown in a study of co-regulation in a mouse F2 intercross [20] that groups of highly correlated transcripts linked to the same genomic location can be identified in a functionally informed genome-wide correlation analysis. Here we show across multiple tissues that highly correlated expression profiles are a consistent motif of such trans-eQTL clusters. These results suggest a significant degree of functional relatedness of the genes making up the cluster (Table 5).
Expression correlation between trans-eQTL cluster transcripts and cis-eQTL genes located in the linkage region has been previously described as a method of identifying candidate regulators and applied in yeast [30] and Arabidopsis [7]. Here we show a strong relationship between the distance of the cis-eQTL from the cluster peak of linkage and the strength of correlation (Figure 5), which was not observed when unlinked transcripts located in the region were similarly tested (Figure S1). This, along with the observation that there is no association between distance from the peak of linkage and heritability of the cis-eQTL genes (Figure S2), suggests that genotype similarity at the linkage region, rather than the genetic influence on gene expression underlies the relationship. On this basis, correlation-based methods of prioritising candidates for genetic regulation of trans-eQTL clusters would be improved by taking into account the distance between the cluster linkage region and the map location of the candidate transcript. We therefore suggest that it may be of interest instead to identify outliers; those cis-eQTL genes whose average correlation with the trans-eQTL cluster genes significantly deviates positively from the general negative regression trend. We were able to find 54 cis-eQTLs with significant positive Z-scores (Z>2) (Table S4), and consider that these may be worthy of further investigation into the possibility that their correlation with the cluster transcripts has a biological basis.
In this study, we demonstrate the power of computational analysis of eQTL datasets across multiple tissues to provide new insights into the genome-wide correlation structure in gene expression data. Our findings consistently show that correlation of cis-eQTL genes' expression profiles is primarily indicative of similarity of genetic inheritance, as measured by the correlation of SDPs at the transcript location. Whereas, correlation between trans-eQTLs can frequently be explained in terms of co-regulation by a common linkage region even though correlated transcripts forming trans-eQTL clusters are located throughout the genome. The observation of functional enrichment within clusters is suggestive of a relationship between co-expression and function. Finally, we inform investigations of candidate regulators of trans-eQTL clusters by indicating that genetic linkage strongly influences co-expression of trans-eQTL cluster genes and candidate regulatory genes.
Materials and Methods
The eQTL dataset
The gene expression dataset used in this study derives from the BXH/HXB panel of 29 RI strains produced from progenitor strains SHR and BN.Lx previously as described [5], [9]. Gene expression was measured in four tissues using Affymetrix GeneChips with appropriate biological and technical replication. For the retroperitoneal fat, kidney and adrenal gland tissues, Rat Expression Array 230A GeneChips were used, on which 15,923 transcripts are represented. Rat Genome 230 2.0 GeneChips, on which 31,099 transcripts are represented (including the same 15,923 as on 230A), were used for left ventricle. The summary value for each expression trait (as described in [5]) was used in the generation of the eQTLs upon which this analysis was performed. This was achieved through the application of a genome-wide linkage methodology, testing for linkage between each expression trait and the same 1,011 microsatellite markers in each of the four study tissues. QTL Reaper ( http://www.genenetwork.org/qtlreaper.html ) was used to carry out this analysis. The software calculates a likelihood ratio statistic (LRS) for each combination of marker and probeset, and uses permutation to estimate an empirical genome-wide probability of obtaining such a score, accounting for multiple testing [5]. The eQTLs used in this analysis are significant at genome-wide p<0.05.
Cis- and trans-eQTLs
The categorisation of eQTLs as cis or trans was determined, following the merging of linkages to tightly linked markers, by eQTL Explorer [31] (http://web.bioinformatics.ic.ac.uk/eqtlexplorer). Cis-eQTLs were defined empirically as having a peak of linkage within 10 Mb of the physical location of the probeset as described previously [9]. Those eQTLs mapped to locations that are 10 Mb or more apart, or where the probeset is located on a different chromosome to the peak of linkage are defined as trans-eQTLs. Probeset location data is obtained from Affymetrix NetAffx (http://www.affymetrix.com/analysis/index.affx) and checked using EnsEMBL (http://www.ensembl.org/) (v42) data. Probesets that map to more than one place in the genome according to the latter were removed from the analysis using SCAMPA (http://microarray.csc.mrc.ac.uk/scampa/), as were those that do not map at all – in which case the cis/trans definition may be inaccurate. The resultant filtered set used in the correlation analysis consists of a total of 8,269 eQTLs across the four tissues, 3,240 of which are defined as cis-eQTLs and the remaining 5,029 as trans-eQTLs.
Trans-eQTL clusters
A ‘trans-eQTL cluster’ was defined as 10 or more co-localising trans-eQTLs. Trans-eQTL clusters within 1 Mb of one another were merged following initial detection of co-ordinated linkage of at least 10 transcripts to the region. For the purpose of prioritising ‘master regulator’ candidates, the ‘window region’ of a trans cluster was conservatively defined as the region of the genome 50 Mb either side of the physical map location of the peak of linkage of the cluster prior to any merging.
Correlation of Expression Data
Correlation of expression levels of genes underlying eQTLs was carried out using Matlab. A raw Pearson coefficient and associated nominal p-value were calculated for each pair of eQTLs. Additionally, an empirical p-value was determined using a permutation-based method robust against non-normality of the underlying distribution [32]. In each tissue, all pair combinations of cis-eQTLs and of trans-eQTLs were correlated. Separately, all pairs of trans-eQTLs within each of the 81 trans-eQTL clusters were correlated with each other and with the cis-eQTLs in the 50 Mb ‘window region’. All of these analyses entailed the testing of substantial numbers of simultaneous hypotheses. Statistical significance of obtained correlation coefficients, taking this into account, was assessed using the q-value (http://faculty.washington.edu/jstorey/qvalue/) [33] method of estimating the False Discovery Rate (FDR), and defined as q<0.05.
Correlation Analysis of Marker SDPs
The correlation of the SDPs of the markers at the peaks of linkage of the eQTLs in each correlated pair was assessed using a matching coefficient. For each strain, a match in the marker genotypes was scored 1, and a mismatch scored 0. Strains with missing genotype data at the marker locus were not used in the calculation of the matching coefficient, which was assessed by dividing the total number of matches by the total number of strains with no missing genotypes. Because both correlation and anti-correlation of SDPs can explain significant correlation of eQTL transcript expression levels, the two-tailed significance (p<0.05) of the resulting coefficient was found by assessing the probability of the occurrence of all combinations, given the distribution of expected matches.
Assessment of Functional Coherence of Trans-eQTL clusters
The possibility of functional enrichment in the genes that make up the observed trans-eQTL clusters was assessed through testing of their GO annotations using the functional annotation clustering component of the web-based genetic data analysis tool DAVID (http://david.abcc.ncifcrf.gov) [28], [34]. This analysis was performed on 15 clusters consisting of 30 or more eQTLs, since only these clusters provide enough statistical power considering that not all transcripts are annotated. Pathway analysis was performed through the same interface, using data from KEGG (http://www.genome.jp/kegg) [35]. 200 control groups of 47 probesets (47 being the median size of the 15 large trans-eQTL clusters) were randomly generated from the set of all trans-eQTLs across all four tissues and each was tested for enrichment of gene ontology terms in the same way as were the real clusters using DAVID. A Kolmogorov-Smirnov test was used to test the statistical significance of the degree of enrichment found in the 15 large trans-eQTL clusters, compared to the control groups. This analysis was carried out using SPSS v14.
Distance Modelling
In the investigation of the correlation of expression levels of trans-eQTL clusters and colocalising cis-eQTLs, the relationship between correlation coefficient and distance of eQTL from the peak of linkage of the trans-eQTL cluster was tested by performing a regression analysis as follows: For each cluster, the genetic distance between the peak of linkage and each of the cis-eQTLs in the window region was found. The distance was plotted against the averaged absolute correlation coefficient of the expression profile measured by that probeset and those of the trans-eQTLs making up the cluster. The data for all of the clusters in each tissue was then combined to produce the plots shown in Figure 5, and linear regression was performed on the data. In addition, the relationship between heritability data (as described in Petretto et al [9]) and cis-eQTL distance from the peak of linkage was tested (Figure S2). Z-scores were calculated from the vertical distance of each cis-eQTL from the regression line, following testing for normality of the distribution of vertical distances. For comparative purposes, the analysis as described was also performed using all of the probesets in the ‘window region’ (as opposed to just cis-eQTLs) (Figure S1).
Supporting Information
Scatter plots showing correlation of genes underlying cluster trans-eQTLs with all probesets mapped to within 50 Mb of the linkage region, plotted against the physical distance of the probeset from the peak of linkage in a) fat, b) kidney, c) adrenal, d) LV.
(6.79 MB TIF)
Z-score of cis-eQTL distance from regression line ( Figure 5 ) plotted against probeset heritability in a) fat, b) kidney, c) adrenal or d) LV.
(3.24 MB TIF)
Outcomes of correlation analysis of pairs of cis-eQTL genes (q<0.05), whose peaks of linkage are located more than 50 cM apart.
(0.03 MB DOC)
Outcomes of correlation analysis of trans-eQTL genes (q<0.05) whose peaks of linkage are located 50 cM or less apart.
(0.05 MB DOC)
Outcomes of correlation analysis of expression profiles of genes forming trans-eQTL clusters
(0.14 MB DOC)
Outcomes of correlation of cis-eQTL genes in the ‘window regions’ of trans-eQTL clusters with cluster-forming genes.
(0.14 MB DOC)
cis-eQTL genes located within the window region of a trans-eQTL cluster positively deviating (Z>2) from regression of cluster-averaged correlation coefficient against distance of cis-eQTL from linkage region ( Figure 5 ).
(0.08 MB DOC)
Supplementary information on functional enrichment analysis of large (>30 transcripts) trans-eQTL clusters.
(0.04 MB DOC)
Acknowledgments
We thank the MRC CSC Computing Department for providing computational facilities including support for large-scale statistical analysis using MatLab and SPSS software and general technical assistance, and Centre for Bioinformatics at Imperial College for supplying server space and general technical assistance. We also thank Anuj Goel for helpful comments and suggestions, and Rizwan Sarwar and Liqun He for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: We acknowledge funding to ICG from the Wellcome Trust (069962/Z/02/Z); to TJA from the Medical Research Council Clinical Sciences Centre, the Wellcome Trust Cardiovascular Functional Genomics award; to TJA and SAC from the Fondation Leducq; to EP, NJD, SAC and JM from the Medical Research Council; to SAC from The Department of Health UK and British Heart Foundation; to NH from the German Ministry of Science and Education (BMBF/NGFN); to MP from the Grant Agency of the Czech Republic; to MP and VK from the Ministry of Education of the Czech Republic; MP is an international research scholar of the Howard Hughes Medical Institute; and to NH, MP and TJA from the EU-funded EURATools Integrated Project. Sponsors/funders played no direct role in the design or conduct of this study.
References
- 1.Jansen RC, Nap J-P. Genetical genomics: the added value from segregation. Trends in Genetics. 2001;17:388–391. doi: 10.1016/s0168-9525(01)02310-1. [DOI] [PubMed] [Google Scholar]
- 2.Damerval C, Maurice A, Josse JM, de Vienne D. Quantitative trait loci underlying gene product variation: a novel perspective for analyzing regulation of genome expression. Genetics. 1994;137:289–301. doi: 10.1093/genetics/137.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley M, Molony CM, Weber TM, Devlin JL, Ewens KG, et al. Genetic analysis of genome-wide variation in human gene expression. Nature. 2004;430:743–747. doi: 10.1038/nature02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrabian M, Allayee H, Stockton J, Lum PY, Drake TA, et al. Integrating genotypic and expression data in a segregating mouse population to identify 5-lipoxygenase as a susceptibility gene for obesity and bone traits. Nat Genet. 2005;37:1224–1233. doi: 10.1038/ng1619. [DOI] [PubMed] [Google Scholar]
- 5.Hubner N, Wallace CA, Zimdahl H, Petretto E, Schulz H, et al. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat Genet. 2005;37:243–253. doi: 10.1038/ng1522. [DOI] [PubMed] [Google Scholar]
- 6.Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- 7.Keurentjes JJB, Fu J, Terpstra IR, Garcia JM, van den Ackerveken G, et al. Regulatory network construction in Arabidopsis by using genome-wide gene expression quantitative trait loci. Proceedings of the National Academy of Sciences. 2007;104:1708–1713. doi: 10.1073/pnas.0610429104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan S, Huang RS, Zhang W, Bleibel WK, Roe CA, et al. Genetic Architecture of Transcript-Level Variation in Humans. The American Journal of Human Genetics. 2008;82:1101–1113. doi: 10.1016/j.ajhg.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petretto E, Mangion J, Dickens NJ, Cook SA, Kumaran MK, et al. Heritability and tissue specificity of expression quantitative trait loci. PLoS Genet. 2006;2:e172. doi: 10.1371/journal.pgen.0020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005;37:710–717. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Schadt EE, Wang H, Wang X, Ingram-Drake L, et al. Identification of Pathways for Atherosclerosis in Mice: Integration of Quantitative Trait Locus Analysis and Global Gene Expression Data. Circ Res. 2007;101:e11–30. doi: 10.1161/CIRCRESAHA.107.152975. [DOI] [PubMed] [Google Scholar]
- 12.Doss S, Schadt EE, Drake TA, Lusis AJ. Cis-acting expression quantitative trait loci in mice. Genome Res. 2005;15:681–691. doi: 10.1101/gr.3216905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng H, Vera I, Che N, Wang X, Wang SS, et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proceedings of the National Academy of Sciences. 2007;104:4530–4535. doi: 10.1073/pnas.0607620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petretto E, Sarwar R, Grieve I, Lu H, Kumaran MK, et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet. 2008;40:546–552. doi: 10.1038/ng.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pravenec M, Churchill PC, Churchill MC, Viklicky O, Kazdova L, et al. Identification of renal Cd36 as a determinant of blood pressure and risk for hypertension. Nature Genetics. 2008;40:952–954. doi: 10.1038/ng.164. [DOI] [PubMed] [Google Scholar]
- 16.Deutsch S, Lyle R, Dermitzakis ET, Attar H, Subrahmanyan L, et al. Gene expression variation and expression quantitative trait mapping of human chromosome 21 genes. Hum Mol Genet. 2005;14:3741–3749. doi: 10.1093/hmg/ddi404. [DOI] [PubMed] [Google Scholar]
- 17.Goring HHH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat Genet. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 18.Tu Z, Wang L, Arbeitman MN, Chen T, Sun F. An integrative approach for causal gene identification and gene regulatory pathway inference. Bioinformatics. 2006;22:e489–496. doi: 10.1093/bioinformatics/btl234. [DOI] [PubMed] [Google Scholar]
- 19.Yvert G, Brem RB, Whittle J, Akey JM, Foss E, et al. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat Genet. 2003;35:57–64. doi: 10.1038/ng1222. [DOI] [PubMed] [Google Scholar]
- 20.Lan H, Chen M, Flowers JB, Yandell BS, Stapleton DS, et al. Combined expression trait correlations and expression quantitative trait locus mapping. PLoS Genet. 2006;2:e6. doi: 10.1371/journal.pgen.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Delano DL, Mitro N, Su SV, Janes J, et al. Gene Set Enrichment in eQTL Data Identifies Novel Annotations and Pathway Regulators. PLoS Genetics. 2008;4 doi: 10.1371/journal.pgen.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petretto E, Mangion JM, Pravenec M, Hubner N, Aitman TJ. Integrated gene expression profiling and linkage analysis in the rat. Mammalian genome. 2006;17:480–489. doi: 10.1007/s00335-005-0181-1. [DOI] [PubMed] [Google Scholar]
- 23.Fuller T, Ghazalpour A, Aten J, Drake T, Lusis A, et al. Weighted gene coexpression network analysis strategies applied to mouse weight. Mammalian Genome. 2007;18:463–472. doi: 10.1007/s00335-007-9043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum PY, Chen Y, Zhu J, Lamb J, Melmed S, et al. Elucidating the murine brain transcriptional network in a segregating mouse population to identify core functional modules for obesity and diabetes. Journal of Neurochemistry. 2006;97:50–62. doi: 10.1111/j.1471-4159.2006.03661.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghazalpour A, Doss S, Zhang B, Wang S, Plaisier C, et al. Integrating genetic and network analysis to characterize genes related to mouse weight. PLoS Genet. 2006;2:e130. doi: 10.1371/journal.pgen.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cervino AC, Li G, Edwards S, Zhu J, Laurie C, et al. Integrating QTL and high-density SNP analyses in mice to identify Insig2 as a susceptibility gene for plasma cholesterol levels. Genomics. 2005;86:505–517. doi: 10.1016/j.ygeno.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Pravenec M, Gauguier D, Schott JJ, Buard J, Kren V, et al. Mapping of quantitative trait loci for blood pressure and cardiac mass in the rat by genome scanning of recombinant inbred strains. J Clin Invest. 1995;96:1973–1978. doi: 10.1172/JCI118244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis G, Sherman B, Hosack D, Yang J, Gao W, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biology. 2003;4:R60. [PubMed] [Google Scholar]
- 29.Khatri P, Draghici S. Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics. 2005;21:3587–3595. doi: 10.1093/bioinformatics/bti565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bing N, Hoeschele I. Genetical genomics analysis of a yeast segregant population for transcription network inference. Genetics. 2005;170:533–542. doi: 10.1534/genetics.105.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller M, Goel A, Thimma M, Dickens NJ, Aitman TJ, et al. eQTL Explorer: integrated mining of combined genetic linkage and expression experiments. Bioinformatics. 2006;22:509–511. doi: 10.1093/bioinformatics/btk007. [DOI] [PubMed] [Google Scholar]
- 32.Churchill GA, Doerge RW. Empirical Threshold Values for Quantitative Trait Mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storey JD, Tibshirani R. Statistical significance for genomewide studies. PNAS. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D, Sherman B, Tan Q, Collins J, Alvord WG, et al. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biology. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Research. 2008;36:D480. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots showing correlation of genes underlying cluster trans-eQTLs with all probesets mapped to within 50 Mb of the linkage region, plotted against the physical distance of the probeset from the peak of linkage in a) fat, b) kidney, c) adrenal, d) LV.
(6.79 MB TIF)
Z-score of cis-eQTL distance from regression line ( Figure 5 ) plotted against probeset heritability in a) fat, b) kidney, c) adrenal or d) LV.
(3.24 MB TIF)
Outcomes of correlation analysis of pairs of cis-eQTL genes (q<0.05), whose peaks of linkage are located more than 50 cM apart.
(0.03 MB DOC)
Outcomes of correlation analysis of trans-eQTL genes (q<0.05) whose peaks of linkage are located 50 cM or less apart.
(0.05 MB DOC)
Outcomes of correlation analysis of expression profiles of genes forming trans-eQTL clusters
(0.14 MB DOC)
Outcomes of correlation of cis-eQTL genes in the ‘window regions’ of trans-eQTL clusters with cluster-forming genes.
(0.14 MB DOC)
cis-eQTL genes located within the window region of a trans-eQTL cluster positively deviating (Z>2) from regression of cluster-averaged correlation coefficient against distance of cis-eQTL from linkage region ( Figure 5 ).
(0.08 MB DOC)
Supplementary information on functional enrichment analysis of large (>30 transcripts) trans-eQTL clusters.
(0.04 MB DOC)