Abstract
Background
Despite widespread use of low-dose psychostimulants for the treatment of attention deficit hyperactivity disorder (ADHD), the neural basis for the therapeutic actions of these drugs are not well-understood. We recently demonstrated that low-dose methylphenidate (MPH) increases catecholamine efflux preferentially within the prefrontal cortex (PFC), suggesting the PFC is a principal site of action in the behavioral-calming and cognition-enhancing effects of low-dose psychostimulants. To better understand the neural mechanisms involved in the behavioral actions of low-dose stimulants, the current study examined the effects of low-dose MPH on the discharge properties of individual and ensembles of PFC neurons.
Methods
Extracellular activity of multiple individual PFC neurons was recorded in freely moving rats using multi-channel recording techniques. Behavioral studies identified optimal, working memory-enhancing doses of intraperitoneal MPH. The effects of these low-doses of MPH on PFC neuronal discharge properties were compared to: 1) the effects of high-dose MPH on PFC neuronal discharge; 2) the effects of low-dose MPH on neuronal discharge within the somatosensory cortex.
Results
Only working memory-enhancing doses of MPH increased the responsivity of individual PFC neurons and altered neuronal ensemble responses within the PFC. These effects were not observed outside the PFC (i.e. within somatosensory cortex). In contrast, high-dose MPH profoundly suppressed evoked discharge of PFC neurons.
Conclusions
These observations suggest that preferential enhancement of signal processing within the PFC, including alterations in the discharge properties of individual PFC neurons and PFC neuronal ensembles, underlie the behavioral/cognitive actions of low-dose psychostimulants.
Keywords: Psychostimulants, ADHD, Dopamine, Norepinephrine, Attention, Working Memory, Principal component analysis, Multi-channel recording, Prefrontal cortex
Methylphenidate (MPH; Ritalin®) and other psychostimulants, are used extensively in the treatment of attention deficit/hyperactivity disorder (ADHD; see review 23,44). Importantly, low doses of these drugs exert behavioral-calming and cognition-enhancing actions in normal human and animal subjects (6,10,30,32,33,35,40). These effects are in contrast to the locomotor-activating and cognition-impairing actions of higher doses of these drugs (31,37). Given their widespread use, it is surprising that the neural mechanisms involved in the behavioral/cognitive actions of low-dose psychostimulants remain poorly understood
The prefrontal cortex (PFC) plays a critical role in the regulation of higher cognitive function, impulsivity and behavioral activity (for review: 7). Thus, it is of interest that low and cognition-enhancing doses of MPH increase extracellular levels of catecholamines preferentially within the PFC (10,22,29,30). These observations are consistent with evidence implicating PFC dysfunction in the neuropathology of ADHD (2,14,40). Combined, these observations suggest that the cognition-enhancing and therapeutic actions of low-dose stimulants involve the modulation of PFC neuronal function. However, currently the nature of these modulatory actions is unclear.
The current studies were designed to better understand the impact of low-dose psychostimulants on PFC circuit function. Behavioral studies first determined the degree to which low-dose intraperitoneal (IP) MPH affects PFC-dependent spatial working memory in rats, identifying a dose range that produced an inverted-U shaped facilitation/impairment. Subsequent studies determined the effects of these low-doses of MPH, as well as a higher and behaviorally-activating dose, on spontaneous and evoked discharge of individual neurons and neuronal ensembles within the PFC of unanesthetized rats. Further studies compared the effects of a cognition-enhancing dose of MPH on PFC neuronal discharge to actions of this dose on neuronal activity within the somatosensory cortex.
Results obtained indicate that the behavioral-calming and cognition-enhancing actions of low-dose psychostimulants involve a selective enhancement in the signal processing abilities of PFC neurons and alterations in the distributed representation of afferent input within the PFC. These actions contrast with the profound suppression of PFC neuronal responsivity by higher doses of MPH associated with behavioral activation and cognitive impairment. Combined, these observations provide novel insight into the neural mechanisms and circuitry associated with the therapeutic actions of low-dose psychostimulants.
Experimental Procedures
Animals
Male Sprague–Dawley rats (300–450 g; Charles River, Wilmington, MA) were singly-housed on a 13-h/11-h light/dark cycle and provided ad lib access to food and water. All procedures were in accordance with NIH guidelines and were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Drugs
MPH (Sigma Chemical Co., Saint Louis, MO) was dissolved in 0.9% saline. The temporal resolution of intraperitoneal (IP) drug delivery is superior over oral administration and thus was viewed as preferable for the current electrophysiological studies. Previous studies demonstrated that 2.0 mg/kg orally-administered and 0.5 mg/kg IP-administered MPH are equipotent in terms of plasma levels and effects on catecholamine efflux (10). In these studies, 2.0 mg/kg oral, but not 8.0 mg/kg, MPH improved working memory. Thus, the current studies examined the behavioral and electrophysiological effects of IP-administered MPH at 0.25 mg/kg, 0.5 mg/kg, 1.0 mg/kg, and 2.0 mg/kg as well as a substantially higher dose of 15.0 mg/kg.
Spatial working memory testing
Animals (n=15) were trained and tested in a T-maze-based delayed alternation task of spatial working memory similar to that described previously (10). Animals were rewarded when they entered the maze arm not chosen on the previous trial. A delay between trials was adjusted until performance was stable and within the range of 60–80% (range=10–100 secs). Stable performance was defined as two consecutive days in which performance did not differ by more than 10%. Animals were administered vehicle or MPH 20-minutes prior to testing. Changes in the correct number of trials were analyzed using a repeated-measures (dose) ANOVA. Post-hoc comparisons were performed using the Fisher LSD test.
Electrophysiological Recordings
Surgery
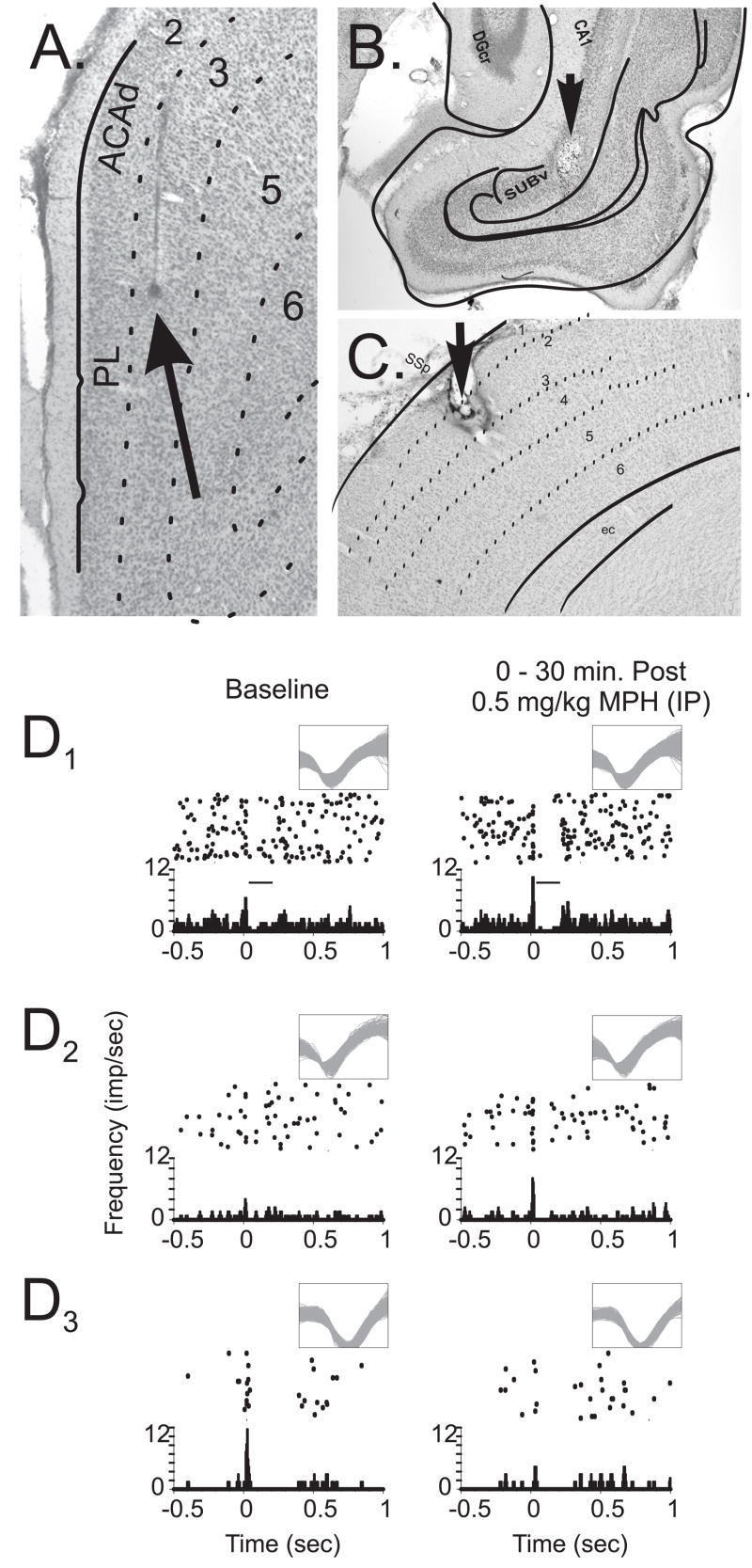
Animals (n=13) were implanted with microelectrode arrays (SB103, NB Labs, Dennison, TX) into the PFC (A+3.2, L±1.2; V−3.0) and a stimulation electrode (SNEX100-15, David Kopf, Tujunga, CA) into the CA1-subiculum region of the hippocampus (A−6.72, L±5.5, V−6.5). PFC microelectrode arrays were oriented in a rostral-caudal direction targeting the prelimbic subregion (layers III–V; Figure 1a–b). Additionally, two animals were implanted with microelectrode arrays into the barrel field (BF) somatosensory cortex (A−2.5, L±5.8, V−1.2) and a stimulating electrode around a single vibrissa (C3) of the whisker pad. BF cortex arrays were implanted such that with a rostral-caudal orientation, the majority of microelectrodes were in layers II/III–V of the C3 barrel (Figure 1c). Halothane anesthesia (Halocarbon Laboratories, River Edge, NJ; 1–4% in air) was used for PFC implants and chloral hydrate (390 mg/kg; Sigma, St. Louis, MO)-pentobarbital (25 mg/kg; Abbot Laboratories, North Chicago, IL) was used for BF cortex implants. Electrodes were attached to the skull with dental acrylic (Plastics One, Roanoke, VA) and animals were allowed to recover for 5–10 days. Following each experiment, electrode placements were marked and examined in ferrocyanide-reacted, counter-stained sections (Figure 1; and 19).
Figure 1.
Example photomicrographs of electrode placements and representative peri-stimulus time histograms (PSTH). A) 4X photomicrograph of the location of a recording microwire implanted into the prelimbic area of the PFC in layer III (Arrow Tip). B) Stimulating electrode placement within the CA1-subiculum region of the hippocampus (Arrow Tip; 10 X). C) Placement of the electrode tip in the somatosensory cortex (4X). D) Example cumulative raster and associated PSTH from three recorded neurons of the PFC before (Baseline) and after 0.5 mg/kg MPH. MPH administration produced four characteristic modulatory actions that included: facilitation of stimulus-evoked excitatory responses (D1), gating of otherwise subliminal afferent input (D2), suppression of excitatory responsiveness (D3), and facilitated stimulus-evoked inhibition (D1). X-axis = time (sec.) before and after stimulus presentation (0.0 sec); histogram Y-axis = spikes/sec for a given time bin (0.005 sec); raster Y-axis = trial number (top row, first trial). Inset waveforms indicate neural discrimination and recording across experimental conditions. Inset bar (D1) indicates the inhibitory response window for this example neuron. (PL, prelimbic; ACAd, anterior cingulate area, dorsal; DGcr, dentate gyrus crest; CA1 region of the hippocampus; SUBv, ventral subiculum; SSp, primary somatosensory cortex; ec, entorhinal cortex)
Recording sessions
Animals were tested and videotaped in sound-attenuating chambers (9), provided ad lib access to food and water, and habituated to the experimental procedure for at least two hours prior to experimentation. Multi-channel electrophysiological hardware and software (RASPUTIN, Plexon Inc, Dallas, TX) were used to record EEG (surface-depth) and simultaneously amplify, discriminate, and record action potential waveforms from putative single neurons (“units”) of the PFC or BF cortex, as previously described (19). During habituation to the chamber, repeated uniform biphasic current pulses were delivered through the CA1-subiculum or whisker pad electrodes and adjusted to elicit stimulus-evoked responses from at least 25% of the recorded units.
Baseline neuronal discharge/responsivity was recorded for 60-minutes in 15-minute blocks, each comprised of 5-minutes of spontaneous discharge followed by 10-mintues of stimulation of the CA1-subiculum (0.3–1.2 μA @ 0.25 Hz × 10-minutes=150 stimulations) or whisker pad (0.5–3 mA @ 0.5 Hz, 1 ms duration × 10-minutes=300 stimulations). Vehicle or MPH (0.25, 0.5, 1.0, 2.0, or 15.0 mg/kg) was administered IP following baseline recording. Spontaneous and stimulus-evoked PFC/BF cortex activity was then assessed in 15-minute blocks for the subsequent 90–180 minutes.
Analysis of electrophysiological data
The effects of MPH on EEG and the magnitude and timing of stimulation-evoked discharge were analyzed similar to that described previously (11,19,20). EEG (0.7–170 Hz; 1.0 kHz sample rate) recorded from the PFC or BF cortex was hand-scored off-line for desynchronized and slow-wave (≥2x desynchronous voltage) activity (see: 11). EEG analyses were used to assess 1) wake-promoting actions of MPH and 2) control for potential effects of fluctuations in sleep-wake state on the activity/reactivity of cortical neurons. Video-recordings confirmed that the majority of desynchronized activity was associated with waking. All electrophysiological analyses were limited to periods of nonsynchronous EEG. Criteria used to verify that individual waveforms originated from a single neuron (19,20) included characterization of: 1) waveform peak voltage, 2) waveform slope(s), 3) scattergram of the first two principal components of the action-potential’s sampled voltages, and 4) spike train auto-correlegram (19 see also: 20).
Stimulus-evoked discharge patterns of individual neurons were analyzed by quantifying peri-stimulus time histograms (PSTH) generated from equal numbers of randomly selected stimulus presentations during baseline and post-treatment periods (1di–iii; 19 and also: 15–17,20). Excitatory response windows were defined by sustained discharge rates exceeding 3 SD of spontaneous discharge. The peak response (peak–pre-stimulus average) and latency-to-peak that occurred within the excitatory window was determined. Stimulus-evoked inhibition or post-excitatory inhibition windows were defined by a 1 SD reduction in spontaneous discharge. Both the magnitude (pre-stimulus average–inhibition) and duration of inhibition were quantified. Treatment-induced changes in these measures were represented as percent change from baseline. In a number of cases, evoked-responses were initially absent or minimal during baseline recordings (< 3 SD above spontaneous rates), but became prominent and achieved significance following treatment; this modulatory action was classified as “gating”.
Discharge pattern relationships between many simultaneously recorded PFC neurons were also examined using principal component analysis (PCA). PCA eigenfunctions were generated by first decomposing the individual spike trains by PCA using correlation matrixes and varimax rotations as previously described (16 see also: 15,17,20). Second, individual spike trains were weighted with eigenvalues generated by PCA decomposition, summed across neurons, and plotted as a PSTH. The post-stimulus eigenfunction excitatory peak height was calculated for PC1, whereas peak-to-trough distance was determined for PC2. Differences in PCA eigenfunctions before and after treatments were analyzed to determine whether MPH produces an alteration in the distributed activity of an ensemble of simultaneously recorded PFC neurons (17).
Data were analyzed using a mixed two-way repeated-measures ANOVA (time, treatment). Post-hoc comparisons were performed using the Fisher LSD test.
RESULTS
Effects of IP MPH on Spatial Working Memory
The effects of IP administered low-dose MPH (vehicle, n=12; 0.5 mg/kg, n=12; and 2.0 mg/kg, n=8) on performance in a spatial working memory task were first examined. IP MPH produced an inverted-U shaped dose-dependent facilitation of working memory (dose, F(2,32)=9.882, p=0.001): 0.5 mg/kg MPH improved whereas 2.0 mg/kg impaired performance (Figure 2). Based on these observations, we examined the effects of varying doses of IP MPH on PFC neuronal discharge activity, including doses that spanned the range associated with cognition enhancement (0.25, 0.5, 1.0 and 2.0 mg/kg) as well as a higher dose that produced robust locomotor activation and stereotypy (15.0 mg/kg).
Figure 2.
Low-dose MPH improves performance measures of spatial working memory in an inverted-U dose-dependent manner. Change in accuracy (% correct trials ± SEM) is plotted for vehicle (VEH), 0.5 mg/kg MPH, and 2.0 mg/kg MPH administered IP. Performance was significantly increased following 0.5 mg/kg MPH and significantly impaired following 2.0 mg/kg MPH. *P < 0.05 vs. vehicle-treatment.
Effects of MPH on EEG activity state
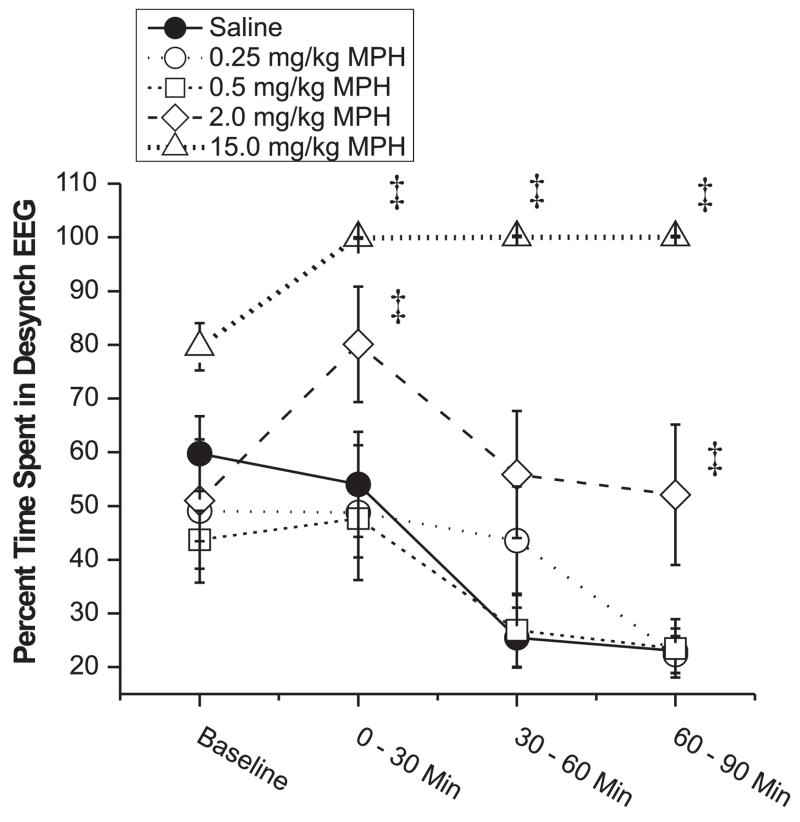
EEG-activating effects of MPH were analyzed for all animals used in the PFC recording studies (n=13). MPH dose-dependently affected the duration of desynchronized (low-voltage, high-frequency) EEG activity (dose, F(5,43)=16.0, p < 0.001; time, F(3,129)=7.9, p < 0.001; dose×time, F(15,129)=1.71, p=0.056). The lowest doses of MPH (0.25, 0.5, and 1.0 mg/kg) lacked arousal-promoting actions (Figure 3). 2.0 mg/kg MPH produced a modest though statistically-significant increase in the duration of desynchronized EEG that was not accompanied by an increase in locomotor activity beyond that associated with normal waking (data not shown). In contrast, the highest dose tested (15.0 mg/kg) produced a robust EEG activation accompanied by locomotor activation and stereotypy (repetitive sniffing, head movements and licking; data not shown) typical of high-dose stimulants (28). For these and all subsequent analyses, the 1.0 mg/kg dose produced an effect in between that observed with the 0.5 mg/kg and 2.0 mg/kg doses. To improve readability of Figure 3 and all subsequent figures, data for the 1.0 mg/kg dose are not plotted.
Figure 3.
The effects of low-dose MPH on the amount of desynchronized EEG activity. The percent of time (mean ± SEM) animals exhibited desynchronized activity per 30-minute epoch is plotted for baseline (30-minutes) and post-treatment (90-minutes) conditions. MPH significantly increased time spent in a desynchronized EEG state only at the two highest doses tested (2.0 mg/kg and 15.0 mg/kg). Subjects used for each dose: Vehicle = 12, 0.25 mg/kg = 6, 0.5 mg/kg = 14, 2.0 mg/kg = 14, 15.0 mg/kg = 3. *P < 0.05, ‡ P < 0.01 vs. vehicle-treatment.
Effects of MPH on spontaneous neuronal discharge within the PFC
The baseline mean spontaneous discharge rate of PFC neurons was 0.84±0.11 Hz (SEM). ANOVA indicated a significant effect of MPH on spontaneous discharge rate of PFC neurons (dose, F(5,885)=2.02, p=0.073; time, F(2,1770)=6.824, p=0.0011; dose×time, F(10,1770)=5.249, p < 0.001). Post-hoc analyses indicated that the lowest doses of MPH (0.25–2.0 mg/kg) produced a modest increase in spontaneous discharge rates of PFC neurons (Figure 4). The largest increase occurred with the lowest dose examined (0.25 mg/kg) and was confined to the later portion of the recording session (i.e. 1.22-fold above baseline during the 60–90 minute post-treatment epoch; see Figure 4). In contrast, 15.0 mg/kg MPH modestly reduced spontaneous discharge rates of PFC neurons (1.32-fold reduction) in the 30–90 minutes post-treatment epochs.
Figure 4.
Modest changes in spontaneous discharge of PFC neurons following low- and high-dose MPH. The percent change (± SEM) from baseline for the mean spontaneous discharge rates of individual neurons recorded from the PFC is shown. Low-dose MPH produced a small increase in spontaneous discharge rates 30–90 minutes post-treatment. This effect was largest for the 0.25 mg/kg dose, with the maximal increase observed at this dose in the 60–90-minute post-treatment epoch. Between 0.5 mg/kg and 2.0 mg/kg, progressively higher doses had progressively smaller effects on spontaneous discharge activity. In contrast, the highest dose of MPH (15.0 mg/kg) significantly reduced spontaneous neuronal discharge. These effects began to dissipate within 2 hours of MPH administration (data not shown). Scale is the same as that used in Figure 5A to better permit comparison of drug effect on spontaneous vs. excitatory evoked discharge. Numbers of neurons analyzed for each dose are listed in Table 1. *P < 0.05, ‡ P < 0.01 vs. vehicle-treatment.
Effects of MPH on evoked discharge within the PFC
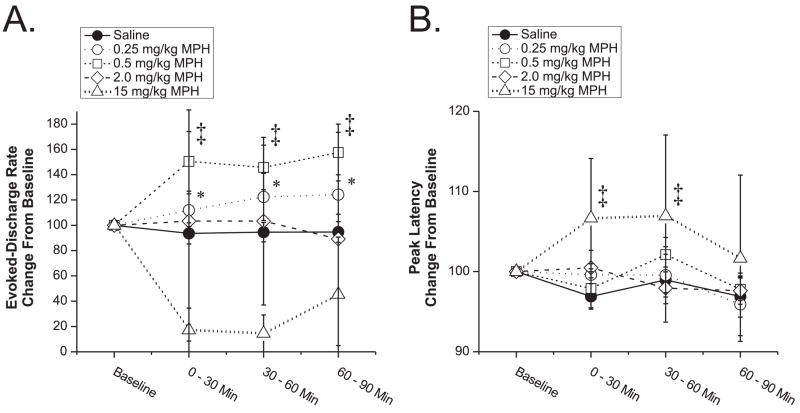
Neurons that were excited by CA1-subiculum stimulation during baseline conditions (24% of recorded neurons, Table 1), responded with an average evoked response of 5.53±0.78 Hz. This relatively modest response indicates stimulation intensities were relatively low and close to threshold for evoking excitatory discharge. MPH produced dose-dependent alterations in evoked discharge of PFC neurons that followed an inverted-U dose-response curve (Figure 5a; dose, F(5,176)=3.325, p=0.0067; time, F(2,352)=0.631; dose×time, F(10,352)=0.391). Thus, lower doses of MPH (0.25–0.5 mg/kg) facilitated excitatory evoked-responses (1.58-fold; e.g. Fig. 1D1), while increasing doses no longer facilitated (1.0 mg/kg) and then suppressed evoked discharge (2.0 and 15.0 mg/kg).
Table 1.
Number of Verified Single Units Used for Analyses.
| Evoked
|
Inhibition
|
||||||
|---|---|---|---|---|---|---|---|
| Single Neurons | Spontaneous | Magnitude | Latency | Magnitude | Duration | ||
| PFC | Saline | 234 | 223 | 59 | 35 | 112 | 114 |
| 0.25 mg/kg | 144 | 138 | 36 | 27 | 46 | 42 | |
| 0.5 mg/kg | 246 | 233 | 63 | 33 | 131 | 121 | |
| 1.0 mg/kg | 245 | 225 | 38 | 27 | 52 | 49 | |
| 2.0 mg/kg | 199 | 156 | 40 | 28 | 59 | 65 | |
| 15.0 mg/kg | 66 | 47 | 7 | 7 | 21 | 22 | |
|
|
|||||||
| Total | 1134 | 1022 | 243 | 157 | 421 | 413 | |
| BFC | 0.5 mg/kg | 64 | 14 | 12 | 14 | ||
Waveforms from individual single neurons recorded from either the prefrontal cortex (PFC) or somatosensory barrel field cortex (BFC) were classified using online and offline criteria. The spike train discharge from each single neuron was quantified from several features of the PSTH analysis before and after saline or one of several doses of MPH (0.25, 0.5, 1.0, 2.0, or 15.0 mg/kg).
Figure 5.
Effects of low-dose MPH on stimulus-evoked excitatory response properties. Plotted are the percent change from baseline (± SEM) of the peak stimulus-evoked excitatory discharge (A) and latency (B) of PFC neurons to CA1-subiculum stimulation. A) Increasing doses of MPH produced an inverted-U facilitation/suppression of the peak stimulus-evoked excitatory discharge. A maximal facilitatory effect (157% of baseline) was observed with the 0.5 mg/kg dose. Further increases in MPH dose resulted in a reduction in this effect to near baseline levels at the 2.0 mg/kg dose and well below baseline levels at the 15.0 mg/kg dose. B) The average latency-to-peak discharge of PFC neurons following hippocampal stimulation was modestly but significantly increased only following the highest dose of MPH tested. 1.0 mg/kg MPH produced effects on both peak stimulus-evoked excitatory discharge and latency-to-peak discharge that were intermediate to 0.5 mg/kg and 2.0 mg/kg and not significantly different from vehicle (data not shown for clarity). In summary, low-dose MPH produced an inverted-U shaped increase in the magnitude of excitatory evoked discharge of PFC neurons in the absence of a change in response latency. Numbers of neurons analyzed for each dose are listed in Table 1. *P < 0.05, ‡ P < 0.01 vs. vehicle-treatment.
Beyond the net facilitatory effect of low-dose MPH on excitatory responses, low-dose MPH (0.5 mg/kg, 1.0 mg/kg) also produced a substantial ‘gating’ effect (Fig. 1D2 and Table 2; Chi-Square: 0.5, p=0.0187; 1.0, p=0.0568 compared to saline). Gating is characterized as the emergence of robust stimulus-evoked discharge to previously subthreshold excitatory input (see: 43 and Methods). Thus, cognition-enhancing doses of MPH increase both the magnitude of the excitatory response of individual PFC neurons and the number of PFC neurons responding to excitatory input.
Table 2.
Distribution of Verified Single Units that Expressed “Gating” Following Vehicle or Methylphenidate.
| BF Sensory Cortex | Frontal Cortex | |||
|---|---|---|---|---|
| % Cells Gating | Cell Count | % Cells Gating | Cell Count | |
| Saline | 26.3 % | 80 | ||
| 0.25 mg/kg | 29.1 % | 55 | ||
| 0.5 mg/kg | 8.8 % | 34 | 35.1 % | 94 |
| 1 mg/kg | 18.2 % | 11 | 32.6 % | 43 |
| 2 mg/kg | 22.4 % | 49 | ||
| 15 mg/kg | 40.0 % | 25 | ||
Shown are the number and percentage of neurons displaying “gating” following treatment with vehicle or varying doses of MPH. The percentage of cells that were “gated” following 0.5 mg/kg MPH was significantly higher (Chi-square; p=0.0187) relative to saline (vehicle) treatment.
The latency-to-peak stimulus-evoked excitatory discharge was 38.8±9.1 ms for CA1-subiculum stimulation responsive neurons. Low-dose MPH had minimal effects on the latency-to-peak stimulus-evoked discharge (Figure 5b; dose, F(5,90)=3.069, p=0.0133; time, F(2,180)=0.721; dose×time, F(10,180)=1.040). Only the highest dose of MPH (15.0 mg/kg) produced a small (0.07-fold), but significant, increase in peak latency.
Effects of MPH on evoked inhibition within the PFC
CA1-subiculum stimulation evoked an inhibitory response in a relatively large subset of PFC neurons (41% of recorded neurons; Table 1 and Fig. 1D1). Stimulus-evoked inhibition was either observed in the absence of an evoked excitatory response or as part of a biphasic excitatory-inhibitory response. Evoked inhibition was characterized by a decrease in spontaneous discharge rate from 0.84±0.11 Hz to 0.14±0.04 Hz with a duration of 727±64 ms. Stimulus-induced inhibition of PFC neurons was significantly altered by MPH (Figure 6a; dose, F(5,376)=3.66, p=0.003; time, F(2,752)=0.365; dose×time, F(10,752)=1.36). Low-dose MPH (0.5 mg/kg) increased the magnitude of inhibitory responses 1.54-fold (more inhibition) during the first 30 minute post-treatment epoch, whereas 2.0 mg/kg MPH did not significantly affect the inhibitory response magnitude. At the highest dose (15.0 mg/kg), MPH decreased the magnitude of the inhibitory response 1.81-fold (less inhibition). Decreases in inhibition observed at this dose in part stems from MPH-induced decreases in spontaneous discharge (see above).
Figure 6.
Inhibitory response magnitude and latency actions of low-dose MPH. The percent change from baseline inhibition (A; baseline discharge - discharge during the inhibitory response) and the percent change in duration of the inhibitory response (B) are shown (±SEM) following vehicle or MPH. A) Increasing doses of MPH produced an inverted-U shaped facilitation of the magnitude of inhibition (area under the peri-stimulus time histogram curve). In the 0–30 minute post-treatment epoch, inhibition was maximally facilitated at the 0.5 mg/kg dose of MPH. In contrast, the highest dose of MPH decreased inhibitory response magnitude. B) The duration of stimulus-evoked inhibition was significantly reduced only following low doses of MPH (0.25 mg/kg–0.5 g/kg). The effects of 1.0 mg/kg MPH were quantitatively similar to 2.0 mg/kg on both magnitude of inhibition and duration of stimulus-evoked inhibition (data not shown for clarity). In summary, low-dose MPH produced an inverted-U shaped increase in the magnitude of inhibitory responses of PFC neurons while simultaneously decreasing the duration of evoked inhibition. Numbers of neurons analyzed for each dose are listed in Table 1. *P < 0.05, ‡ P < 0.01 vs. vehicle-treatment.
The duration of inhibition was also significantly effected by MPH (dose, F(5,375)=3.334, p=0.006; time, F(2,750)=0.224; dose×time, F(10,750)=0.778). Low-dose MPH (0.25–0.5 mg/kg) reduced the duration of inhibition, with a maximal effect (1.28-fold reduction) observed following 0.5 mg/kg (Figure 6b). Higher doses of MPH (2.0–15.0 mg/kg) did not significantly affect duration of the inhibitory response. Thus, only at a low and cognition-enhancing dose (0.5 mg/kg), MPH simultaneously increased the magnitude of inhibitory responses of PFC neurons while decreasing the duration of these inhibitory responses.
Effects of MPH on distributed activity of ensembles of PFC neurons
Distributed patterns of activity across ensembles of interconnected neurons likely represent additional levels of information coding beyond discharge rates and patterns of individual neurons (15 see also: 8,16,17,19,20,25–27,39). Principal component analysis (PCA) identifies correlated activity across spike trains of an ensemble of neurons, with each increasing component accounting for residual correlated activity from the previous component. Figure 7 plots the first two PCA eigenvectors derived from these analyses. As shown in Figure 7a, punctate CA1-subiculum stimulation produced an upward deflection in the first PC eigenfunction (PC1). MPH produced an inverted-U dose-dependent increase in the post-stimulus peak of activity of PC1 (dose, F(5,81)=2.785, p=0.049; time, F(2,36)=2.01; dose×time, F(10,36)=0.22), with the 0.5 mg/kg dose maximally increasing PC1 6.7-fold (Figure 7b). In contrast, CA1-subiculum stimulation elicited a biphasic response from the second PC eigenfunction (PC2). MPH significantly affected the response of PC2 (dose, F(5,16)=5.266, p=0.005; time, F(2,32)=3.607, p=0.038; dose×time, F(10,32)=1.727). Across the lowest doses tested (0.25–0.5 mg/kg), MPH dose-dependently reduced the magnitude of PC2 (Figure 7d), with a maximal reduction (6.8-fold) at 0.5 mg/kg. These analyses indicate that low-dose MPH alters the relationships between neuron activity patterns across an ensemble of PFC neurons in a non-uniform manner (i.e. decreased PC2, increased PC1) suggesting a reduction in the complexity of distributed neuronal discharge within the PFC.
Figure 7.
Distributed discharge patterns across PFC neural ensembles are altered by low-dose MPH. Peri-stimulus time histograms (PSTHs) illustrate the response of the first (A) and second (B) PC eigenfunction for a single simultaneously recorded ensemble of PFC neurons during baseline conditions. PCA analysis was only conducted on subjects with a minimum of 10 simultaneously recorded neurons (range = 10–34). As in Fig. 1, PSTH X-axis represents time (sec) before and after stimulus presentation (0.0 sec); the Y-axis indicates frequency for a given time bin (0.005 sec). The magnitude of the response to CA1-subiculum stimulation for each PC1 eigenfunction was calculated as peak height (Panel A grey line; a–b). Typically, PC2 was biphasic and contained a trough of negative values. Therefore the magnitude of response for each PC2 eigenfunction was calculated as the peak-to-trough height (Panel B grey line; a–b). C) The response magnitude of PC1 was dose-dependently increased following 0.25 mg/kg–0.5 mg/kg MPH and then returned to baseline levels following higher doses 1.0 mg/kg–15.0 mg/kg. D) PC2 demonstrated a dose-dependent reduction in magnitude following low-dose MPH (0.25 mg/kg–0.5 mg/kg). Number of ensembles analyzed for each dose: Vehicle = 5, 0.25 mg/kg = 5, 0.5 mg/kg = 7, 1.0 mg/kg = 6, 15.0 mg/kg = 2. *P < 0.05, ‡ P < 0.01 vs. baseline.
Effects of MPH on spontaneous and stimulus-evoked neuronal discharge within the somatosensory barrel field (BF) cortex
Limited additional studies examined the effects of 0.5 mg/kg MPH on the activity of somatosensory BF cortical neurons. BF cortical neurons responded to stimulation of the rat’s whisker pad similar to that previously described (20,22). During baseline, BF cortical neurons exhibited a mean spontaneous discharge of 2.08±0.34 Hz (SEM), mean sensory-evoked discharge of 35.7±3.8 Hz, and a mean latency-to-peak stimulus-evoked excitatory discharge of 7.66±0.4 ms. MPH (0.5 mg/kg) did not significantly affect spontaneous discharge (Figure 8a; time, F(3,60)=1.40) or either the magnitude (Figure 8b; time, F(3,46)=0.16) or latency-to-peak stimulus-evoked excitatory discharge (Figure 8c; time, F(3,46)=0.01).
Figure 8.
Somatosensory BF cortex (BFC) neuron discharge properties are minimally affected by low-dose MPH. Shown are the effects of 0.5 mg/kg MPH on spontaneous discharge (A), stimulus-evoked discharge (B), or the latency-to-peak discharge (C) of BFC and PFC neurons (mean ± SEM). PFC data are re-graphed from Figures 1 & 2 for comparison purposes. This dose of MPH had no noticeable effects on these discharge properties of BFC neurons. This insensitivity of BF neurons is in contrast to that seen in the PFC at this dose of MPH). Numbers of neurons analyzed for each dose are listed in Table 1. *P < 0.05, ‡ P < 0.01 vs. baseline.
Discussion
The current study provides the first demonstration of a close relationship between the cognition-enhancing actions of low-dose psychostimulants and drug-induced increases in responsivity of individual PFC neurons and PFC neuronal ensembles. Facilitation of PFC neuronal responsiveness (both excitatory and inhibitory) and increases in the response of highly correlated activity distributed across neural ensembles (PC1) were observed only following low and clinically-relevant doses of MPH that lack EEG- and locomotor-activating effects and improve PFC-dependent cognition. Low-dose MPH-induced alterations in PFC neuronal responsiveness were associated with relatively minimal change in the spontaneous discharge activity of PFC neurons. Modestly higher doses of MPH that also lacked locomotor-activating effects, but impaired PFC-dependent cognition, had minimal effects on PFC neuronal discharge properties. In contrast to that seen with low-dose MPH, a locomotor-activating/stereotypy-inducing dose of MPH potently suppressed evoked discharge of individual PFC neurons and modestly suppressed spontaneous discharge. Importantly, low-dose MPH-induced facilitation of neuronal responsivity was selective to the PFC and was not observed in the somatosensory cortex. These observations suggest that the cognition-enhancing actions of low-dose psychostimulants involve the selective enhancement of information processing within the PFC via actions at both the single neuron and neuronal ensemble levels.
Clinically-relevant doses of MPH preferentially impact the PFC
Low-doses of psychostimulants improve PFC-dependent working memory and other cognitive processes while suppressing impulsivity and locomotor activity. Importantly, these actions are not confined to ADHD and are observed in normal human and animal subjects (6,10,30,32,33,35,36,40). The current studies demonstrate that stimulant-induced facilitation of PFC-dependent cognition is not dependent on the oral route of administration, when doses are adjusted to yield comparable and clinically-relevant plasma concentrations (10).
Despite widespread clinical use of psychostimulants, the neural mechanisms responsible for their cognition-enhancing/behavioral-calming actions are poorly understood. Imaging studies implicate PFC dysfunction in ADHD (14,40). Moreover, lesions of the PFC or PFC-catecholamine fibers result in behavioral attributes similar to those seen in ADHD, including attentional deficits and hyperactivity (for review: 4). Recent microdialysis studies demonstrate that clinically-relevant doses of MPH increase NE and DA efflux preferentially within the PFC (10,22,29,30). The current studies further indicate that, only at these low and cognition-enhancing doses, MPH increases evoked responding of PFC neurons and enhances highly correlated discharge-pattern relationships between neurons of the PFC. The facilitatory action of low-dose MPH on PFC neuronal responsivity contrasts with the profound suppression of evoked discharge observed at higher doses and the minimal effects of low-dose MPH on evoked discharge of somatosensory cortical neurons (see: 22). Combined, these studies indicate a unique sensitivity of the PFC to low-dose MPH. This sensitivity is likely a critical factor in the therapeutic actions of low-dose psychostimulants.
It is important to note this hypothesis does not imply that all behavioral/therapeutic effects of low-dose stimulants are dependent on actions within the PFC (see: 21). In particular, stimulant actions outside the PFC may be of greater relevance in patients requiring higher doses of psychostimulants for therapeutic efficacy, given the more widespread actions of MPH on catecholamine efflux with increasing dose (10,22).
Functional relevance of MPH-induced changes in signal processing within the PFC: single unit observations
The current study demonstrates that cognition-enhancing doses of MPH increase the magnitude of both excitatory and inhibitory responses of PFC neurons while simultaneously reducing the duration of the inhibitory response. Low-dose MPH also produced ‘gating’, resulting in a larger number of PFC neurons responsive to CA1-subiculum input. Combined, these observations suggest low-dose MPH increases both the sensitivity of PFC neurons and the pool of responsive PFC neurons in a more complex manner than simply regulating the level of PFC excitability (i.e. gain of neuronal activity), consistent with known actions of catecholamines on cortical neurons (4,6,18,34,38,45,46).
High doses of MPH, associated with locomotor activation and stereotypy, modestly suppressed spontaneous discharge, consistent with previous observations with amphetamine (24). In contrast, high-dose MPH produced a profound suppression of evoked responses, essentially rendering the PFC non-responsive and functionally taking it “off-line”. It is likely that these actions contribute to the cognition-impairing and behaviorally-activating actions of high-dose psychostimulants (31,37).
Functional relevance of MPH-induced changes in signal processing within the PFC: Ensemble observations
Information from the hippocampus to the PFC is likely in the form of a sparse and distributed code (8,25–27,39). Given this, it is of particular interest that low and cognition-improving doses of MPH substantially altered the distributed representation of PFC responses to hippocampal afferent input: simultaneously enhancing PC1 while suppressing PC2. In contrast to that of sensory systems (16), the functional significance of PC1 and PC2 derived from PFC neuron recordings is currently unclear. Nonetheless, these observations suggest that low-dose MPH alters the relationship of firing patterns within a population of PFC neurons, reorganizing the representation of afferent input within this population of neurons. This may, in part, involve an increase in the pool of responsive neurons (gating) observed with these doses of MPH. Functionally these actions may suggest that, within the PFC dominant signals (PC1) are enhanced while less redundant information (i.e. potential noise, PC2) is suppressed following low-dose MPH. These network actions could not have been predicted from single-unit recordings alone, indicating the utility of population based measures of simultaneously recorded neuron discharge for better understanding the neural mechanisms underlying the cognitive/behavioral effects of psychostimulants.
Methodological Considerations
Ventral CA1-subiculum stimulation in the home cage offers many advantages for initially assessing the impact of low-dose MPH on PFC neuronal activity, including precise quantification of PFC neuronal responses to afferent input under well-controlled conditions. The hippocampus CA1-subiculum conveys behaviorally-important information to the PFC (25,38). However, punctate stimulation of the CA1-subiculum is unlikely to provide much behaviorally-relevant information. Nonetheless, the current observations demonstrate significant facilitation of low-dose MPH on PFC neuron responsivity to afferent input from this behaviorally-significant pathway. To better understand the functional significance of MPH-induced alterations in PFC neuronal responsivity, ongoing studies in our laboratory have extended the current approach to animals engaged in a PFC-dependent cognitive task (e.g. working memory). Of particular interest in these studies is whether low-dose MPH uniformly affects all task-related neuronal discharge within the PFC or whether MPH selectively affects a subset of task-related discharge (e.g. delay-related activity).
Neurotransmitter bases for MPH-induced improvement in signal processing within the PFC
Previous microdialysis studies indicate that low-dose MPH preferentially increases NE and DA efflux within the PFC (10). NE and DA exert a complicated array of actions on PFC neuronal activity and PFC-dependent cognition that are concentration- and receptor-specific (for reviews: 1,7,12). Available evidence indicates that postsynaptic α2-receptors (α2A) facilitate, whereas α1-receptors impair PFC-dependent cognition (6). In contrast, D1 and D2 receptors exert inverted-U type modulatory actions on PFC-dependent cognition (41). Consistent with these observations, MPH-induced improvement in working memory performance is prevented by pretreatment with both a D1 and an α2-antagonist (7).
These behavioral actions of NE and DA receptors are paralleled by effects of these receptors on PFC neuronal discharge activity (3–5,41,42). For example, in a saccade-based task of working memory, α2A-receptor stimulation strengthens delay-related activity of PFC neurons for stimuli located in a neuron’s preferred direction (“signal”), whereas moderate D1 receptor stimulation suppresses responses to stimuli located in non-preferred directions (“noise”; 3–5,41,42). Therefore, we hypothesize that the ability of low-dose MPH to enhance responsivity of PFC neurons at least involves stimulation of postsynaptic α2-receptors. Additional studies have demonstrated that noradrenergic α1-receptor stimulation and high levels of D1 receptor stimulation suppress delay-related discharge of PFC neurons (5,13). These latter observations suggest that the ability of high dose MPH to suppress evoked discharge of PFC neurons likely involves the actions of both α1-receptors and D1-receptors.
Summary
Despite the widespread use of low-doses of psychostimulants, the neural substrates underlying the cognition-enhancing/behavioral-calming actions of these drugs are poorly understood. The current observations indicate that cognition-enhancing doses of psychostimulants increase the responsiveness of individual PFC neurons and modify representations of afferent input distributed across ensembles of PFC neurons. Combined with previous neurochemical observations (10), these observations indicate a unique sensitivity of the PFC to low-dose psychostimulants and provide a perspective on low-dose psychostimulants alterations of neuronal, ensemble, and cognitive functions that are likely critical components in the therapeutic actions of low-dose psychostimulants in the treatment of ADHD.
Acknowledgments
The authors are grateful for the expert technical assistance of Mr. Tim Stellick. Dr. Berridge has received lecture fees from Shire Pharmaceuticals and DOV Pharmaceuticals. Dr. Devilbiss reports no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Arnsten AF, Robbins TW. Neurochemical modulation of prefrontal cortical function in humans and animals. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. [Google Scholar]
- 2.Arnsten AF. Modulation of prefrontal cortical-striatal circuits: relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol. 2001;85:333–341. [PubMed] [Google Scholar]
- 3.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 4.Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- 9.Berridge CW, Bolen SJ, Manley MS, Foote SL. Modulation of forebrain electroencephalographic activity in halothane-anesthetized rat via actions of noradrenergic beta-receptors within the medial septal region. J Neurosci. 1996;16:7010–7020. doi: 10.1523/JNEUROSCI.16-21-07010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 15.Chapin JK. Population-Level Analysis of Multi-Single Neuron Recording Data: Multivariate Statistical Methods. In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. New York: CRC Press; 1999. pp. 193–228. [Google Scholar]
- 16.Chapin JK, Nicolelis MA. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–40. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- 17.Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 2000;37:273–82. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Devilbiss DM, Waterhouse BD. Determination and quantification of pharmacological, physiological, or behavioral manipulations on ensembles of simultaneously recorded neurons in functionally related neural circuits. J Neurosci Methods. 2002;121:181–198. doi: 10.1016/s0165-0270(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 20.Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): a neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity) Dev Psychopathol. 2005;17:807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drouin C, Page M, Waterhouse B. Methylphenidate enhances noradrenergic transmission and suppresses mid- and long-latency sensory responses in the primary somatosensory cortex of awake rats. J Neurophysiol. 2006;96:622–632. doi: 10.1152/jn.01310.2005. [DOI] [PubMed] [Google Scholar]
- 23.Greenhill LL. Clinical effects of stimulant medication in ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 31–71. [Google Scholar]
- 24.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory Amino Acid Pathway from the Hippocampus to the Prefrontal Cortex. Contribution of AMPA Receptors in Hippocampo-prefrontal Cortex Transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 26.Jensen O. Reading the hippocampal code by theta phase-locking. Trends Cogn Sci. 2005;9:551–553. doi: 10.1016/j.tics.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- 28.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 29.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- 30.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- 32.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta MA, Sahakian BJ, Robbins TW. Comparative psycholpharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 303–331. [Google Scholar]
- 34.Ramos BP, Colgan LA, Nou E, Arnsten AF. beta2 adrenergic agonist, clenbuterol, enhances working memory performance in aging animals. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 36.Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6(Suppl 1):S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- 37.Segal DS. Behavioral characterization of d- and l-amphetamine: neurochemical implications. Science. 1975;190:475–477. doi: 10.1126/science.1166317. [DOI] [PubMed] [Google Scholar]
- 38.Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Turner RS, DeLong MR. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull. 1988;21:425–32. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- 44.Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619–623. doi: 10.1001/jama.292.5.619. [DOI] [PubMed] [Google Scholar]
- 45.Williams GV, Castner SA. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Yang CR, Seamans JK, Gorelova N. Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology. 1999;21:161–194. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]