Abstract
Environmental stressors impact physiology and behavior in many species of animals. These effects are partly mediated through changing concentrations of glucocorticoids, which also vary with reproductive state and social conditions. Prior research has focused largely on seasonal breeders, but the close temporal linkage between season and reproductive state in these species hinders ability to disentangle environmental effects from those of the animal’s reproductive status. Here we assessed the effects of environmental challenges on the fecal glucocorticoid (fGC) levels of non-seasonal breeders, female baboons (Papio cynocephalus) of Amboseli, Kenya. Amboseli is characterized by a long dry season, during which food and water become scarce, and by extreme temperatures above 40°C in the shade during some months of the year. We found that after accounting for female reproductive status and individual variability, females exhibited higher fGC levels during the dry season than during the wet season. Further, during the wet season, fGC levels were higher in months of high average daily maximum temperatures. During the dry season, fGC levels were elevated both in hotter months and in months during which the baboons spent a relatively high proportion of time feeding. In spite of these stressors, female baboons reproduce during all months of the year in Amboseli, unlike most other mammals in this environment. This may be attributable to their extreme adaptability, specifically their diversified diet, and their ability to modify their behavior, including their activity profiles.
Keywords: environmental stressors, chronic stress, glucocorticoids, reproductive status, feeding behavior, female baboons, Papio, heat stress, dry season
INTRODUCTION
Environmental stressors such as drought, extremes of hot or cold temperatures, and food shortage impact reproduction and behavior in many species of birds (Lynn et al. 2003; Wingfield, 1984) and mammals (Bronson, 1985; Gwazdauskas, 1985; Nelson, 1993; Nelson et al., 1989), including humans (Bronson, 1995) and non-human primates (Alberts et al., 2005, Beehner et al., 2006b; Brockman and van Schaik, 2005; Sapolsky, 1986). These effects are in part mediated via increases in glucocorticoid (GC) concentrations (Astheimer et al., 1992; Kitaysky et al., 2001b, 2003; reviewed by Boonstra, 2004; Landys et al., 2006; Romero, 2002 and Wingfield and Kitaysky, 2002). The classic illustration of the ecologically adaptive nature of the GC response (e.g. Nelson, 2005, p.15) is that of an acute stressor, such as a predator attack, where the GC response leads to an immediately advantageous rapid mobilization of energy that is necessary for ‘flight or fight’, enhancing gluconeogenesis and shutting down processes that are not essential for immediate survival, such as growth, reproduction, and immune function. However, chronically elevated GC levels can have deleterious consequences for the animal as a result of ongoing, rather than brief, suppression of growth, reproduction, and immune defense (Boonstra, 2004, McEwen, 1998; Sapolsky et al., 2000; Wingfield, 2005).
Several approaches have been taken to evaluate the effect of environmental stressors on animal behavior and physiology. The majority of research has been on single acute stressors, either approached through experimental laboratory studies or, in a few cases, through non-experimental evaluation of acute natural stressors in the field. In experimental laboratory studies, researchers have usually created such stressors by exposing animals to various single environmental stressors for short period: extreme heat (Larsson et al., 1983; Lowe et al., 2002; Olsson et al., 1995, 1996; review by Silanikove, 2000); cold temperatures (DeVries et al., 1997; Filipovic, 2007); food or water deprivation (Astheimer et al., 1992; Dunlap, 1995; Li et al., 2000; Lynn et al., 2003; Tsuma et al., 1996; see also review by Harvey et al., 1984). A few experiments have used a combination of two acute stressors simultaneously, e.g. heat stress in either food deprived animals (Olsson et al., 1995) or in water deprived animals (Lowe et al., 2002; Olsson et al., 1996, see also review by Silanikove, 2000).
Another approach has evaluated acute environmental challenges in natural contexts by examining changes of GC concentrations in free-ranging animals following intense, relatively brief stressors such as severe storms (Astheimer et al., 1992, 1995; Wingfield and Kitaysky, 2002; Wingfield and Ramenofsky, 1997). As expected for an acute stressor, in all of these studies researchers observed a rapid and short activation of the hypothalamic-pituitary-axis (HPA), resulting in elevation of GC levels and inhibition of functions not needed for survival in the immediate future.
A third approach is reflected in a small but growing body of literature from both laboratory and field settings that has begun to explore the consequences of long-term, chronic rather than acute, exposure to environmental stressors. Examples have included water or food restriction for several weeks for small rodents in laboratory settings (Harper and Austad, 2000; Kitaysky et al., 2001a; Nelson, 1993; Nelson et al., 1989), and harsh seasons such as winter or dry season in free-ranging animals (Cavigelli, 1999; Huber et al., 2003; Lynch et al., 2002; Pereira et al., 2006; Romero et al., 1997; 2000; Saltz and White, 1991; Strier et al., 1999; Wilson and Wingfield, 1994; Wingfield, 1984; review by Boonstra, 2004 and by Reeder and Kramer, 2005). Because all the species studied under these conditions have been seasonal breeders, however, it has not been clear to what extent the elevated GC concentrations occur in response to the inclement weather conditions or to the inherent physiological challenges characteristic of the animals’ particular life history stages, e.g. the reproductive season (nesting, pregnancy, lactation). Investigations of non-seasonally-breeding species may therefore provide new insights by revealing the effect of environmental factors on animal physiology during the full range of life history stages. Two short projects on non-seasonal breeders, baboons in two parts of Africa, provide a first step in this direction. Their findings suggested that persistent inclement weather conditions, e.g. drought (anubis baboons, Sapolsky, 1986) or winter (chacma baboons, Weingrill et al., 2004), also led to elevated GC concentrations. Here we build on these two studies and on those from the experimental literature to evaluate chronic natural environmental stressors at various life history stages over a four year period.
The subjects of our study were the female members of five groups of wild yellow baboons (Papio cynocephalus). Baboons reproduce throughout the year, thereby experiencing predictable life history differences in physiological demands in all ecological conditions. Baboons also often live in highly challenging semi-arid tropical habitats that are characterized by both predictable and unpredictable environmental variability. This is particularly true of the study population, which inhabits the Amboseli basin of Kenya, a semi-arid savannah that is characterized by strong seasonal patterns of rainfall and by high rainfall variability from year to year. Our goal was to assess the effects of non-acute, long-term environmental stressors, particularly dry season, and thermal extremes, on the physiology and behavior of females in different maturation and reproductive states.
METHODS
All protocols were non-invasive and comply with relevant regulations in Kenya (Kenya Research Permit MOEST 13/001/C351 Vol. II) and the United States (Princeton IACUC 1689, 9 November 2007).
Study site and study population
The Amboseli basin, located south of the equator in East Africa (2°40’S, 37°15’E, 1100m altitude), is a semi-arid short-grass savannah ecosystem located in an ancient lake basin at the base of Mount Kilimanjaro. The environment is characterized by a predictable 5 month-long dry season (June through October) that is without rain and during which availability of food and drinking water declines as the season progresses. The remaining months of the year are ones in which rainfall is highly and unpredictably variable. Rainfall is also highly variable across years, and drought conditions are produced when the normal dry season is unpredictably extended at either end by failure of normal rains. Consequently, the Amboseli baboons experience a mixture of predictable and unpredictable rainfall variability, as the 5-month dry season is predictable whereas the wet season is highly variable in how much rain falls.
In contrast, temperature is both more predictable and less variable than rainfall, both within and across years. As is usual in arid tropical environments, temperature varies more within a 24hr period than between months and across years; although temperature varies both seasonally and annually, it does so less than rainfall. For the baboons, conditions of thermal heat stress are most likely to occur from midday through mid-afternoon, and the risk of thermal stress is higher in some months than in others. In addition, the animals are likely to experience uncomfortably cool conditions around dawn during the cooler months of June and July.
The adult females in the present study were members of five social groups of wild-feeding baboons, for which individual life-history data have been collected for almost four decades (e.g. Alberts et al., 2005; see www.princeton.edu/~baboon for a complete bibliography and the Baboon Project data collection Monitoring Guide). Physiological data have been obtained for known individuals through non-invasive collection and subsequent analysis of freshly deposited feces starting in late 1999; fecal samples for the present project were available for 2002 through 2005, and all other data for this project, discussed below, cover the same years.
Amboseli weather data
Daily records of rainfall and minimum and maximum temperature (Tmin and Tmax, respectively) were obtained using a rain gauge and min-max thermometer at the research field camp within a 2-17 km of the ranges of the various groups, (Altmann et al., 2002). Over the several decades of the Baboon Project research, annual rainfall in Amboseli has varied from 150 mm to more than 550 mm but with no trend or predictability (Alberts et al., 2005; Altmann et al., 2002). In contrast, both average maximum and minimum daily temperatures gradually increased from the 1970s through the early to mid 1990’s (Altmann et al., 2002). Particularly in recent years, monthly average daily maximum air temperatures at the shaded camp thermometer are sometimes above 34°C, a temperature that is close to baboons’ normal core body temperature of 38°C, and daily maximum temperatures above 40°C are occasionally recorded.
Female reproductive state
Demographic and reproductive records were drawn from our long-term database, BABASE. Data used to determine reproductive state were recorded on a near-daily basis. On each observation day, for each female we recorded the color of the paracallosal skin (an indication of pregnancy or recent parturition; Altmann, 1970), presence of any menstrual bleeding, and both the size and condition (turgescent, deturgescent) of the sexual swelling (Gesquiere et al., 2007). For analysis, females were categorized as Pre-reproductive (before their first sexual swelling), Cycling (exhibiting a cyclical pattern of sex skin turgescence, followed by deturgescence and then menstruation), Pregnant (cessation of sexual swellings without evidence of menstruation and followed by subsequent confirmation; see Beehner et al., 2006a) or Post-partum (the period of post-partum amenorrhea, from parturition until resumption of cycling).
Hormone data
Fecal sample collection, storage, and extraction were as described previously (Khan et al., 2002; Lynch et al., 2003), no more than one sample collected from an individual on any day. The samples were then assayed for glucocorticoids (fGC) by radioimmunoassay (Khan et al., 2002; Lynch et al., 2003; full laboratory protocols available at www.princeton.edu/~baboon). The primary antibody in the Corticosterone kit for rats and mice (ICN Diagnostics, Costa Mesa, CA) cross-reacts with major cortisol metabolites present in baboon feces (Wasser et al., 2000). Inter-assay coefficients of variation were 13.6% and 10.7% (n=49), respectively for a low and high control. Intra-assay coefficients of variation were below 6% for both the low and high control (any duplicate above 15% was re-assayed). All hormone values are expressed as ng per g of dry feces.
For this study we used all fecal hormone data for females 2-20 years old and in all reproductive states excluding fecal samples that were obtained the first week after parturition (Altmann et al., 2004; Nguyen et al., 2008). The resulting records included a total of approximately 7000 samples collected from over 130 females. Because fecal samples were collected ad libidum, sample numbers were variable across females and months; some females had no sample for a particular month and in some cases a female was sampled on as many as 16 days in a month. For analyses of female fGC, we minimized the effect of uneven sampling among females as follows: for each month, each female was first assigned to a reproductive state (see above), and a single, average, fGC was calculated across all the female’s samples during the month. (For months in which a female transitioned between two states, her fGC concentration was averaged within each reproductive state, such that this female received two records for fGC for that month, one for each reproductive state). As a result, most females contributed only one (average) value to analyses for any month, and sometimes contributed two (average values) or none.
Activity budgets
The proportion of time spent in various major activities was established using point sampling at one-minute intervals within 10-minute focal samples (Altmann, 1974). On each minute we recorded the activity of the focal baboon, categorized into six exhaustive and mutually exclusive behaviors: feeding (including food processing), walking while not feeding, grooming, being groomed, other social activities, or resting (see Alberts et al., 2005). Although females were the subject of a focal sample up to 14 times (140 points) in a month, we calculated a single value for each female in any month in which she was sampled at all. To do so, we estimated the proportion of time spent in each activity for the month as the proportion of sample points for which that activity was recorded, i.e. females were represented in the analyses by a single value for a month.
Data analysis and presentation
We first characterized weather, behavioral and fGC variability across calendar months using descriptive statistics in order to provide a broad context for subsequent statistical analyses that dissected the relationships among these variables. For the weather data, we calculated monthly values within each year, and then means and variability of these monthly values across years, i.e. the N for each calendar month was 4, one value for each year. The monthly rainfall measure was the cumulative rainfall during that month, and monthly temperature measures were the mean daily Tmin and Tmax for the month (Figure 1a).
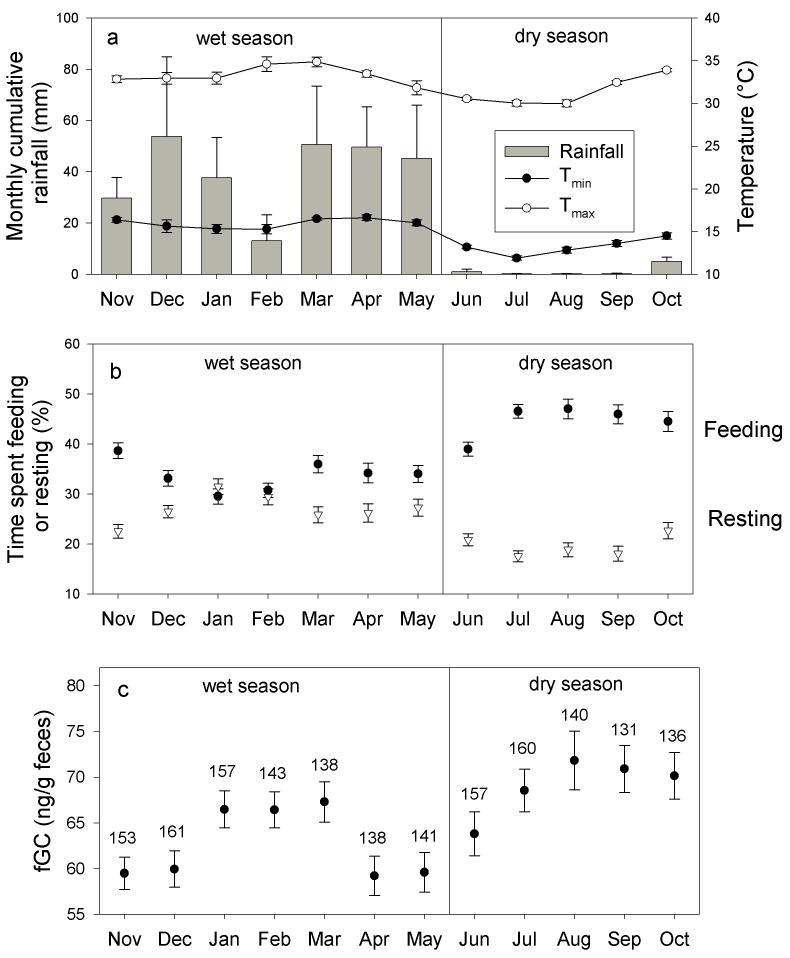
Figure 1.
Monthly and seasonal patterns of (a) Tmin, Tmax and rainfall, (b) proportion of time spent feeding and resting, and (c) fGC. Months are ordered according to ‘hydological year’, beginning with November, the first month of the wet season (as in, e.g., Altmann et al., 2002). (a), each monthly value of Tmin, Tmax and cumulative rainfall represents a mean (± SE) across the 4 years of this study (2002-2005), i.e. N=4 in this case. (b,c), the time spent feeding or resting and fGC represents the mean across female by reproductive state (see Methods). The sample size, N, represents the number of females × reproductive status.
For behavioral and fGC measures, we calculated the monthly behavior and fGC values as described in the Activity budgets and Hormone data sections above. Then for each calendar month, e.g. January, we calculated the average value across years for each female within a reproductive state; e.g. if a female was pregnant in January during 2 of the 4 study years, we averaged the female’s 2 January values for use in the analyses so that this female contributed a single value to analyses of pregnant females in January. As a result, for each reproductive state, each female contributed to a value for up to 12 calendar months for behavior and fGC, (average of 6.89 calendar months per female). To further minimize unequal contributions among females, for analyses across all calendar months, within each reproductive state we only used the subset of females for which we had monthly fGC values for at least 7 of the 12 calendar months. This reduced data set included 46 Pre-reproductive, 42 cycling, 42 pregnant, and 59 post-partum females for inclusion in our analyses.
We performed statistical tests on log fGC because it was the best transformation for meeting distributional assumptions of tests. For presentation purposes, however, we provide the raw fGC values.
Factors predicting fGC levels
To analyze sources of variance in fGC concentrations, we first constructed an overall general linear model (GLM) using SPSS 14.0 (SPSS Inc., 2005) to predict (log-transformed) fGC means for calendar months. The predictor variables for each month included one categorical variable: reproductive stage, and three continuous variables: mean Tmax, cumulative rainfall, proportion of time spent feeding. To allow for potential individual differences, we also entered baboon’s identity as a random factor. We note, however, that inclusion of female identity in the models leads to heterogeneity of variances (p<0.05 in Levene’s Test of Error Variance), and homogeneity is not improved by alternative data transformations. Tabachnick and Fidell (2001) suggest using more stringent significance values (0.025 or 0.01) in interpreting model results.
When a pair of predictor variables is highly correlated, generally only one should be included in the GLM, although various authorities suggest different cutoff levels. We followed that of Tabachnick and Fidell (2001) and using a cutoff of 0.60, neither Tmin nor time spent resting were included in the GLM. Tmin was strongly correlated with both Tmax and rainfall (Pearson correlation=0.781 and 0.859, respectively), and time spent resting was strongly correlated with time spent feeding (Pearson correlation=-0.615). Our decision on which of the correlated variables to retain was based on their predictive ability. When Tmin was entered in the GLM instead of Tmax or time spent resting was entered instead of time spent feeding, neither variable significantly predicted fGC (F=0.091, p=0.762 for Tmin, and F=0.501, p=0.479 for time spent resting. The positive association between rainfall and Tmax (Pearson Correlation=0.534) fell below the exclusion criterion.
Subsequent analyses were then based on the results of the initial GLM model (see Results). Briefly, we performed matched pair nonparametric tests within individual between seasons for each reproductive condition. For this analysis, we calculated for each female a mean across wet months and a mean across dry months within each reproductive state. Because each female’s fGC value for the wet season was compared to her own fGC value for the dry season we did not limit our data set to those females with at least 7 months of fGC data for this analysis. Rather, all females with a mean fGC value for both the wet season and the dry season were included in the matched paired analysis. In a final analysis, then, we sought to elucidate the potentially very different weather impacts during each of these seasons by constructing separate wet season and dry season GLMs, similar to the initial overall GLM analysis.
RESULTS
Broad patterns of variability across months
The striking seasonality of rainfall in Amboseli during these years is apparent in Figure 1a; monthly cumulative rainfall was always less than 10 mm in each of the dry season months across the 4 years of this study. Also clear is that within the wet season months, rainfall differed considerably among months (from February’s average of 13 mm to December’s average of 54 mm across years). Rainfall was also highly variable from year to year: cumulative wet season rainfall across these 4 consecutive years ranged more than two-fold from a low of 184 mm in 2003 to 393 mm in 2002. Temperatures exhibited less dramatic but similar variability between and within seasons (Figure 1a). Seasonal averages across years for dry season monthly means tended to be cooler (Tmin=13.2°C, Tmax=31.4°C) than those for wet season months (Tmin=16.0°C, Tmax=33.4°C). Within seasons, average Tmin and Tmax differed by 1 to 4°C across months. Further, although temperature varied relatively little across these 4 years, the most variable months were in the wet season, in particular December and February (Figure 1a).
Feeding time, too, exhibited variability between seasons and across months within seasons. Feeding time was generally lower in the wet season months than in the dry season months (34% vs. 45%, Mann-Whitney U: Z=-10.683, p<0.001; Figure 1b), the exception being that feeding time in the first month of the wet season (November) was the same, 39%, as that in the first month of the dry season (June), indicating the slight lag in each case for major changes in rainfall to translate into differences in plant productivity. As expected, resting time exhibited a pattern opposite in direction from feeding time; females spent less time resting during the dry season than during the wet season (20% vs. 27% Mann-Whitney U: Z=-8.607, p<0.001; Figure 1b).
As with the weather and behavioral parameters, mean monthly fGC differed both between and within seasons (figure 1c). During wet season months, fGC was lower than during dry season months. The four months with lowest fGC were in the wet season, and the four with highest fGC were in the dry season. Only during the first dry season month (June) was fGC concentration significantly lower than during the remaining months of the dry season (Mann-Whitney U: Z=3.410, p=0.001); fGC in June was intermediate between the higher and lower wet season values. These general patterns across seasons and months suggest that within dry and wet seasons, different sources of environmental variability might be associated with fGC variability. Subsequent GLM and other analyses provided formal and more detailed quantitative evaluation of these environmental factors that predict fGC levels.
Factors predicting GC concentrations
We first conducted a GLM analysis across all calendar months as described in the methods. Four variables contributed significantly to variation in fGC levels in this overall GLM after taking other variables into account: rainfall (F=39.590, p<0.001, B=-0.001), Tmax (F=13.613, p<0.001, B=+0.009), female’s reproductive state (F=41.292, p<0.001), and female identity (F=2.908, p<0.001). In contrast, feeding time didn’t predict fGC levels (F=1.834, p=0.176). Females had elevated levels of fGC in months with low rainfall (as suggested in Fig. 1) and in hot months. These findings and the fact that the dry season encompasses all the months with extremely low rainfall (and includes only months with extremely low rainfall) led us next to directly compare wet and dry seasons in a within-female comparison within each reproductive state (i.e. controlling for the two significant non-environmental fGC predictors) and then to construct separate GLM for the wet and dry seasons.
Within female comparison of wet/dry seasons
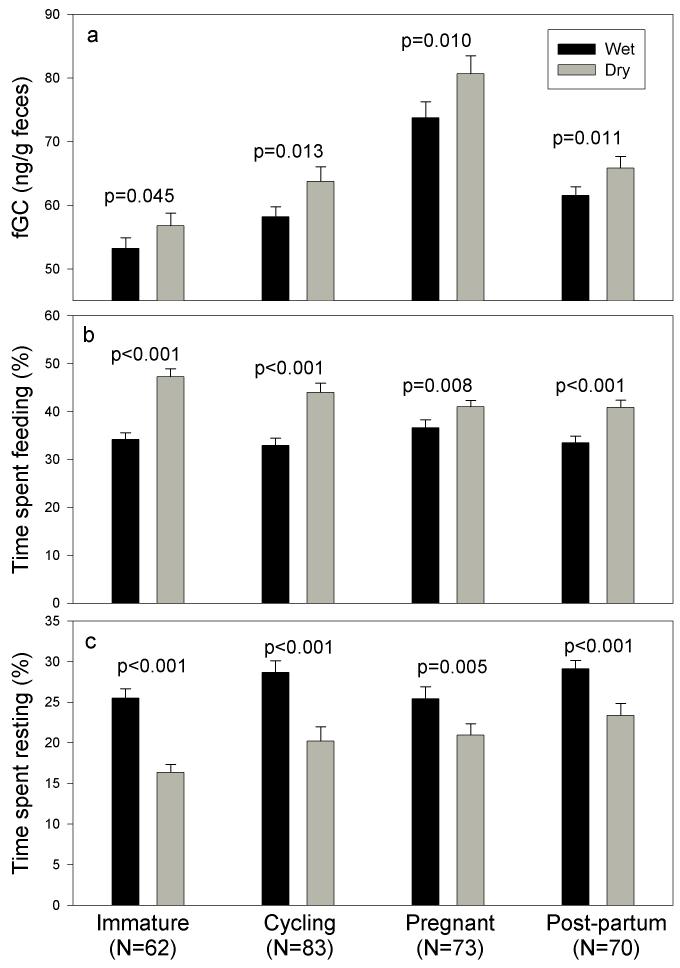
Differences in fGC between wet and dry seasons were confirmed in the within-female paired comparison. Females of each reproductive status experienced significantly higher fGC levels during dry seasons than during wet seasons (Pre-reproductive: Z=2.001, p=0.045, Cycling: Z=2.473, p=0.013, Pregnant: Z=2.593, p=0.010, Post-partum: Z=2.547, p=0.011; Figure 2a). Increases in GC from wet to dry season ranged from 7% for Pre-reproductive and Post-partum females to 9.5% for Cycling and Pregnant females. Moreover, during the dry season, when food availability declined, females spent more time feeding and less time resting than in the wet season (Figure 2b and 2c). The largest increases in feeding time from wet to dry season were experienced by Pre-reproductive and Cycling females (38% and 34% increases respectively), and the smallest increases in feeding time were seen in Pregnant and Post-partum females (14% and 24% respectively).
Figure 2.
Comparison of (a) fGC levels, (b) feeding and (c) resting behavior of females for whom data were available in the same reproductive state during both the wet and the dry season. Each value in the graph represents the mean ± SE across females for wet and dry season. N represents the number of females used in the matched paired comparison for each reproductive category. Significance was determined using the Wilcoxon Signed Ranks Test.
Environmental factors predicting fGC concentration within wet seasons or dry seasons
Because wet and dry seasons differed so pervasively in several respects, we next constructed separate GLMs to predict fGC within each season to gain further insights into how environmental factors impact fGC. Rainfall and Tmax were moderately positively associated (Pearson Correlation=0.534) across all calendar months, and inclusion of an interaction term between these two in the first GLM, reduced the predictive ability of both and produced a marginally significant interaction term (not shown). This further suggests the value of creating separate models for the two seasons because one major predictor of high fGC, low rainfall, occurs primarily in the dry season months, and the other, high Tmax occurs predominantly in the wet season months. During the wet season, rainfall and Tmax have a much lower association and in the opposite direction of the overall association, negative rather than positive (Pearson Correlation= -0.256), i.e. Tmax tends to be higher in drier wet season months than in wet season months with higher rainfall. Predictor variables for each seasonal model included reproductive state, average Tmax,, and average feeding time; individual was again included as a random factor. For the wet season model we also included cumulative monthly rainfall; we did not do so in the dry season model because rainfall was negligible during dry season.
In both wet season and dry season models, reproductive status and female identity significantly predicted fGC concentrations as they had in the overall GLM (wet season: F=22.935, p<0.001 for reproductive status, and F=2.050, p<0.001 for female identity; dry season: F=18.269, p<0.001 for reproductive status, and F=2.012, p<0.001 for female identity). However, the separate seasonal models also revealed between season differences in environmental impacts on fGC. During the wet season, when both mean fGC and mean Tmax, were more variable and, therefore, any relationships between the two were easier to detect, higher mean Tmax values were associated with high concentrations of fGC (F=13.417, p<0.001, B=+0.017). Neither variability in rainfall nor in time spent feeding predicted variability in fGC during the wet season once the other variables were taken into account (F=1.579, p=0.209 for rainfall and F=0.296, p=0.587 for time spent feeding). Predicting environmental sources of fGC variability across dry season months was more complex; both Tmax and the time spend feeding were associated with fGC, albeit less strongly than in the wet season. As in the wet season, fGC levels were higher in hotter months (F=5.959, p=0.015, B=+0.009); they were also higher in months when females spent more time feeding (F=5.890, p=0.016, B=+0.001).
DISCUSSION
Individual and reproductive state differences in fGC
Glucocorticoid concentrations were greater during pregnancy than in other reproductive stages, a pattern that has been demonstrated for females of many primate species (e.g. baboons: French et al., 2004; marmosets: Smith and French, 1997; tamarins: Ziegler et al., 1995; lemurs: Cavigelli, 1999; and humans: Lockwood et al., 1996; McLean and Smith, 1999). Such non-pathological hypercortisolemia during pregnancy is the result of the production of placental corticotrophin-releasing hormone (Goland et al., 1994). After parturition, concentrations of fGC in the post-partum Amboseli females returned to the levels of cycling females (see also Beehner et al., 2006a; for similar decline in other primates see e.g. Bahr et al., 1998; Bardi et al., 2003; Ziegler et al., 1995). The marked individual differences we detected in fGC levels can arise from a range of genetic and ontogenetic sources that are beyond the scope of the present study; elucidating those will require a focused investigation in itself.
Environmental conditions and fGC variability
Overlaid upon variability associated with reproductive status, we detected a strong signature of environmental conditions on fGC concentrations after controlling for individual differences. Females of all reproductive states had higher fGC levels in the dry season than in the wet one (see also Sapolsky, 1986 for similar results for male anubis baboons in drought vs. non-drought years). Baboons, like humans and unlike most other vertebrates, are relatively aseasonal in their reproduction, and conceptions occur frequently throughout the year (Alberts et al., 2005). Reproduction throughout the year means that baboon females sometimes experience pregnancy and infant care during relatively lush seasons, and at other times they experience these reproductive states during harsh seasons or even drought years, facilitating insight into potentially interacting effects of environmental and reproductive factors on GC. Cumulative effects of multiple stressors or challenges may result in GC reaching pathological levels at some times more than others, or more readily for some females than for others (Romero, 2004). For example, females that are pregnant during the dry season simultaneously experience the hypercortisolemia of pregnancy and a stress response to the dry season. The cumulative effects of multiple challenges on GC concentrations may be sufficiently great that females are at higher risk of reproductive failure; such hypercortisolemia may contribute to the reduced rates of cycling and conception and the increased rates of fetal loss during droughts that we have documented in Amboseli (Beehner et al., 2006b).
Females also had higher fGC concentrations in hotter months. The finding of heat stress is consistent with experimental studies (e.g. Hussain et al., 1992; Larsson et al., 1983; Lowe et al. 2002) in which animals that were exposed to extreme heat experienced elevated GC concentrations. In Amboseli, the wet season encompasses the summer months. February and March are particularly hot; on average across the 4 years of the present study, for 63% of the days in February and 74% of those in March the weather station Tmax was at least 34°C, a temperature that is close to baboons’ normal core body temperature of 38°C. In some years Amboseli experiences an ‘inter-rain’ season of lower rainfall during the January through March period (Struhsaker, 1967; Altmann et al., 2002), especially so during drought years. In such years, environmental stressors may resemble to some extent those in October at the end of normal dry season. Furthermore, heat stressors may be greater than suggested by weather station temperatures. Animals’ ‘perceived environmental temperature’ will often be considerably above weather station values, depending on habitat, sun exposure, humidity and behavior (Hill et al., 2004). Humidity, which is extremely low in Amboseli’s dry season, is, higher in the wet season months of summer. As various foods become available during the wet season, each perhaps for only short periods as in the case of various flowers or small shrub fruits (Alberts et al., 2005), the baboons shift habitat use to take advantage of emerging opportunities. The emerging foods vary greatly in the extent to which feeding on them entails sun exposure during harvesting as well as during travel to the feeding sites. As a result of the baboons ‘eclectic omnivory’ (Alberts et al., 2005; Altmann, 1998) and the heterogeneity of environmental stressors entailed by various foods, high temperatures may differ in impacts during different months and years.
To a lesser extent, high Tmax was associated with elevate fGC during the dry as well as the wet season, but several potential environmental stressors co-vary during the dry season, as indicated by the positive association between fGC and time spent feeding during that season. At the onset of the dry season in June, temperatures are cool, a diversity of foods are available, and drinking water is still of high quality and relatively accessible in many locations, which probably explains the relatively low fGC levels in June. As the dry season progresses, the Amboseli baboons become increasingly dependent on grass corms, an underground food source that requires considerable processing and that is found on the open, unshaded, grassland rather than woodland and shrub areas (Alberts et al., 2005; Altmann, 1998). The increase in food processing time entailed by corm-feeding may explain the increase in time spent feeding observed from July to October. Associated with the changes in feeding time, increased ambient temperatures, and increased exposure to sun, we found that female baboons had elevated fGC concentrations from July to October. These data are consistent with those reported in the literature for various animal species subjected to food deprivation (Astheimer et al., 1992; Harper and Austad, 2000; Kitaysky et al., 2001a; Tsuma et al., 1996). Moreover, water sources become progressively more scarce, smaller in size, and poorer in quality in Amboseli as the dry season progresses. Water is often considered the major limiting factor in African savannahs (Lamprey, 1964), and water shortage is a potent stressor in laboratory experiments (Nelson, 1993; Nelson et al., 1989). For the Amboseli baboons, utilizing the few remaining available late dry season water sources also requires the baboons to travel more, be more exposed to the sun, and to compete for access with other baboon groups, other wildlife, and local pastoralists with their livestock.
Finally, because all environmental stressors tend to be greater as the dry season progresses, we expected that the highest fGC levels would occur at the end of the dry season, in September and October. However, both fGC and feeding time plateaued during these two months. We hypothesize that the baboons shift from a strategy of seeking more food and more water to one of avoiding heat, minimizing energy expenditure and water needs, and avoiding exposure to water that is increasingly of poor quality as well as being scarce. A behavioral response of this sort has been reported for chacma baboons during the long days of southern African summers (Hill, 2006), and the present findings demonstrate a parallel physiological response. Such ‘strategy switching’ may be more general than previously appreciated. Indeed, strategy switching may have an important role in response to changing environments and may set limits on the extent to which animals are able to adapt to environmental change.
Although short-term, acute stressors result in several-fold increases in GC levels in some studies (Astheimer et al., 1992; Boonstra et al., 2001; Jessop et al., 2000), the elevations in fGC concentrations found in our study were much less (<20%), and were similar to that reported by Sapolsky (1986) in his comparison of male Anubis baboons in drought vs. non-drought years. The environmental stressors here and in Sapolsky’s comparison are long term and chronic, rather than acute, and may therefore have major impacts through smaller, persistent and cumulative effects. Such stressors may be particularly deleterious when conditions are both unpredictable and more extreme than usual in degree or duration. In addition, Sapolsky (1986) reported more adverse changes in male reproductive hormones (testosterone) than in GC in anubis baboons during drought, and our preliminary evidence for Amboseli baboons suggests they, too, experience more adverse changes in reproductive hormone concentrations than in GC (Gesquiere et al., in preparation).
In summary, female baboons, independent of their reproductive stage and despite individual differences, experienced environmental stress in Amboseli as reflected in elevated GC concentrations Nonetheless, unlike the situation for many other species, but similar to humans, the extreme adaptability of baboons (e.g. their ability to modify their time budgets, diet and perhaps landscape use) mitigates the consequences of this environmental stress, and no complete cessation of reproductive function ordinarily occurs during unfavorable months. Moreover, the highly suggestive individual differences in fGC that we documented here indicate a potential mechanism of life history variability on which natural selection may operate, an important topic for future investigation.
ACKNOWLEDGEMENTS
Supported by NSF IBN-0322613, NSF BSE-0323553 and R03 MH65294, and the Chicago Zoological Society. Thanks to the Office of the President, Republic of Kenya, the Kenya Wildlife Services, its Amboseli staff and Wardens, the Institute of Primate Research, the National Museums of Kenya, and the members of the Amboseli-Longido pastoralist communities. Particular thanks go to the Amboseli field team who contributed to sample and data collection (R.S. Mututua, S. Sayialel, and J.K. Warutere). Thanks to T. Fenn for database assistance, and to C. Markham, P. Onyango, J. Beehner for their input on data analysis or for providing comments on earlier drafts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long term change in a savannah environment. In: Brockman DK, van Schaik CP, editors. Primate Seasonality: Implications for Human Evolution. Cambridge University Press; 2005. pp. 157–196. [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behav. 1974;49:227–267. doi: 10.1163/156853974x00534. reprinted in Foundations of Animal Behavior, L.D. Houck & L.C. Drickamer, eds. U Chicago Press, 1996. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC, Altmann SA, Roy SB. Dramatic change in local climate patterns in the Amboseli basin. Afr. J. Ecol. 2002;40:248–251. [Google Scholar]
- Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. Life-history correlates of steroid concentrations in wild peripartum baboons. Am. J. Primatol. 2004;64:95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- Altmann SA. The pregnancy sign in savannah baboons. Lab. Anim. Dig. 1970;6:7–10. [Google Scholar]
- Altmann SA. Foraging for Survival: Yearling Baboons in Africa. University of Chicago Press; Chicago: 1998. [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scand. 1992;23:355–365. [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm. Behav. 1995;29:442–457. doi: 10.1006/hbeh.1995.1276. [DOI] [PubMed] [Google Scholar]
- Bahr NI, Pryce CR, Dobeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol. Behav. 1998;64:429–437. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum cortisol levels and mother-infant interactions in Japanese macaques. Am. J. Phys. Anthropol. 2003;120:298–304. doi: 10.1002/ajpa.10150. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Horm. Behav. 2006a;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behav. Ecol. 2006b;17:741–750. [Google Scholar]
- Boonstra R. Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr. Comp. Biol. 2004;44:95–108. doi: 10.1093/icb/44.2.95. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Hubbs AH, Lacey EA, McColl CJ. Seasonal changes in glucocorticoid and testosterone concentrations in free-living arctic ground squirrels from the boreal forest of the Yukon. Can. J. Zool. 2001;79:49–58. [Google Scholar]
- Brockman DK, van Schaik CP, editors. Primate Seasonality: Implications for Human Evolution. Cambridge University Press; 2005. [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Seasonal variation in human reproduction: environmental factors. Q. Rev. Biol. 1995;70:141–164. doi: 10.1086/418980. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim. Behav. 1999;57:935–944. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Gerber JM, Richardson HN, Moffatt CA, Demas GE, Taymans SE, Nelson RJ. Stress affects corticosteroid and immunoglobulin concentrations in male house mice (Mus musculus) and prairie voles (Microtus ochrogaster) Comp. Biochem. Physiol. A Physiol. 1997;118:655–663. doi: 10.1016/s0300-9629(97)87355-0. [DOI] [PubMed] [Google Scholar]
- Dunlap KD. Hormonal and behavioral responses to food and water deprivation in a lizard (Sceloporus occidentalis): implications for assessing stress in a natural population. J. Herpetol. 1995;29:345–351. [Google Scholar]
- Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. The effect of repeated physical exercise on hippocampus and brain cortex in stressed rats. Ann. N.Y. Acad. Sci. 2007;1096:207–219. doi: 10.1196/annals.1397.087. [DOI] [PubMed] [Google Scholar]
- French JA, Koban T, Rukstalis M, Ramirez SM, Bardi M, Brent L. Excretion of urinary steroids in pre- and postpartum female baboons. Gen. Comp. Endocrinol. 2004;137:69–77. doi: 10.1016/j.ygcen.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm. Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Goland RS, Josak S, Conwell I. Placental corticotropin-releasing hormone and the hypercortisolism of pregnancy. Am. J. Obstet. Gynecol. 1994;171:1287–1291. doi: 10.1016/0002-9378(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Gwazdauskas FC. Effects of climate on reproduction in cattle. J. Dairy Sci. 1985;68:1568–1578. doi: 10.3168/jds.S0022-0302(85)80995-4. [DOI] [PubMed] [Google Scholar]
- Harper JM, Austad SN. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol. Biochem. Zool. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- Harvey S, Phillips JG, Rees A, Hall TR. Stress and adrenal function. J. Exp. Zool. 1984;232:633–645. doi: 10.1002/jez.1402320332. [DOI] [PubMed] [Google Scholar]
- Hill RA. Thermal constraints on activity scheduling and habitat choice in baboons. Am. J. Phys. Anthropol. 2006;129:242–249. doi: 10.1002/ajpa.20264. [DOI] [PubMed] [Google Scholar]
- Hill RA, Weingrill T, Barrett L, Henzi SP. Indices of environmental temperatures for primates in open habitats. Primates. 2004;45:7–13. doi: 10.1007/s10329-003-0054-8. [DOI] [PubMed] [Google Scholar]
- Huber S, Palme R, Arnold W. Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus) Gen. Comp. Endocrinol. 2003;130:48–54. doi: 10.1016/s0016-6480(02)00535-x. [DOI] [PubMed] [Google Scholar]
- Hussain SMI, Fuquay JW, Younas M. Estrous cyclicity in nonlactating and lactating Holsteins and Jerseys during a Pakistani summer. J. Dairy Sci. 1992;75:2968–2975. doi: 10.3168/jds.s0022-0302(92)78060-6. [DOI] [PubMed] [Google Scholar]
- Jessop TS, Hamann M, Read MA, Limpus CJ. Evidence for a hormonal tactic maximizing green turtle reproduction in response to a pervasive ecological stressor. Gen. Comp. Endocrinol. 2000;118:407–417. doi: 10.1006/gcen.2000.7473. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen. Comp. Endocrinol. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. [B] 2001a;171:701–709. doi: 10.1007/s003600100230. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001b;12:619–625. [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 2003;43:140–149. doi: 10.1016/s0018-506x(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Lamprey HF. Estimation of the large mammal densities, biomass, and energy exchange in the Tarangire Reserve and the Masai steppe in Tanganyika. E. afr. Wildl. J. 1964;20:1–46. [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Larsson K, Einarsson S, Lundstrom K, Hakkarainen J. Endocrine effects of heat stress in boars. Acta Vet. Scand. 1983;24:305–314. doi: 10.1186/BF03546734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BT, Christopherson RJ, Cosgrove SJ. Effect of water restriction and environmental temperatures on metabolic rate and physiological parameters in sheep. Can. J. Anim. Sci. 2000;80:97–104. [Google Scholar]
- Lockwood CJ, Radunovic N, Nastic D, Petkovic S, Aigner S, Berkowitz GS. Corticotropin-releasing hormone and related pituitary-adrenal axis hormones in fetal and maternal blood during the second half of pregnancy. J. Perinat. Med. 1996;24:243–251. doi: 10.1515/jpme.1996.24.3.243. [DOI] [PubMed] [Google Scholar]
- Lowe TE, Gregory NG, Fisher AD, Payne SR. The effects of temperature elevation and water deprivation on lamb physiology, welfare, and meat quality. Aust. J. Agric. Res. 2002;53:707–714. [Google Scholar]
- Lynch JW, Ziegler TE, Strier KB. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm. Behav. 2002;41:275–287. doi: 10.1006/hbeh.2002.1772. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen. Comp. Endocrinol. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Lynn SE, Breuner CW, Wingfield JC. Short-term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird. Horm. Behav. 2003;43:150–157. doi: 10.1016/s0018-506x(02)00023-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol. Metab. 1999;10:174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Simulated drought affects male reproductive function in deer mice (Peromyscus maniculatus bairdii) Physiol. Zool. 1993;66:99–114. [Google Scholar]
- Nelson RJ, Frank D, Bennett SA, Carter CS. Simulated drought influences reproduction in male prairie voles. Physiol. Behav. 1989;46:849–852. doi: 10.1016/0031-9384(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, editor. An Introduction to Behavioral Endocrinology. Third Edition Sinauer Associates, Inc.; Sunderland, Massachsetts: 2005. [Google Scholar]
- Nguyen N, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Late pregnancy glucocorticoid levels predict responsiveness in wild baboon mothers (Papio cynocephalus) Anim. Behav. 2008 doi:1016/ j.anbehav 2007.09.035. [Google Scholar]
- Olsson K, Josater-Hermelin M, Hossaini-Hilali J, Hydbring E, Dahlborn K. Heat stress causes excessive drinking in fed and food deprived pregnant goats. Comp. Biochem. Physiol. A Physiol. 1995;110:309–317. doi: 10.1016/0300-9629(94)00186-w. [DOI] [PubMed] [Google Scholar]
- Olsson K, Josater-Hermelin M, Hossaini-Hilali J, Cvek K, Hydbring E, Dahlborn K. Reproductive period affects water intake in heat-stressed dehydrated goats. Comp. Biochem. Physiol. A Physiol. 1996;113:323–331. doi: 10.1016/0300-9629(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Pereira RJG, Duarte JMB, Negrao JA. Effects of environmental conditions, human activity, reproduction, antler cycle and grouping on fecal glucocorticoids of free-ranging Pampas deer stags (Ozotoceros bezoarticus bezoarticus) Horm. Behav. 2006;49:114–122. doi: 10.1016/j.yhbeh.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Reeder DM, Kramer KM. Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J. Mammal. 2005;86:225–235. [Google Scholar]
- Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Romero LM, Ramenofsky M, Wingfield JC. Season and migration alters the corticosterone response to capture and handling in an Arctic migrant, the white-crowned sparrow (Zonotrichia leucophrys gambelii) Comp. Biochem. Physiol.C Pharmacol. Toxicol. Endocrinol. 1997;116:171–177. doi: 10.1016/s0742-8413(96)00208-3. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM, Wingfield JC. Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 2000;118:113–122. doi: 10.1006/gcen.1999.7446. [DOI] [PubMed] [Google Scholar]
- Saltz D, White GC. Urinary cortisol and urea nitrogen responses to winter stress in mule deer. J. Wildl. Manage. 1991;55:1–16. doi: 10.7589/0090-3558-27.1.41. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrine and behavioral correlates of drought in wild olive baboons (Papio anubis) Am. J. Primatol. 1986;11:217–227. doi: 10.1002/ajp.1350110303. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Silanikove N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000;67:1–18. [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1997;42:253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Strier KB, Ziegler TE, Wittwer DJ. Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides) Horm. Behav. 1999;35:125–134. doi: 10.1006/hbeh.1998.1505. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. Ecology of vervet monkeys (Cercopithecus Aethiops) in the Masai-Amboseli Game Reserve, Kenya. Ecol. 1967;48:891–904. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th edition HarperCollins; New York: 2001. [Google Scholar]
- Tsuma VT, Einarsson S, Madej A, Kindahl H, Lundeheim N. Effect of food deprivation during early pregnancy on endocrine changes in primiparous sows. Animal Reproduction Sci. 1996;41:267–278. [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Gray DA, Barrett L, Henzi SP. Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm. Behav. 2004;45:259–269. doi: 10.1016/j.yhbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Wingfield JC. Seasonal and interpopulational variation in plasma levels of corticosterone in the side-blotched lizard (Uta stansburiana) Physiol. Zool. 1994;67:1025–1049. [Google Scholar]
- Wingfield JC. Influence of weather on reproduction. J. Exp. Zool. 1984;232:589–594. doi: 10.1002/jez.1402320327. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. The concept of allostasis: coping with a capricious environment. J. Mammal. 2005;86:248–254. [Google Scholar]
- Wingfield JC, Kitaysky AS. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Int. Comp. Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Ramenofsky M. Corticosterone and facultative dispersal in response to unpredictable events. ARDEA. 1997;85:155–166. [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm. Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]