Abstract
The genetic causes of most of the disorders of cornification have been uncovered. We now face the significant task of delineating how these mutations result in specific phenotypes. Because the permeability barrier resides in the extracellular lipid-enriched domains of the stratum corneum, it was anticipated that disorders of lipid metabolism would perturb the lamellar membrane structures of the extracellular domains and would result in a defective barrier. Unanticipated was the finding that inherited disorders of corneocyte proteins also exhibit, to varying degrees, an impaired permeability barrier. The effect of these corneocyte mutations on barrier function have shed light on how corneocytes interact with the intercellular lamellae to provide the barrier. In some entities, an impaired scaffold leads to fragmented and foreshortened lamellar membranes (e.g., transglutaminase-deficient lamellar ichthyosis, loricrin keratoderma). In others, there is impaired lamellar body secretion (e.g., epidermolytic hyperkeratosis) and altered lipid processing (e.g., Netherton syndrome), leading to deficiency of lamellar membrane structures. The combined insights from delineation of the pathogenesis of lipid metabolic defects and corneocyte protein abnormalities can be used to develop a function-driven model of disease pathogenesis. This model will aid in the development of more targeted approaches to therapy and in understanding some systemic complications of these disorders.
Introduction
The ichthyoses comprise a large group of scaling disorders with diverse etiology 1–4. To date, more than 25 genes have been identified that encode a wide spectrum of epidermal proteins, including enzymes of lipid metabolism and of peptide cross-linking, proteases and their inhibitors, epidermal structural proteins, and proteins involved in cellular communication, signaling and gene transcription. Abnormalities in any of these components result in a rather stereotypic epidermal response with epidermal hyperplasia and the formation of excess stratum corneum (SC) accompanied by abnormal (delayed and/or disordered) desquamation, with visible accumulation of squames (scales) on the skin’s surface – the clinical hallmark of all the ichthyoses (Table 1). In certain instances, there is at least transiently increased skin fragility or blistering, as in Netherton syndrome and epidermolytic hyperkeratosis (EHK). In this review, we examine the role of corneocyte proteins in disorders of cornification using a new, function-driven model of pathogenesis. The role of lipid metabolic defects in these disorders is reviewed elsewhere 5.
TABLE 1.
Pathogenesis of Ichthyoses: Corneocytes vs. Extracellular Lipid Matrix
| 1. | Ichthyosis is a final common pathway for errors in genes expressed in the epidermis |
| 2. | Genes encoding a wide spectrum of epidermal proteins have been shown to cause ichthyosis |
| 3. | Impaired stratum corneum barrier function results from divergent mechanisms depending on the underlying gene mutation |
| 4. | Although diverse molecular defects underlie the various subtypes, ichthyoses are characterized by a stereotypic epidermal response with acanthosis, hyperkeratosis, and extracellular permeability barrier abnormalities |
A Function-Driven Model of Disease Pathogenesis in Disorders of Cornification
As more has been learned about these disorders, various classification systems have been proposed. In the 1960’s, Frost and Weinstein offered a classification based upon epidermal kinetics, in which disorders were designated either primarily retention hyperkeratoses (delayed desquamation with normal rates of epidermal renewal) or hyperproliferative states 6. In the 1980’s Elias and Williams advanced a morphological classification with disorders affecting the intercellular lipids (“mortar”) and those affecting the structural proteins of the corneocyte (“bricks”)7, 8. As further insights have been gained into the relationships between stratum corneum structures and the barrier functions of epidermis 9, it is now possible to integrate the epidermal kinetics model with the “Bricks and Mortar” model. This new, function-driven model provides a framework for understanding how such a wide and disparate group of genetic errors results in the ichthyosis phenotype. Of particular importance in the function-driven model is the observation that epidermal permeability barrier function is abnormal to a varying extent in virtually all of these disorders (Table 2).
Table 2.
Principles of a Function-Driven Model of Disorders of Cornification (with Examples)
| 1. | The Permeability Barrier is abnormal to varying degrees in all ichthyoses. Barrier defects may arise due to: | ||||
| a. | Abnormal lipid composition of intercellular membranes leading to lamellar/nonlamellar phase separation | ||||
| i. | Inborn errors of lipid metabolism resulting in over- or under- representation of key SC lipids (i.e., cholesterol, free fatty acids, ceramides) that form the lamellar membranes and/or the introduction of other lipid species. (X-Linked I.; NLSD; Refsum disease, ARCI-Lipoxygenase pathway) | ||||
| ii. | Failure of lipid processing to key SC lipids due to: | ||||
| 1. | Deficiency of lipid processing enzymes | ||||
| a. | Loss of function mutations (Neonatal Gaucher D.) | ||||
| b. | Failure of lamellar body delivery (EHK, CEDNIK Syndrome) | ||||
| c. | Proteolytic attack (Netherton) | ||||
| 2. | Increase in SC pH leading to inhibition of lipid processing enzymes with acidic pH optima (?I. vulgaris) | ||||
| b. | Paucity of lamellar membranes due to failure of: | ||||
| i. | Lamellar body organellogenesis (Harlequin I.) | ||||
| ii. | Lamellar body secretion (EHK; CEDNIK syndrome) | ||||
| c. | Abnormal cornified envelope scaffold for organization of lamellar membranes (TGMI-def. ARCI; Loricrin K.) | ||||
| 2. | Homeostatic responses attempt to repair the barrier defect. | ||||
| a. | These responses include: | ||||
| i. | increased epidermal lipid synthesis | ||||
| ii. | epidermal hyperplasia | ||||
| iii. | inflammation | ||||
| b. | Because of the genetic defect, the barrier defect cannot be repaired and these homeostatic responses are sustained. | ||||
| c. | The severity of the barrier defect determines the intensity of the homeostatic repair responses. | ||||
| d. | Environmental conditions modify the barrier defect. | ||||
| i. | The need for a permeability barrier in the aqueous, in utero environment is much reduced. | ||||
| ii. | At birth, the xeric stress of post-natal life augments the barrier repair homeostatic responses. In ichthyoses with severe barrier defects, this stress results in a striking “phenotypic shift” (Harlequin I; EHK; Collodion baby phenotypes). | ||||
| 3. Desquamation is also impaired in all ichthyoses. Abnormal desquamation can be mediated by: | |||||
| a. | Delayed or accelerated corneodesmosome proteolysis | ||||
| i. | Protease inhibition (X-linked I.) or failure of protease delivery leads to delayed corneodesmosome degradation (Harlequin I; EHK) | ||||
| ii. | Loss of protease inhibition leads to accelerated corneodesmosome degradation (Netherton) | ||||
| b. | Epidermal hyperplasia with incomplete terminal differentiation | ||||
| i. | Entombed lamellar bodies in hyperplastic corneocytes with failure of protease delivery (Hyperplastic ARCI phenotypes) | ||||
| ii. | Elevated SC pH inhibiting proteases with acidic pH optima (?I. vulgaris) | ||||
| c. | Decreased corneocyte hydration with loss of hydration-related corneocyte swelling exerting mechanical stress on intercellular connections | ||||
| i. | Deficiency of filaggrin breakdown products (?I. vulgaris) | ||||
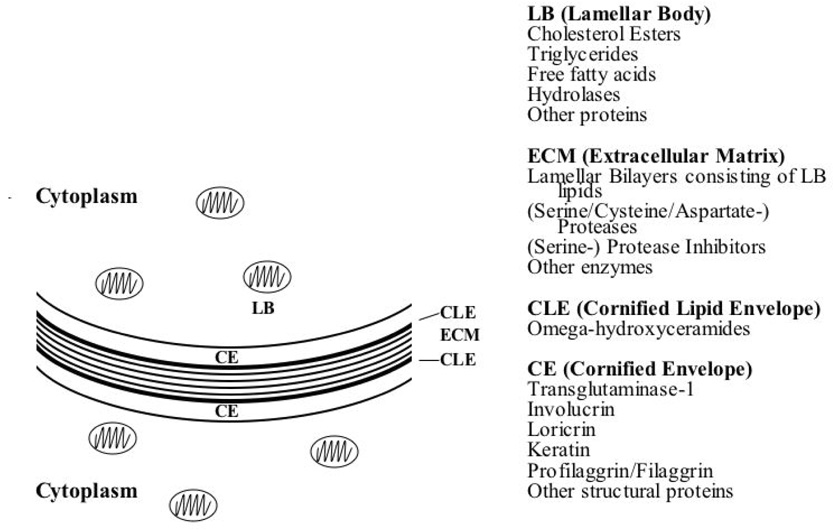
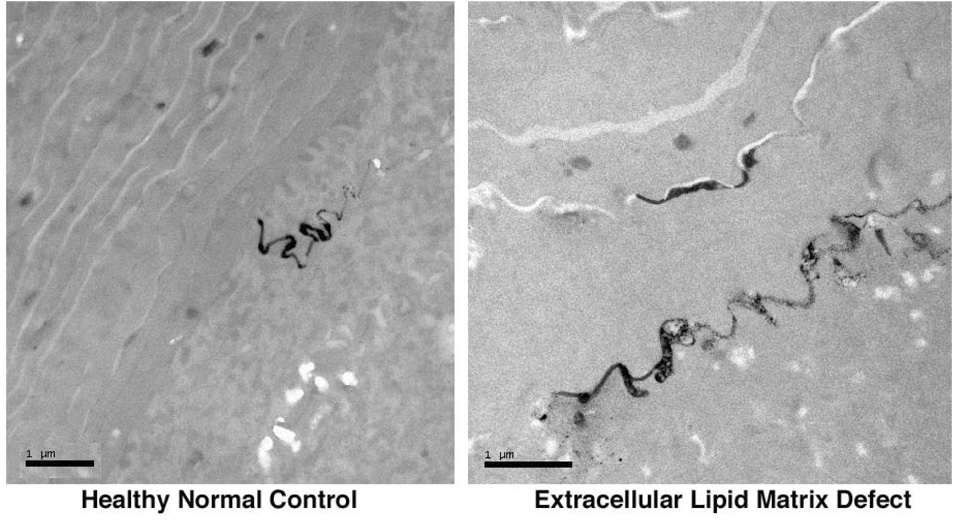
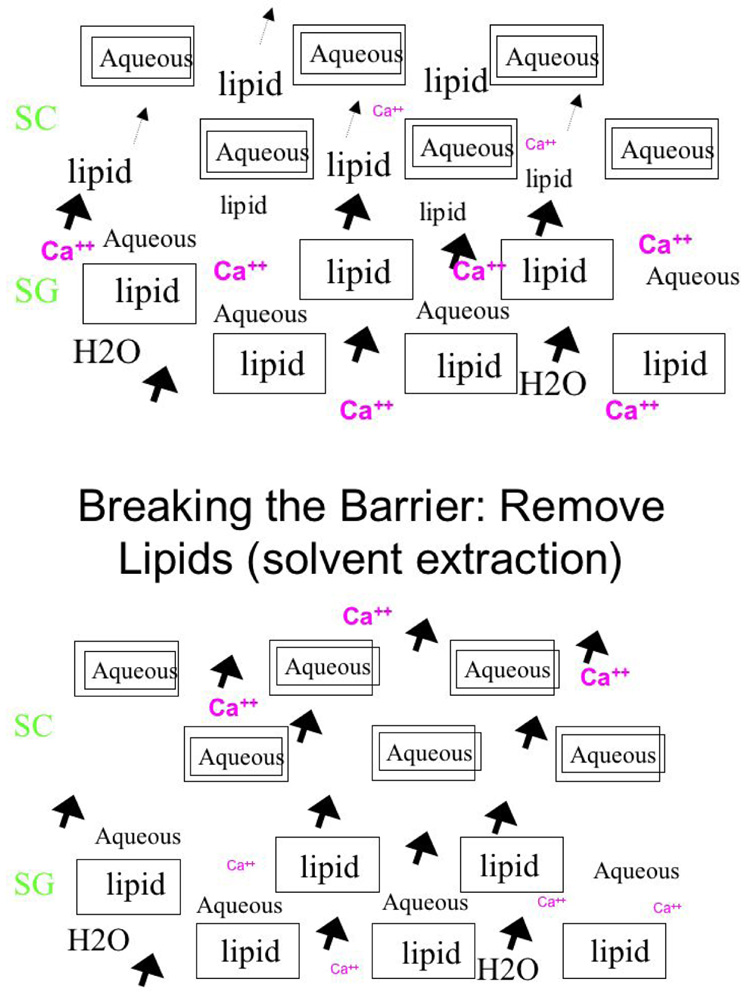
The stratum corneum (SC) comprises a unique, two-compartment system of protein-enriched corneocytes, embedded in a lipid-enriched extracellular matrix. SC lipids are composed of extremely hydrophobic species, and organized into repeating arrays of lamellar membranes (Fig. 1). These membranes provide the permeability barrier, which can be demonstrated ultrastructurally using a small, electron-dense water-soluble tracer molecule, lanthanum that follows the outward movement of water in the epidermis. In normal epidermis, the movement of lanthanum is halted at the stratum granulosum – SC interface by the interposition of lamellar membrane structures in the intercellular domain of the SC 10, 11. If the lamellar structures are inherently abnormal, as in many ichthyoses, or removed from the SC, as for example, by solvent extraction, transepidermal water loss (TEWL) rates increase, and lanthanum then can be seen to penetrate the SC through the extracellular pathway (Fig. 2). Acute experimental perturbations of the permeability barrier (e.g., through solvent extraction or tape strippings) result in a series of homeostatic responses aimed at repairing the barrier 12, 13. In the first wave of responses, occurring within minutes of barrier disruption, the loss of the high calcium milieu bathing the stratum granulosum (SG), signals secretion of preformed lamellar bodies from the upper SG (“Deliver more lipid, now!”) (Fig. 3). In the second phase, occurring within hours and signaled in part by release of preformed IL-1α from SC stores, epidermal lipid synthesis increases (“Make more lipid!”). In the third phase, within a day and also in response to cytokine signaling, epidermal DNA synthesis increases (“Make more cells!”). In normal human epidermis, these responses result in repair of the permeability barrier within about 3 days. In disorders of cornification, where a mutation produces a barrier defect that cannot be corrected by these homeostatic responses, the repair efforts (hypermetabolism, hyperplasia) do not terminate. Thus, a mutation that produces a barrier defect will invariably be associated with epidermal hyperplasia.
Figure 1. Key stratum corneum components.
Figure 2. Lanthanum penetration through the extracellular pathway.
In normal control skin (left panel), electron dense lanthanum tracer permeation is halted at the stratum granulosum – SC junction, whereas in ichthyosis vulgaris (right panel), tracer permeates beyond the junction, but remains restricted within the extracellular lamellar lipid pathway. Freshly obtained skin biopsies were exposed to 4% colloidal lanthanum nitrate tracer in 0.05 M Tris buffer, pH 7.4, for one hour, containing 2% glutaraldehyde and 2% paraformaldehyde, followed by post-fixation in OsO4. Magnification bars = 1 µm (left panel), and = 2 µm (right panel).
Figure 3. Two compartment model of SC.
In normal control skin (upper panel), the calcium ion gradient peaks in the lower SC, whereas after barrier disruption via solvent extraction of extracellular matrix lipids (lower panel) there is a rapid loss of the SC calcium gradient.
The corneocytes ‘bricks’ are constituents of the permeability barrier by two mechanisms. First, corneocytes serve as a critical scaffold, required for the organization of the extracellular lipid matrix into its characteristic lamellar pattern. Second, through formation of multiple, overlapping layers of cells, they generate a tortuous, intercellular pathway that impedes the egress of water 14. In addition to providing the framework for the permeability barrier, corneocytes subserve several other critical functions; including resistance to mechanical insults, as well as hydration and pliability, an additional set of functions related to the humidity-dependent hydrolysis of filaggrin into amino acids and their deiminated products (see below).
Another SC function that is universally impaired in the ichthyoses is desquamation. Normal desquamation is an invisible process, whereby single corneocytes at the skin surface are invisibly swept away. Normal desquamation represents an orderly process of loss of corneocyte cohesion, which is mediated by corneodesmosomes, intercellular protein connectors 15–17. Corneodesmosome degradation is mediated by several proteases, and regulated by protease inhibitors and pH 18, 19. In the predominantly retention hyperkeratoses (e.g., ichthyosis vulgaris), desquamation is the SC function that is primarily affected.
Due to the energy losses that accompany evaporative water loss, infants and children with severe phenotypes can exhibit growth failure due to increased caloric requirements 20, 21. Other functional deficiencies include heat intolerance in patients with more severe generalized ichthyoses (e.g., lamellar ichthyosis), due to obstruction of sweat ducts. In certain ichthyoses there is also an increased susceptibility to cutaneous and systemic infections. Although not yet confirmed experimentally, a plausible scenario for the infectious complication is as follows: Certain SC lipids (e.g., free fatty acids) and anti-microbial peptides (defensins and cathelicidins) are lamellar body-derived residents of the SC intercellular domains that provide a first line of defense against microbial invasion (innate immune system). Failure of lamellar body secretion (e.g., EHK) or of lipid processing (required for generation of free fatty acids)(e.g., Netherton syndrome) or proteolytic inactivation of anti-microbial peptides (e.g., Netherton syndrome) may therefore account for the propensity for bacterial and fungal infections in EHK, as well as bacterial and viral infections in Netherton syndrome 22–24.
“Lipid” versus “Corneocyte” Defect
Irregularities of epidermal lipid metabolism were among the first to be shown to cause disorders of cornification 25–29. For example, lack of steroid sulfatase in recessive x-linked recessive ichthyosis directly results in altered lamellar membrane structure and function. In contrast, the inherent attenuation of corneocytes in avian 30 and mammalian skin 31 was not accompanied by gross barrier abnormalities. Hence, corneocyte structural proteins were initially thought to primarily provide mechanical resilience to the SC. Nevertheless, evidence from human studies suggests that even primary corneocyte protein abnormalities are accompanied by substantial barrier impairment 32–38. Defects in the cornified envelope scaffold result in barrier abnormalities both in lamellar ichthyosis 32, 33, 39, and in loricrin keratoderma 37, supporting a critical scaffold function of the corneocyte for the permeability barrier. In contrast, mutations in keratins 1 or 10 in EHK provoke a barrier abnormality by interfering with lamellar body exocytosis (in this instance a cytoskeletal, rather than a scaffold abnormality) 35. Furthermore, in Netherton syndrome, unopposed epidermal protease activity results in desmosomal degradation and compensatory acceleration of lamellar body secretion 36, 38. The phenotypes vary rather widely in these entities, as do their pathogenic mechanisms. Yet, despite the primary defect affecting either corneocyte structural proteins or proteolytic enzymes impairing corneocyte-to-corneocyte adhesion, the permeability barrier abnormality in all instances is due to enhanced water movement between rather than through the defective corneocytes (Table 2).
Transglutaminase 1-Deficient Autosomal Recessive Congenital Ichthyosis (TGM1-deficient ARCI)
Clinical characteristics
The group of autosomal recessive congenital ichthyoses (ARCI) includes lamellar ichthyosis (LI) and nonbullous congenital erythroderma (CIE), and comprises a spectrum of phenotypes ranging from large dark plate-like scales and often little to no erythema (LI) to a fine, lighter scaling pattern, often with prominent erythema (CIE), with many intermediate phenotypes. Patients with this spectrum of phenotypes present at birth encased in a shiny, coherent coating, the ‘collodion membrane’. During the first post-natal weeks, as the hyperkeratotic membrane is shed, it is replaced by scaling and lichenification that involves the entire body including the intertriginous areas, palms, soles and scalp. In rare cases, the phenotype may be very mild (“self-resolving collodion baby”) or focal 40. Although there rarely is corneal involvement, patients can show marked facial tautness with severe ectropion.
Molecular defect
At least one third of ARCI are caused by mutations in transglutaminase-1 (TGM1), on chromosome 14 41–43, resulting in impaired cornified envelope crosslinking 44. To date, over 50 different mutations have been identified in this gene. Some mutations are proposed to destabilize a hydrophobic pocket, distorting the active site of the enzyme, and resulting in loss of activity 45. Transglutaminase-1 not only crosslinks proteins, but also sphingolipids to the cornified envelope 46, 47. Ex vivo gene replacement of TGM1, followed by transplantation to SCID mice, has been successfully used on the ARCI phenotype 48. Recent progress in delineating the genetic spectrum of ARCI has uncovered several additional gene mutations, many of which appear to disrupt epidermal lipid metabolism 5, 49–51. A clear correlation between these genotypes and the spectrum of ARCI phenotypes has yet to be established.
Consequences of TGM1 mutations for morphology and function
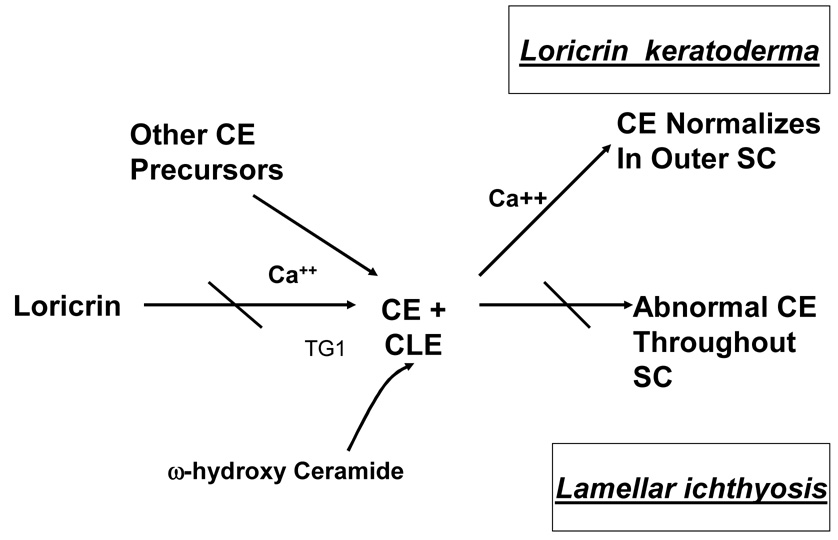
The epidermis in TGM1-deficient ARCI is acanthotic, often with a prominent granular layer. There is marked orthohyperkeratosis and occasional focal parakeratosis. On electron microscopy of the SC, the cornified envelopes are focally attenuated 32 and the resilience of corneocytes to boiling in sodium dodecyl sulfate (SDS) and dithiothreitol (DTT) is diminished 47, 52. Moreover, prominent abnormalities in extracellular lipid structures are evident ultrastructurally, with truncation and fragmentation of extracellular lamellar membrane arrays in regions where the cornified envelope is attenuated 32, as well as minor abnormalities in spacing of lamellar bilayers on electron microscopy 33 and x-ray diffraction 53. Functionally, these membrane structural abnormalities correlate with a modest increase in rates of TEWL under basal conditions 32, 33, 39, 53. However, interpretation of prior TEWL data is difficult, because several of these studies were conducted before genotyping was available, i.e., it is uncertain if all ARCI genotypes exhibit similar barrier impairments. Nevertheless, in biopsies from patients with proven TGM1-deficient ARCI, increased movement of lanthanum tracer through the intercellular pathway of the SC has been demonstrated 32, confirming a permeability barrier abnormality. Although the lamellar membranes are fragmented and truncated in TGM1-deficient ARCI, the cornified lipid envelope, a lipid membrane cross-linked to the outer surface of the cornified envelope is preserved, and omega-hydroxy-ceramide content, the lipid species cross-linked to the cornified envelope is normal 32, 54. This suggests that the barrier defect is not due to failure to form or attach a cornified lipid envelope, and indicates further that enzymes other than TGM1 also participate in omega-hydroxy-ceramide crosslinking. Thus, the extracellular lamellar membrane abnormalities are best explained by the lack of a proper scaffold, consistent with the putative role of the cornified envelope as necessary for organization of the extracellular matrix into lamellar bilayers (Fig. 4, Table 3).
Figure 4. Scaffold abnormalities in lamellar ichthyosis and loricrin keratoderma.
Table 3.
Function-Driven Model of Generalized Disorders of Cornification: Defects of the Corneocyte Proteins
| Disorder | 1° Defect | Downstream effect | SC Function | Homeostatic response |
|---|---|---|---|---|
| TGM1-def. ARCI & Loricrin K. | Fragile cornified envelope | Abnormal scaffold for lamellar membranes | ↑↑ TEWL | ↑↑ Epidermal hyperplasia |
| Epidermolytic HK | Disrupted keratin | Impaired lamellar body secretion | ↑↑ TEWL | ↑↑ Epidermal hyperplasia |
| ↓↓ CD digestion | ↓↓ Desquamation | |||
| Ichyosis vulgaris | ↓ Filaggrin | ↓↓ Corneocyte hydration | ↓↓ Hydration | ↓ Desquamation |
| ↑ SC pH (?) | ||||
| Netherton syndrome | Disrupted corneocyte adhesion | Abnormal scaffold | ↑↑ CD digestion | Epidermal hyperplasia ↑↑ Desquamation |
Loricrin Keratoderma
Clinical characteristics
Loricrin keratoderma exhibits a characteristic, honeycomb-like, palmo-plantar keratoderma on the acral extremities including palms and soles; a generalized fine-scaling; hyperkeratotic knuckle pads on the dorsal surface of the fingers; and, often, constricting bands encircling the fingers and/or toes (pseudoainhum) 55–61. While these features are similar to those of classic Vohwinkel syndrome 62, loricrin keratoderma lacks the neurosensory deafness of classic Vohwinkel syndrome, and additionally it exhibits the generalized scaling described above. This distinct phenotype is also referred to as Vohwinkel syndrome with ichthyosis, Camisa variant of Vohwinkel syndrome, or Vohwinkel disease limited to the skin.
Molecular defect
Loricrin keratoderma is caused by dominantly inherited mutations in the loricrin gene localized within the epidermal differentiation complex (EDC) encoded on chromosome 1q21. Loricrin is a glycine-serine-cysteine-enriched protein synthesized in the stratum granulosum, that along with profilaggrin, localizes to keratohyalin granules 63–65. In the outermost granular cell, loricrin migrates to the cell periphery, where it is deposited beneath the plasma membrane, and cross-linked to several other cytosolic proteins (e.g., involucrin, small proline rich proteins [SPRRs], elafin, repetin, S100, and up to ≈20 others) by TGM1 to form the cornified envelope. In loricrin keratoderma the mutations result in elongation of the C-terminal domain of the loricrin protein. The mutant loricrin is misdirected to the nuclei of the granular layer, fails to reach the cornified envelope and is retained in the parakeratotic SC 55, 66.
Consequences of loricrin mutation for morphology and function
In loricrin keratoderma, the epidermis exhibits epidermal hyperplasia, hypergranulosis, marked hyperkeratosis and parakeratosis, with characteristic roundish (rather than flattened) retained nuclei in the lower SC 58, 67. Corneocyte-to-corneocyte cohesion is impaired, as demonstrated by ready removal of SC by tape stripping 37. Functionally, SC hydration is markedly decreased in the hyperkeratotic/honeycomb-type skin sites, and basal rates of TEWL are increased. Disorganized lamellar bilayers together with penetration of the water soluble, electron-dense tracer, lanthanum, through the extracellular space beyond the stratum granulosum-SC junction indicate that the increased water loss occurs predominantly via extracellular domains 37. Bilayer abnormalities occur adjacent to regions in the lower SC in which discontinuities and attenuation of the cornified envelope are present. However, cornified envelope dimensions partially normalize in the outer SC, apparently due to ongoing, compensatory incorporation of other cornified envelope peptides (Fig. 4). This normalization correlates with persistence of abundant calcium in the extracellular spaces of the SC (due to the defective barrier), where it is in the correct location to activate TGM1. A primary pathogenic role of the cornified envelope scaffold abnormality in loricrin keratoderma is underscored by the presence of a normal lamellar body secretory system, with largely unimpeded secretion of lamellar body contents, with a normal corneocyte lipid envelope, and normal bound omega-hydroxy-ceramide content 37. In summary, the permeability barrier abnormality in loricrin keratoderma can be linked to a defective cornified envelope scaffold in the inner SC layers (Fig. 4). Thus, both TGM1-deficient ARCI and loricrin keratoderma share a common disease pathomechanism. In TGM1-deficient ARCI, the cornified envelope cross-linking enzyme is defective, while in loricrin keratoderma, its major substrate is deficient (Table 3).
Epidermolytic Hyperkeratosis (EHK)
Clinical characteristics
A characteristic feature in many EHK patients is a phenotypic shift from widespread blistering at birth to prominent hyperkeratosis during later life. Due to the blistering phenotype of the newborn, EHK was previously termed bullous congenital ichthyosiform erythroderma (BCIE) to distinguish it from the autosomal recessive, non-bullous ARCI group. Erythroderma is prominent in some patients, but absent in others. The hyperkeratosis in EHK often is generalized, and it may either involve or spare the palms and soles 68. In the generalized variants, typically, involvement of flexural and extensor surfaces of large joints (elbows, knees) is accentuated, sometimes with massive hyperkeratosis, giving a peculiar, ridged appearance. Flexural scales often become secondarily colonized by bacteria, producing a foul odor. Variants with particularly widespread and thick, porcupine-like (hystrix) hyperkeratosis (but lack of an early blistering phase) have been termed ichthyosis hystrix of Curth and Macklin. A milder variant of EHK, ichthyosis bullosa of Siemens (IBS), is characterized by peeling as well as annular, hyperkeratotic plaques preferentially over joints, shins and the periumbilical region. There are also localized forms, which are more limited to acral and flexural surfaces plus the palms/soles, or distributed in a linear pattern along the lines of Blaschko; i.e., as epidermal nevi. Epidermolytic hyperkeratosis strictly limited to palms and soles has been termed Vörner’s palmoplantar keratoderma (PPK).
Molecular defect
Mutations in keratin 1 (chromosome 12q13) and keratin 10 (chromosome 17q21-q22) have been identified as the cause of the classic generalized form of EHK 69 70–77. Localized forms are due to chromosomal mosaicism 66, 78–83. The offspring of patients with mosaic or nevoid variants of EHK due to K1 or K10 mutations are at risk for generalized forms of the disease. As many as half of all cases have no family history of the disease, indicating a high frequency of spontaneous mutations. The wide spectrum of clinical variability is predominantly between kindreds 84, 85. In the milder IBS variant, mutations in another keratin expressed in the granular layer, K2e, have been identified. In families with the EHK limited to palms and soles (Vörner’s) mutations in keratin 9 have been identified.
Keratinopathies are inherited as autosomal dominant traits, and function in a dominant-negative manner to disrupt the keratin filament network within the keratinocyte cytosol. The main components of these networks are the keratins, the most abundant proteins produced during the vectorial process of epidermal differentiation 86. Typically, one acidic (type I) keratin heterodimerizes with one basic (type II) keratin and two such dimers are arranged in an antiparallel and staggered configuration (protofilament) 87, 88. In turn, two protofilaments comprise a protofibril, of which four assemble to form the 10nm keratin intermediate filament. Within these filaments, the most abundant epidermal keratins, keratins 1 and 10, become linked to the cornified envelope (CE) 89–91. In the disrupted state, keratin filaments retract from their attachments beneath desmosomal plaques, forming clumps or perinuclear shells 92. Skin fragility due to mechanical trauma, which is most pronounced in neonatal EHK, is an expected consequence of mutations in keratin 1 or 10, analogous to the fragile skin phenotype of epidermolysis bullosa, where mutations in the basally expressed keratins, K5 or 14, produce cytoskeletal defects 93–95 and result in a mechanobullous phenotype.
Consequences of K1/10 mutations for morphology and function
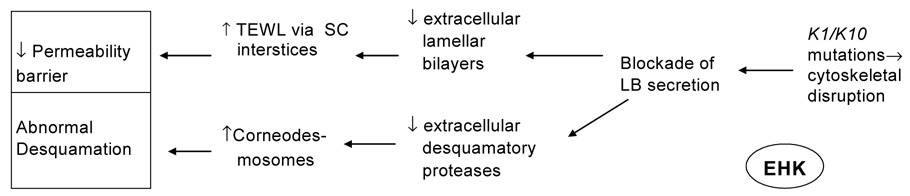
The histopathology of EHK is distinctive. Underlying a massive hyperkeratosis there is a characteristic degeneration of the upper epidermal cell layers, which stimulated coinage of the term “epidermolytic”. Large basophilic intracellular deposits are present in the upper epidermal layers that resemble enlarged keratohyalin granules, but which instead are composed of clumped keratin filaments 96. These changes are evident ultrastructurally in cases where the light microscopic features are mild and non-diagnostic. Whereas basal TEWL rates are elevated by approximately three-fold in EHK, recovery rates are faster than in age-matched control skin 35. There is no defect either in the cornified envelope, or in the adjacent cornified lipid envelope; hence, a corneocyte scaffold abnormality does not explain the barrier abnormality. Although the histologic appearance of the upper nucleated cell layers suggests increased fragility as the cause of the barrier abnormality in EHK 97, this was not confirmed experimentally. Instead, the water-soluble tracer, colloidal lanthanum, penetrates through the extracellular compartment, with little evidence of tracer accumulation within corneocytes 35. Thus, despite the fragility of nucleated keratinocytes in EHK, tracer neither enters the corneocyte nor permeates through the abnormal, intracellular route. Increased intercellular permeability in EHK, instead, correlates with decreased quantities and defective organization of extracellular lamellar bilayers (Fig. 5), which in turn can be attributed to incomplete secretion of lamellar bodies from SG cells, resulting in many “entombed” lamellar body remnants within corneocytes. Yet, this secretory defect can be temporarily overridden, since after acute barrier disruption, a rapid release of preformed lamellar body contents is observed in conjunction with increased organelle contents in the extracellular spaces, thereby accounting for the accelerated recovery kinetics in EHK (Table 3). Loss of calcium from the outer SG cells is also observed in EHK following acute barrier disruption, which may provide the signal for the rapid exocytosis of lamellar bodies that results in accelerated repair kinetics 35. Thus, the baseline permeability barrier abnormality in EHK can be attributed to impaired lamellar body secretion (resulting in deficiency of lamellar membranes and failure of lipid processing due to impeded delivery of lipid processing enzymes), rather than to corneocyte fragility or an abnormal cornified envelope scaffold, a defect that can be overcome in part by external applications of stimuli for barrier repair. The impaired lamellar body secretion at baseline could derive from disruption of the cytoskeletal framework, as has been shown in other secretory cells 98, suggesting further that the intermediate filament framework plays a role in lamellar body secretion. Although the impaired desquamation in EHK could also be related to a failure to deliver lamellar body-derived desquamatory proteases, this mechanism still needs to be investigated. Similarly, the increased infection rate may be due to failed delivery of lamellar body-derived antimicrobial peptides (Fig. 5).
Figure 5. LB secretory defect in EHK.
While the overall number of LB is normal in EHK, arrays of individual organelles remain restricted beneath the apical plasma membrane of outermost granular (SG) cells (A, B, arrowheads). C: Lack of LB secretion results in a paucity of extracellular deposits at the SG-SC interface (asterisks). RuO4 post-fixation. Magnification bars = 0.5 µm.
The dramatic “phenotypic shift” in the neonatal period from a mechanobullous phenotype, where blistering from trauma predominates, to a hyperkeratotic phenotype is probably driven by post- natal environmental changes. Immersed in the aqueous, intrauterine environment, the fetus has a limited need for a highly competent permeability barrier; hence, the predominance of the “pure” keratinopathy-induced mechanobullous phenotype in the newborn with EHK. At birth, the need for a competent barrier becomes paramount and barrier repair homeostatic responses are initiated (through loss of extracellular calcium and cytokine release) with induction of epidermal hyperplasia. This is associated with expression of the wound healing keratins, K6 and K16 99–101, and it also involves c-myc and 14-3-3 proteins 97. Expression of keratins 6 and 16 has been observed in both human EHK 83, 102, and in mouse models of EHK 69, 103–105. Down-regulation of keratins 1 and 10 under conditions of epidermal hyperplasia and/or substitution of the unaffected hyperproliferative keratin pairs in keratin filament formation, may result in amelioration of the blistering in the mature EHK phenotype 106.
Ichthyosis vulgaris
Clinical characteristics
Ichthyosis vulgaris represents the most common disorder of cornification. The phenotype is rarely evident before 3–6 months after birth, but usually manifests during childhood and becomes more severe with age. Its prevalence appears to vary with geographical region. The highest prevalence was estimated to be up to 1 in 250 in British school children with dry skin 107. In contrast, studies from Japan 108 and Mexico 109 estimated lower prevalences (i.e., 1: 1000 and 2000). Individuals with ichthyosis vulgaris commonly display rather mild generalized fine scaling with flexural sparing. The extensor surfaces of the extremities are usually most affected. Involvement of palms and soles (hyperlinearity) is common as is the association with keratosis pilaris and atopic dermatitis. It is difficult clinically to differentiate between mild ichthyosis vulgaris and the xerosis that accompanies atopic dermatitis, which is not surprising in view of the common mutations found in these disorders (see below). Varying clinical criteria for the diagnosis and/or geographical variation in the mutation frequency may account for the discrepant prevalence rates reported in the literature.
Molecular defect
Common genetic variants in the gene encoding for filaggrin (FLG), located in the epidermal differentiation complex (EDC) on chromosome 1q21 110, 111, have recently been proposed to cause ichthyosis vulgaris 112–117. This was long suspected, because Sybert et al. had demonstrated that profilaggrin and its proteolytic product, filaggrin, are reduced or absent in ichthyosis vulgaris, correlating with instability of the profilaggrin message 118 and a long-recognized paucity in keratohyalin within the stratum granulosum 119 120. The delay in identifying the genetic basis of the FLG deficiency in ichthyosis vulgaris was due to its exceptionally long and highly repetitive gene sequence. Interestingly, even in the absence of overt mutations, there is a range from 10–12 FLG repeats within the gene, variability which may account for more subtle phenotypic differences 121, and it could further explain the discrepant prevalence data (see above). In IV, there is a dose effect wherein heterozygous patients display a mild or no phenotype, while homozygous and compound heterozygous FLG genotypes exhibit the full ichthyosis vulgaris phenotype. The same FLG mutations have been linked to atopic dermatitis 113, 117, but these are predominantly found in the heterozygous configuration. Overall, the high prevalence of FLG mutations even in phenotypically normal subjects suggests there may be an evolutionary advantage conferred by FLG deficiency.
Profilaggrin is the initial product of translation of the FLG gene. It is found in the keratohyalin granules in the outer nucleated layers of the epidermis, which are responsible for the designation of this layer as the granular layer. Profilaggrin is a large, histidine-rich, highly cationic phosphoprotein, consisting of filaggrin repeats, connected by peptide segments enriched in hydrophobic amino acids 64, 122. Profilaggrin is both dephosphorylated and proteolytically processed during cornification. Immunolocalization studies suggest that processed FLG peptides also associate with the cornified envelope 123. Above the stratum compactum, FLG is deiminated and proteolyzed further into its constituent amino acids and other small molecules 124–126, whose hygroscopic properties underlie SC hydration 127. Their generation is catalyzed by an aspartate protease whose activity is upregulated when relative humidity levels decline <80%, as occurs normally in outer SC 128. In contrast to the cytoplasmic location of the C-terminal FLG monomers, the N-terminal portion of profilaggrin is found in the nucleus consistent with its nuclear localization sequence (S100-like EF-hand), which may indicate a calcium-dependent nuclear function 129.
Consequences of FLG mutations for morphology and function
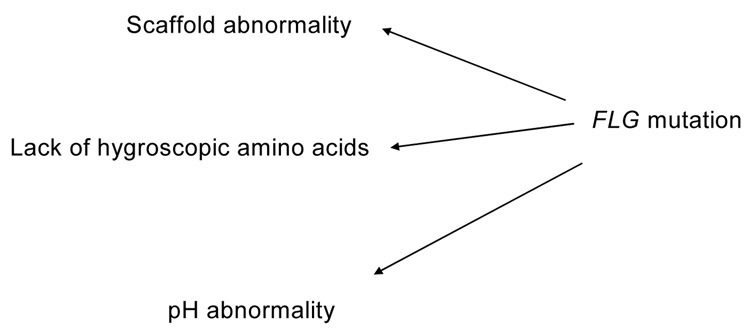
A histologic feature of ichthyosis vulgaris is a reduced or absent granular layer with a paucity of keratohyalin granules and reduced cellular profilaggrin content 120. Absence of the granular layer is independent of body site and season of the year, but correlates with severity of the disease 120, and mutational status. Patients with one mutation (heterozygous) display a reduced granular layer whereas patients with two FLG mutations (homozygous or compound heterozygous) in most instances show a virtually complete lack of a granular layer 112, 115, 116. However, in rare cases, even patients with two mutations still show residual granular cells 130. This appears to depend on the location of the mutation within the gene; i.e., more proximal mutations show complete absence of profilaggrin in homozygotes 112, whereas more distal mutations show residual, yet greatly reduced, truncated profilaggrin species that are not processed to FLG monomers 117. On electron microscopy, residual keratohyalin granules have been described as poorly formed (“crumbly”) 131 or absent 120. Although initially FLG monomers were believed to mediate the collapse of the keratin filament network during cornification, the fact that the keratin intermediate filaments are normal in ichthyosis vulgaris 119, 132 suggests that FLG is not required for keratin aggregation. Therefore, the pathogenesis of the scaling abnormality in ichthyosis vulgaris remains to be resolved. One possible explanation is that a reduction in proteolytic FLG breakdown products results in a lack of osmotically active small molecules that regulate corneocyte hydration 125, 133, 134. In support of this hypothesis, FLG hydrolysis has been shown to be regulated by environmental conditions with increased hydrolysis under conditions of xeric stress 127. In ichthyosis vulgaris, corneocytes would have reduced capacity to imbibe water when exposed to high humidity or during bathing. This could impair desquamation, since outer corneocytes would not “swell and slough” with the friction that accompanies bathing. As FLG has been proposed to participate in the linkage of keratins to the cornified envelope during cornification 123, ichthyosis vulgaris could also be another example of a scaffold abnormality 135. Alternatively, it is also possible that other properties of the FLG degradation products play a pathogenetic role in ichthyosis vulgaris, e.g. FLG degradation contributes to stratum corneum pH, which is an important determinant of permeability barrier function (Fig. 6, Table 3).
Figure 6. Possible mechanisms for barrier abnormality in ichthyosis vulgaris.
Netherton syndrome
Clinical characteristics
Patients with Netherton syndrome (syn. ichthyosis linearis circumflexa, Comèl-Netherton syndrome) display generalized scaling with marked inflammatory features, often with similarities to atopic dermatitis. At or shortly after birth, infants commonly present with generalized erythroderma. In older children and adults, a unique scaling pattern, termed ichthyosis linearis circumflexa, may develop in which serpiginous areas of double-edged scale are present. Patients with Netherton syndrome are at risk for excessive systemic absorption of topical medications as a result of a severe barrier abnormality. The skin changes are typically accompanied by pathognomonic hair shaft defects, called bamboo hair or trichorrhexis invaginata, in which the distal hair segment is telescoped into the proximal part. Only 20–50% of hair may be affected, and this feature may be absent in some patients. Atopy with anaphylactic reactions to food is another feature of the disease.
Molecular defect
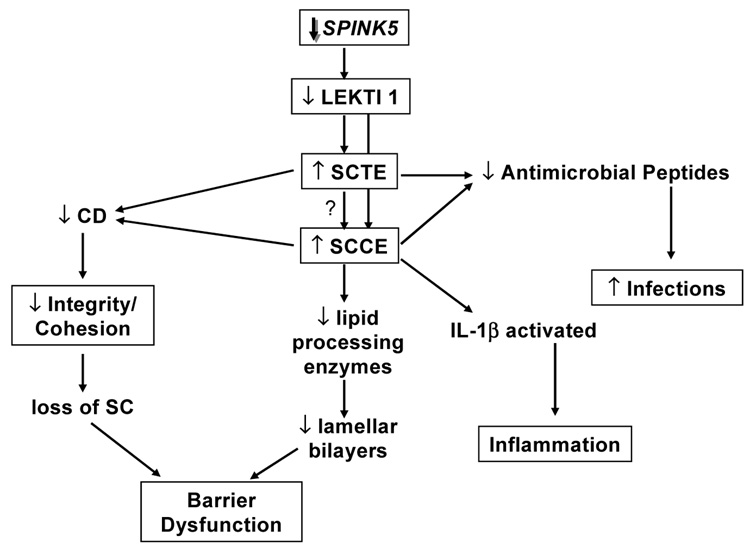
Netherton syndrome is caused by autosomal recessive mutations in the SPINK5 gene, coding for the serine protease inhibitor lymphoepithelial-Kazal-type 5 inhibitor (LEKTI), and presumably resulting in unopposed serine protease activity. A large number of different mutations have been documented in the literature, many of them abolishing enzyme activity, a few mutations merely compromising enzyme function 136–139. LEKTI is expressed in skin, mucous membranes, tonsils and thymus 140, 141. It is cleaved into 15 active domains that suppress proteases. However, the specific biological targets of LEKTI in human tissues are unknown. Among possible targets are the stratum corneum trypsin- and chymotrypsin-like enzymes (SCTE and SCCE, respectively) 18, 142–145, whose defective inhibition by LEKTI could result in both premature corneodesmosome degradation with premature desquamation as well as in the inactivation of other proteins, such as lipid hydrolases required for processing of lamellar body lipids. Other putative targets are trypsin-like serine proteases, including the membrane-type serine protease 1 (MT-SP1). Thus, LEKTI is believed to play a role in corneocyte desquamation, as also suggested by its restricted expression in the granular layer of the epidermis, and the severe cornification abnormality in Netherton syndrome. LEKTI may also be important in the down regulation of inflammatory processes, since the phenotypic finding of atopic manifestations in Netherton syndrome is not seen in other congenital ichthyoses, and polymorphisms in the SPINK5 gene (missense variants) have been associated with atopic disease 146.
Consequences of SPINK5 mutations for morphology and function
Histological analysis shows acanthosis and a thin, variably parakeratotic SC in Netherton syndrome. The histological picture can be very similar to psoriasis, particularly in infants. Perivascular inflammation may be present, and neutrophils have been described to invade the epidermis. On electron microscopy, multilocular vesicles filled with an amorphous substance have been described at the level of the stratum spinosum 147–150, which correlate with premature secretion of lamellar body contents 38. The lamellar body-derived extracellular lamellae are disturbed and interspersed with foci of electron dense material 38. Overall, SC thickness is reduced 151; hence in Netherton syndrome both the quantity and quality of SC membranes are abnormal (Fig. 5) 20.
We showed recently that the severity of the cutaneous phenotype in Netherton syndrome (including the barrier abnormality) correlates inversely with both the extent of LEKTI protein reduction and with the increase in serine protease activity 36. Unrestricted serine protease activation results in loss of SC due to accelerated desmosomal degradation. In contrast to a loss- of- function mutation of SPINK5 in transgenic knock out mice, which results in early post-partum death 152, most humans with Netherton survive, probably due to compensatory, suprabasal upregulation of desmosomal proteins and accelerated lamellar body secretion 36, 38. Nevertheless, the secreted contents of lamellar bodies are not processed into functional lamellar bilayers in Netherton syndrome due to proteolytic degradation of lipid-processing enzymes (Fig. 7, Table 3).
Figure 7. Pathogenesis of Netherton syndrome.
Growth failure is a common complication for infants with Netherton syndrome, and it is attributed predominantly to a severe barrier defect with loss of calories through heat of evaporation 20, 21. Infants with Netherton syndrome are at risk for systemic infections, with significant morbidity and mortality, which maybe a result of proteolytic attack on SC antimicrobial peptides. The antimicrobial peptides, cathelicidin and human β-defensin, SC serine proteases, and their inhibitor, LEKTI, all co-localize to lamellar bodies 153–155. Indeed, a disturbed regulation of antimicrobial peptide (cathelicidin) proteolysis has been linked to LEKTI deficiency in the mouse model 156. There is also an increased susceptibility to cutaneous HPV infections in Netherton syndrome, and cases of HPV-associated, non-melanoma skin cancer have been reported 24, 157–160. Yet, it is not known if these predispositions are related to disturbances in SC innate immunity or to the T-cell abnormalities associated with the atopic state.
Conclusion: Pathways to Abnormal Cornification and Towards a Function-Driven Model of Disease
While it has been useful to categorize the ichthyoses into disorders primarily affecting corneocyte proteins (“the bricks”) vs. those due to lipid metabolic defects (“the mortar”), the complex structure of tissue and functional interdependencies of SC constituents render this simplistic scheme unsatisfactory. The intercellular matrix contains important structural proteins, the corneodesmosome components, as well as enzymes that modulate SC functions, and antimicrobial peptides. Moreover, it is apparent from studies of defects of corneocyte proteins that competent SC extra-cellular membranes and lamellar body secretory system require a structurally preserved corneocyte. Thus, all of the ichthyoses due to corneocyte abnormalities studied to date provoke a defect in the extracellular lamellar membranes and generate a permeability barrier abnormality with accelerated transcutaneous water movement via the SC interstices. Abnormal permeability barrier function drives compensatory manifestations, including epidermal hyperplasia and hyperkeratosis. As the SC serves as a biosensor, transmitting danger signals to the underlying nucleated, epidermal cell layers, any acute or sustained insult that results in barrier impairment stimulates homeostatic repair responses, including both increased synthesis and secretion of lamellar body lipids and a mitogenic stimulus to the epidermis. Hence, the hyperkeratosis in most ichthyoses is largely the result of a hyperproliferative response to the permeability barrier abnormality in these disorders in an attempt to normalize barrier function. Ultimately, the hyperplastic response provides both more corneocytes as ‘bricks’, and more keratinocytes that synthesize lipids (‘mortar’) for the barrier. The second major functional disturbance is abnormal desquamation. The gradual weakening of intercellular connections, through regulated proteolysis of corneodesmosomes and, perhaps, assistance of mechanical debridement by corneocyte hydration leading to normal, invisible, single cell desquamation is altered in the ichthyoses. Development of a function-driven model of pathogenesis, while still a work in progress, will provide a rational classification of this diverse group of genetic disorders that share the similar clinical phenotype of ichthyosis. It can be integrated with prior histometric and morphological (“the bricks and mortar”) models of disease and provides insights into mechanisms of disease and potential complications. Most importantly, it can guide the development of rationale therapeutic approaches 161, 162
Acknowledgments
Editorial Comment:
Over the past 15 years, there have been significant advances in our understanding of the disorders of cornification. Currently, there are more than 25 identified genes involved in the pathogenesis of the ichthyoses. This diverse group of disorders presents many diagnostic as well as therapeutic challenges to the clinician. Unfortunately, advances in treatment lag far behind the exponential growth in the genetic understanding of these diseases.
In this update, Drs. Schmuth, Gruber, Elias and Williams masterfully review the role of corneocyte proteins in the disorders of cornification using a new, function-driven model of pathogenesis. They explain how the effect of corneocyte mutations on barrier function have enhanced our understanding of the critical interactions between corneocytes and the intercellular lamellae in a normal permeability barrier.
Furthermore, their function-driven model provides a framework for understanding how such diverse genetic alterations can result in similar phenotypic features among the numerous disorders of cornification. Despite the many pathogenetic origins of impaired permeability barrier, patients with these conditions share compensatory responses of epidermal hyperplasia and hyperkeratosis.
The authors have contributed greatly to our understanding of the ichthyoses over the years. They are sure to enlighten you with this outstanding update.
Amy Jo Nopper, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Traupe H. A Guide to Clinical Diagnosis, Genetic Counseling, and Therapy. ed 1. New York: Springer Verlag; 1989. p. 253. [Google Scholar]
- 2.Vahlquist A, Ganemo A, Pigg M, Virtanen M, Westermark P. The clinical spectrum of congenital ichthyosis in Sweden: a review of 127 cases. Acta Derm Venereol Suppl (Stockh) 2003:34–47. [PubMed] [Google Scholar]
- 3.Williams ML, Elias PM. From basket weave to barrier. Unifying concepts for the pathogenesis of the disorders of cornification. Arch Dermatol. 1993;129:626–629. doi: 10.1001/archderm.129.5.626. [DOI] [PubMed] [Google Scholar]
- 4.Williams ML, Bruckner A, Nopper A. Textbook of Pediatric Dermatology, in Oxford, UK. In: Harper J, Orange A, Prose N, editors. Generalized Disorders of Cornification (the Ichthyoses) Oxford, UK: Blackwell Science; 2006. pp. 1304–1358. [Google Scholar]
- 5.Elias P, Williams ML, Demerjian M, Feingold K, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyosis: inherited disorders of lipid metabolism. J Lipid Res. 2007 doi: 10.1194/jlr.R800002-JLR200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost P, Weinstein GD, Van Scott EJ. The ichthyosiform dermatoses. II. Autoradiographic studies of epidermal proliferation. J Invest Dermatol. 1966;47:561–567. doi: 10.1038/jid.1966.185. [DOI] [PubMed] [Google Scholar]
- 7.Williams ML, Elias PM. Genetically transmitted, generalized disorders of cornification. The ichthyoses. Dermatol Clin. 1987;5:155–178. [PubMed] [Google Scholar]
- 8.Williams ML, Elias PM. Ichthyosis. Genetic heterogeneity, genodermatoses and genetic counseling. Arch Dermatol. 1986;122:529–531. doi: 10.1001/archderm.122.5.529. [DOI] [PubMed] [Google Scholar]
- 9.Elias PM, Feingold KR, editors. Stratum corneum barrier function: definitions and broad concepts. New York: Taylor & Francis; 2006. Skin Barrier, in New York; pp. 1–4. [Google Scholar]
- 10.Elias PM, Friend DS. The permeability barrier in mammalian epidermis. J Cell Biol. 1975;65:180–191. doi: 10.1083/jcb.65.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias PM, Goerke J, Friend DS. Mammalian epidermal barrier layer lipids composition and influence on structure. J Invest Dermatol. 1977;69:535–546. doi: 10.1111/1523-1747.ep12687968. [DOI] [PubMed] [Google Scholar]
- 12.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 13.Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, Menon GK. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Invest Dermatol Symp Proc. 1998;3:87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- 14.Menon GK, Elias PM. Morphologic basis for a pore-pathway in mammalian stratum corneum. Skin Pharmacol. 1997;10:235–246. doi: 10.1159/000211511. [DOI] [PubMed] [Google Scholar]
- 15.Simon M, Montezin M, Guerrin M, Durieux JJ, Serre G. Characterization and purification of human corneodesmosin, an epidermal basic glycoprotein associated with corneocyte-specific modified desmosomes. J Biol Chem. 1997;272:31770–31776. doi: 10.1074/jbc.272.50.31770. [DOI] [PubMed] [Google Scholar]
- 16.Simon M, Jonca N, Guerrin M, Haftek M, Bernard D, Caubet C, Egelrud T, Schmidt R, Serre G. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001;276:20292–20299. doi: 10.1074/jbc.M100201200. [DOI] [PubMed] [Google Scholar]
- 17.Haftek M, Simon M, Kanitakis J, Marechal S, Claudy A, Serre G, Schmitt D. Expression of corneodesmosin in the granular layer and stratum corneum of normal and diseased epidermis. Br J Dermatol. 1997;137:864–873. [PubMed] [Google Scholar]
- 18.Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, Egelrud T, Simon M, Serre G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 19.Egelrud T. Desquamation in the stratum corneum. Acta Derm Venereol Suppl (Stockh) 2000;208:44–50. doi: 10.1080/000155500750012513. [DOI] [PubMed] [Google Scholar]
- 20.Moskowitz DG, Fowler AJ, Heyman MB, Cohen SP, Crumrine D, Elias PM, Williams ML. Pathophysiologic basis for growth failure in children with ichthyosis an evaluation of cutaneous ultrastructure epidermal permeability barrier function and energy expenditure. J Pediatr. 2004;145:82–92. doi: 10.1016/j.jpeds.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Fowler AJ, Moskowitz DG, Wong A, Cohen SP, Williams ML, Heyman MB. Nutritional status and gastrointestinal structure and function in children with ichthyosis and growth failure. J Pediatr Gastroenterol Nutr. 2004;38:164–169. doi: 10.1097/00005176-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Sedlacek V, Krenar J. [Symptomatology of Comel's linear circumflex ichthyosis (a case associated with genito-anal papillomatosis)] Hautarzt. 1971;22:390–397. [PubMed] [Google Scholar]
- 23.Folster-Holst R, Swensson O, Stockfleth E, Monig H, Mrowietz U, Christophers E. Comel-Netherton syndrome complicated by papillomatous skin lesions containing human papillomaviruses 51 and 52 and plane warts containing human papillomavirus 16. Br J Dermatol. 1999;140:1139–1143. doi: 10.1046/j.1365-2133.1999.02892.x. [DOI] [PubMed] [Google Scholar]
- 24.Weber F, Fuchs PG, Pfister HJ, Hintner H, Fritsch P, Hoepfl R. Human papillomavirus infection in Netherton's syndrome. Br J Dermatol. 2001;144:1044–1049. doi: 10.1046/j.1365-2133.2001.04196.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams ML, Elias PM. Stratum corneum lipids in disorders of cornification: increased cholesterol sulfate content of stratum corneum in recessive x-linked ichthyosis. J Clin Invest. 1981;68:1404–1410. doi: 10.1172/JCI110391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein EH, Jr, Williams ML, Elias PM. Steroid sulfatase, X-linked ichthyosis and stratum corneum cell cohesion. Arch Dermatol. 1981;117:761–763. [PubMed] [Google Scholar]
- 27.Epstein EH, Williams ML, Elias PM. The epidermal cholesterol sulfate cycle. J Am Acad Dermatol. 1984;10:866–868. doi: 10.1016/s0190-9622(84)80144-9. [DOI] [PubMed] [Google Scholar]
- 28.Bonifas JM, Morley BJ, Oakey RE, Kan YW, Epstein EH., Jr Cloning of a cDNA for steroid sulfatase: frequent occurrence of gene deletions in patients with recessive X chromosome-linked ichthyosis. Proc Natl Acad Sci U S A. 1987;84:9248–9251. doi: 10.1073/pnas.84.24.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias PM, Williams ML, Maloney ME, Bonifas JA, Brown BE, Grayson S, Epstein EHJ. Stratum corneum lipids in disorders of cornification. Steroid sulfatase and cholesterol sulfate in normal desquamation and the pathogenesis of recessive X-linked ichthyosis. Journal Of Clinical Investigation. 1984;74:1414–1421. doi: 10.1172/JCI111552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon GK, Brown BE, Elias PM. Avian epidermal differentiation: role of lipids in permeability barrier formation. Tissue Cell. 1986;18:71–82. doi: 10.1016/0040-8166(86)90008-x. [DOI] [PubMed] [Google Scholar]
- 31.Williams ML, Aszterbaum M, Menon GK, Moser AH, Feingold KR, Hoath SB. Preservation of permeability barrier ontogenesis in the intrauterine growth-retarded fetal. Pediatr Res. 1993;33:418–424. doi: 10.1203/00006450-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, Feingold KR, Holleran WM. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghadially R, Williams ML, Hou SY, Elias PM. Membrane structural abnormalities in the stratum corneum of the autosomal recessive ichthyoses. J Invest Dermatol. 1992;99:755–763. doi: 10.1111/1523-1747.ep12614489. [DOI] [PubMed] [Google Scholar]
- 34.Elias P, Man MQ, Williams ML, Feingold KR, Magin T. Barrier function in K-10 heterozygote knockout mice. J Invest Dermatol. 2000;114:396–397. doi: 10.1046/j.1523-1747.2000.00889-2.x. [DOI] [PubMed] [Google Scholar]
- 35.Schmuth M, Yosipovitch G, Williams ML, Weber F, Hintner H, Ortiz-Urda S, Rappersberger K, Crumrine D, Feingold KR, Elias PM. Pathogenesis of the permeability barrier abnormality in epidermolytic hyperkeratosis. J Invest Dermatol. 2001;117:837–847. doi: 10.1046/j.0022-202x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- 36.Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, Houben E, Mauro TM, Leonardsson G, Brattsand M, Egelrud T, Roseeuw D, Clayman GL, Feingold KR, Williams ML, Elias PM. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609–1621. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- 37.Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, Moskowitz DG, Christiano AM, Feingold KR, Elias PM. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis) J Invest Dermatol. 2004;122:909–922. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- 38.Fartasch M, Williams ML, Elias PM. Altered lamellar body secretion and stratum corneum membrane structure in Netherton syndrome: differentiation from other infantile erythrodermas and pathogenic implications. Arch Dermatol. 1999;135:823–832. doi: 10.1001/archderm.135.7.823. [DOI] [PubMed] [Google Scholar]
- 39.Lavrijsen AP, Oestmann E, Hermans J, Boddé HE, Vermeer BJ, Ponec M. Barrier function parameters in various keratinization disorders: transepidermal water loss and vascular response to hexyl nicotinate. British Journal Of Dermatology. 1993;129:547–553. doi: 10.1111/j.1365-2133.1993.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 40.Arita K, Jacyk WK, Wessagowit V, van Rensburg EJ, Chaplin T, Mein CA, Akiyama M, Shimizu H, Happle R, McGrath JA. The South African "bathing suit ichthyosis" is a form of lamellar ichthyosis caused by a homozygous missense mutation, p.R315L, in transglutaminase 1. J Invest Dermatol. 2007;127:490–493. doi: 10.1038/sj.jid.5700550. [DOI] [PubMed] [Google Scholar]
- 41.Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, Bale SJ. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet. 1995;9:279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- 42.Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, Bon A, Lautenschlager S, Schorderet DF, Hohl D. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- 43.Kuramoto N, Takizawa T, Takizawa T, Matsuki M, Morioka H, Robinson JM, Yamanishi K. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest. 2002;109:243–250. doi: 10.1172/JCI13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon S, Djian P, Green H. Inability of keratinocytes lacking their specific transglutaminase to form cross-linked envelopes: absence of envelopes as a simple diagnostic test for lamellar ichthyosis. Proc Natl Acad Sci U S A. 1998;95:687–690. doi: 10.1073/pnas.95.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kon A, Takeda H, Sasaki H, Yoneda K, Nomura K, Ahvazi B, Steinert PM, Hanada K, Hashimoto I. Novel transglutaminase 1 gene mutations (R348X/Y365D) in a Japanese family with lamellar ichthyosis. J Invest Dermatol. 2003;120:170–172. doi: 10.1046/j.1523-1747.2003.19522.x. [DOI] [PubMed] [Google Scholar]
- 46.Nemes Z, Marekov LN, Fesus L, Steinert PM. A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation. Proc Natl Acad Sci U S A. 1999;96:8402–8407. doi: 10.1073/pnas.96.15.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemes Z, Marekov LN, Steinert PM. Involucrin cross-linking by transglutaminase 1. Binding to membranes directs residue specificity. J Biol Chem. 1999;274:11013–11021. doi: 10.1074/jbc.274.16.11013. [DOI] [PubMed] [Google Scholar]
- 48.Choate KA, Kinsella TM, Williams ML, Nolan GP, Khavari PA. Transglutaminase 1 delivery to lamellar ichthyosis keratinocytes. Hum Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 49.Fischer J, Faure A, Bouadjar B, Blanchet-Bardon C, Karaduman A, Thomas I, Emre S, Cure S, Ozguc M, Weissenbach J, Prud'homme JF. Two new loci for autosomal recessive ichthyosis on chromosomes 3p21 and 19p12-q12 and evidence for further genetic heterogeneity. Am J Hum Genet. 2000;66:904–911. doi: 10.1086/302814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krebsova A, Kuster W, Lestringant GG, Schulze B, Hinz B, Frossard PM, Reis A, Hennies HC. Identification by homozygosity mapping of a novel locus for autosomal recessive congenital ichthyosis on chromosome 17p and evidence for further genetic heterogeneity. Am J Hum Genet. 2001;69:216–222. doi: 10.1086/321284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, Sawamura D, Shimizu H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice RH, Crumrine D, Uchida Y, Gruber R, Elias PM. Structural changes in epidermal scale and appendages as indicators of defective TGM1 activity. Arch Dermatol Res. 2005;297:127–133. doi: 10.1007/s00403-005-0591-7. [DOI] [PubMed] [Google Scholar]
- 53.Lavrijsen AP, Bouwstra JA, Gooris GS, Weerheim A, Bodde HE, Ponec M. Reduced skin barrier function parallels abnormal stratum corneum lipid organization in patients with lamellar ichthyosis. J Invest Dermatol. 1995;105:619–624. doi: 10.1111/1523-1747.ep12323752. [DOI] [PubMed] [Google Scholar]
- 54.Paige DG, Morse-Fisher N, Harper JI. Quantification of stratum corneum ceramides and lipid envelope ceramides in the hereditary ichthyoses. British Journal of Dermatology. 1994;131:23–27. doi: 10.1111/j.1365-2133.1994.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 55.Maestrini E, Korge BP, Ocana-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP, Munro CS. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 56.Camisa C, Rossana C. Variant of keratoderma hereditaria mutilans (Vohwinkel's syndrome). Treatment with orally administered isotretinoin. Arch Dermatol. 1984;120:1323–1328. [PubMed] [Google Scholar]
- 57.Camisa C, Hessel A, Rossana C, Parks A. Autosomal dominant keratoderma, ichthyosiform dermatosis and elevated serum beta-glucuronidase. Dermatologica. 1988;177:341–347. doi: 10.1159/000248604. [DOI] [PubMed] [Google Scholar]
- 58.Korge BP, Ishida-Yamamoto A, Punter C, Dopping-Hepenstal PJ, Iizuka H, Stephenson A, Eady RA, Munro CS. Loricrin mutation in Vohwinkel's keratoderma is unique to the variant with ichthyosis. J Invest Dermatol. 1997;109:604–610. doi: 10.1111/1523-1747.ep12337534. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong DK, McKenna KE, Hughes AE. A novel insertional mutation in loricrin in Vohwinkel's Keratoderma. J Invest Dermatol. 1998;111:702–704. doi: 10.1046/j.1523-1747.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi H, Ishida-Yamamoto A, Kishi A, Ohara K, Iizuka H. Loricrin gene mutation in a Japanese patient with Vohwinkel's syndrome. J Dermatol Sci. 1999;19:44–47. doi: 10.1016/s0923-1811(98)00049-8. [DOI] [PubMed] [Google Scholar]
- 61.O'Driscoll J, Muston GC, McGrath JA, Lam HM, Ashworth J, Christiano AM. A recurrent mutation in the loricrin gene underlies the ichthyotic variant of Vohwinkel syndrome. Clin Exp Dermatol. 2002;27:243–246. doi: 10.1046/j.1365-2230.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 62.Vohwinkel KH. Keratoderma hereditaria mutilans (Vohwinkel's syndrome) Arch Dermatol Syphil. 1929;158:354–364. [Google Scholar]
- 63.Mehrel T, Hohl D, Rothnagel JA, Longley MA, Bundman D, Cheng C, Lichti U, Bisher ME, Steven AC, Steinert PM, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 64.Steven AC, Bisher ME, Roop DR, Steinert PM. Biosynthetic pathways of filaggrin and loricrin--two major proteins expressed by terminally differentiated epidermal keratinocytes. J Struct Biol. 1990;104:150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 65.Hohl D, Mehrel T, Lichti U, Turner ML, Roop DR, Steinert PM. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–6636. [PubMed] [Google Scholar]
- 66.Suga Y, Duncan KO, Heald PW, Roop DR. A novel helix termination mutation in keratin 10 in annular epidermolytic ichthyosis, a variant of bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 1998;111:1220–1223. doi: 10.1046/j.1523-1747.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- 67.Maestrini E, Monaco AP, McGrath JA, Ishida-Yamamoto A, Camisa C, Hovnanian A, Weeks DE, Lathrop M, Uitto J, Christiano AM. A molecular defect in loricrin the major component of the cornified cell envelope underlies Vohwinkel's syndrome. Nature Genetics. 1996;13:70–77. doi: 10.1038/ng0596-70. [DOI] [PubMed] [Google Scholar]
- 68.DiGiovanna JJ, Bale SJ. Clinical heterogeneity in epidermolytic hyperkeratosis. Arch Dermatol. 1994;130:1026–1035. [PubMed] [Google Scholar]
- 69.Bickenbach JR, Longley MA, Bundman DS, Dominey AM, Bowden PE, Rothnagel JA, Roop DR. A transgenic mouse model that recapitulates the clinical features of both neonatal and adult forms of the skin disease epidermolytic hyperkeratosis. Differentiation. 1996;61:129–139. doi: 10.1046/j.1432-0436.1996.6120129.x. [DOI] [PubMed] [Google Scholar]
- 70.Rothnagel JA, Dominey AM, Dempsey LD, Longley MA, Greenhalgh DA, Gagne TA, Huber M, Frenk E, Hohl D, Roop DR. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992;257:1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- 71.Cheng J, Syder AJ, Yu QC, Letai A, Paller AS, Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992;70:811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- 72.Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, Steinert PM. A leucine----proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992;70:821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- 73.Chipev CC, Yang JM, DiGiovanna JJ, Steinert PM, Marekov L, Compton JG, Bale SJ. Preferential sites in keratin 10 that are mutated in epidermolytic hyperkeratosis. Am J Hum Genet. 1994;54:179–190. [PMC free article] [PubMed] [Google Scholar]
- 74.Syder AJ, Yu QC, Paller AS, Giudice G, Pearson R, Fuchs E. Genetic mutations in the K1 and K10 genes of patients with epidermolytic hyperkeratosis. Correlation between location and disease severity. J Clin Invest. 1994;93:1533–1542. doi: 10.1172/JCI117132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLean WH, Eady RA, Dopping-Hepenstal PJ, McMillan JR, Leigh IM, Navsaria HA, Higgins C, Harper JI, Paige DG, Morley SM, et al. Mutations in the rod 1A domain of keratins 1 and 10 in bullous congenital ichthyosiform erythroderma (BCIE) J Invest Dermatol. 1994;102:24–30. doi: 10.1111/1523-1747.ep12371726. [DOI] [PubMed] [Google Scholar]
- 76.Yang JM, Nam K, Park KB, Kim WS, Moon KC, Koh JK, Steinert PM, Lee ES. A novel H1 mutation in the keratin 1 chain in epidermolytic hyperkeratosis. J Invest Dermatol. 1996;107:439–441. doi: 10.1111/1523-1747.ep12365483. [DOI] [PubMed] [Google Scholar]
- 77.Arin MJ, Longley MA, Kuster W, Huber M, Hohl D, Rothnagel JA, Roop DR. An asparagine to threonine substitution in the 1A domain of keratin 1: a novel mutation that causes epidermolytic hyperkeratosis. Exp Dermatol. 1999;8:124–127. doi: 10.1111/j.1600-0625.1999.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 78.Paller AS, Syder AJ, Chan YM, Yu QC, Hutton E, Tadini G, Fuchs E. Genetic and clinical mosaicism in a type of epidermal nevus. N Engl J Med. 1994;331:1408–1415. doi: 10.1056/NEJM199411243312103. [DOI] [PubMed] [Google Scholar]
- 79.Joh GY, Traupe H, Metze D, Nashan D, Huber M, Hohl D, Longley MA, Rothnagel JA, Roop DR. A novel dinucleotide mutation in keratin 10 in the annular epidermolytic ichthyosis variant of bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 1997;108:357–361. doi: 10.1111/1523-1747.ep12286491. [DOI] [PubMed] [Google Scholar]
- 80.Kremer H, Lavrijsen AP, McLean WH, Lane EB, Melchers D, Ruiter DJ, Mariman EC, Steijlen PM. An atypical form of bullous congenital ichthyosiform erythroderma is caused by a mutation in the L12 linker region of keratin 1. J Invest Dermatol. 1998;111:1224–1226. doi: 10.1046/j.1523-1747.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 81.Michael EJ, Schneiderman P, Grossman ME, Christiano AM. Epidermolytic hyperkeratosis with polycyclic psoriasiform plaques resulting from a mutation in the keratin 1 gene. Exp. Dermatol. 1999;8:501–503. doi: 10.1111/j.1600-0625.1999.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 82.Sybert VP, Francis JS, Corden LD, Smith LT, Weaver M, Stephens K, McLean WH. Cyclic ichthyosis with epidermolytic hyperkeratosis: A phenotype conferred by mutations in the 2B domain of keratin K1. Am J Hum Genet. 1999;64:732–738. doi: 10.1086/302278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishida-Yamamoto A, McGrath JA, Judge MR, Leigh IM, Lane EB, Eady RA. Selective involvement of keratins K1 and K10 in the cytoskeletal abnormality of epidermolytic hyperkeratosis (bullous congenital ichthyosiform erythroderma) J Invest Dermatol. 1992;99:19–26. doi: 10.1111/1523-1747.ep12611391. [DOI] [PubMed] [Google Scholar]
- 84.Irvine AD, McLean WH. Human keratin diseases: the increasing spectrum of disease and subtlety of the phenotype-genotype correlation. Br J Dermatol. 1999;140:815–828. doi: 10.1046/j.1365-2133.1999.02810.x. [DOI] [PubMed] [Google Scholar]
- 85.Ishida-Yamamoto A, Iizuka H, Manabe M, O'Guin WM, Hohl D, Kartasova T, Kuroki T, Roop DR, Eady RA. Altered distribution of keratinization markers in epidermolytic hyperkeratosis. Arch Dermatol Res. 1995;287:705–711. doi: 10.1007/BF01105793. [DOI] [PubMed] [Google Scholar]
- 86.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 87.Miller RK, Khuon S, Goldman RD. Dynamics of keratin assembly: exogenous type I keratin rapidly associates with type II keratin in vivo. J Cell Biol. 1993;122:123–135. doi: 10.1083/jcb.122.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ming ME, Daryanani HA, Roberts LP, Baden HP, Kvedar JC. Binding of keratin intermediate filaments (K10) to the cornified envelope in mouse epidermis implications for barrier function. J Invest Dermatol. 1994;103:780–784. doi: 10.1111/1523-1747.ep12413024. [DOI] [PubMed] [Google Scholar]
- 89.Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell. 1999;10:4247–4261. doi: 10.1091/mbc.10.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 91.Steinert PM. The complexity and redundancy of epithelial barrier function. J Cell Biol. 2000;151:F5–F8. doi: 10.1083/jcb.151.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anton-Lamprecht I. Genetically induced abnormalities of epidermal differentiation and ultrastructure in ichthyoses and epidermolyses: pathogenesis, heterogeneity, fetal manifestation, and prenatal diagnosis. J Invest Dermatol. 1983;81:149s–156s. doi: 10.1111/1523-1747.ep12540961. [DOI] [PubMed] [Google Scholar]
- 93.Bonifas JM, Rothman AL, Epstein EH., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991;254:1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- 94.Coulombe PA, Hutton ME, Letai A, Hebert A, Paller AS, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 95.Lane EB, Rugg EL, Navsaria H, Leigh IM, Heagerty AH, Ishida-Yamamoto A, Eady RA. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356:244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- 96.Wilgram GF, Caulfield JB. An electron microscopic study of epidermolytic hyperkeratosis. With a special note on the keratinosome as the "fourth" structural factor in the formation of the horny layer. Arch Dermatol. 1966;94:127–143. doi: 10.1001/archderm.94.2.127. [DOI] [PubMed] [Google Scholar]
- 97.Reichelt J, Magin TM. Hyperproliferation induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci. 2002;115:2639–2650. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- 98.Aunis D, Bader MF. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- 99.McGowan K, Coulombe PA. The wound repair-associated keratins 6 16 and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem. 1998;31:173–204. [PubMed] [Google Scholar]
- 100.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 101.Mommers JM, van Rossum PE, van De Kerkhof PC. Changes in keratin 6 and keratin 10 (co-)expression in lesional and symptomless skin of spreading psoriasis. Dermatology. 2000;201:15–20. doi: 10.1159/000018422. [DOI] [PubMed] [Google Scholar]
- 102.Virtanen M, Gedde-Dahl T, Jr, Mork NJ, Leigh I, Bowden PE, Vahlquist A. Phenotypic/genotypic correlations in patients with epidermolytic hyperkeratosis and the effects of retinoid therapy on keratin expression. Acta Derm Venereol. 2001;81:163–170. doi: 10.1080/000155501750376221. [DOI] [PubMed] [Google Scholar]
- 103.Porter RM, Reichelt J, Lunny DP, Magin TM, Lane EB. The relationship between hyperproliferation and epidermal thickening in a mouse model for BCIE. J Inves Dermatol. 1998;110:951–957. doi: 10.1046/j.1523-1747.1998.00218.x. [DOI] [PubMed] [Google Scholar]
- 104.Reichelt J, Bauer C, Porter R, Lane E, Magin V. Out of balance consequences of a partial keratin 10 knockout. J Cell Sci. 1997;110(Pt 18):2175–2186. doi: 10.1242/jcs.110.18.2175. [DOI] [PubMed] [Google Scholar]
- 105.Fuchs E, Esteves RA, Coulombe PA. Transgenic mice expressing a mutant keratin 10 gene reveal the likely genetic basis for epidermolytic hyperkeratosis. Proc Natl Acad Sci U S A. 1992;89:6906–6910. doi: 10.1073/pnas.89.15.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reichelt J, Bussow H, Grund C, Magin TM. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol Biol Cell. 2001;12:1557–1568. doi: 10.1091/mbc.12.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wells RS. Ichthyosis. Br Med J. 1966;2:1504–1506. doi: 10.1136/bmj.2.5528.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okano M, Kitano Y, Yoshikawa K, Nakamura T, Matsuzawa Y, Yuasa T. X-linked ichthyosis and ichthyosis vulgaris: comparison of their clinical features based on biochemical analysis. Br J Dermatol. 1988;119:777–783. doi: 10.1111/j.1365-2133.1988.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 109.Cuevas-Covarrubias SA, Diaz-Zagoya JC, Rivera-Vega MR, Beirana A, Carrasco E, Orozco E, Kofman-Alfaro SH. Higher prevalence of X-linked ichthyosis vs. ichthyosis vulgaris in Mexico. Int J Dermatol. 1999;38:555–556. doi: 10.1046/j.1365-4362.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 110.Compton JG, DiGiovanna JJ, Johnston KA, Fleckman P, Bale SJ. Mapping of the associated phenotype of an absent granular layer in ichthyosis vulgaris to the epidermal differentiation complex on chromosome 1. Exp Dermatol. 2002;11:518–526. doi: 10.1034/j.1600-0625.2002.110604.x. [DOI] [PubMed] [Google Scholar]
- 111.Zhong W, Cui B, Zhang Y, Jiang H, Wei S, Bu L, Zhao G, Hu L, Kong X. Linkage analysis suggests a locus of ichthyosis vulgaris on 1q22. J Hum Genet. 2003;48:390–392. doi: 10.1007/s10038-003-0043-1. [DOI] [PubMed] [Google Scholar]
- 112.Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S, Arseculeratne G, Munro CS, Sergeant A, O'Regan G, Bale SJ, Compton JG, DiGiovanna JJ, Presland RB, Fleckman P, McLean WH. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 113.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 114.Sandilands A, O'Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, Cassidy AJ, Goudie DR, Smith FJ, McLean WH, Irvine AD. Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol. 2006;126:1770–1775. doi: 10.1038/sj.jid.5700459. [DOI] [PubMed] [Google Scholar]
- 115.Gruber R, Janecke A, Fauth C, Utermann G, Fritsch P, Schmuth M. Filaggrin mutations p.R501X and c.2282del4 in ichthyosis vulgaris. Eur J Hum Genet. 2006 Dec 13; doi: 10.1038/sj.ejhg.5201742. Epub: [DOI] [PubMed] [Google Scholar]
- 116.Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, Palmer CN, Smith FJ, McLean WH, Shimizu H. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007;119:434–440. doi: 10.1016/j.jaci.2006.12.646. [DOI] [PubMed] [Google Scholar]
- 117.Sandilands A, Terron-Kwiatkowski A, Hull PR, O'Regan GM, Clayton TH, Watson RM, Carrick T, Evans AT, Liao H, Zhao Y, Campbell LE, Schmuth M, Gruber R, Janecke AR, Elias PM, van Steensel MA, Nagtzaam I, van Geel M, Steijlen PM, Munro CS, Bradley DG, Palmer CN, Smith FJ, McLean WH, Irvine AD. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007 doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 118.Nirunsuksiri W, Zhang SH, Fleckman P. Reduced stability and bi-allelic, coequal expression of profilaggrin mRNA in keratinocytes cultured from subjects with ichthyosis vulgaris. J Invest Dermatol. 1998;110:854–861. doi: 10.1046/j.1523-1747.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 119.Sybert VP, Dale BA, Holbrook KA. Ichthyosis vulgaris: identification of a defect in synthesis of filaggrin correlated with an absence of keratohyaline granules. J Invest Dermatol. 1985;84:191–194. doi: 10.1111/1523-1747.ep12264813. [DOI] [PubMed] [Google Scholar]
- 120.Fleckman P, Brumbaugh S. Absence of the granular layer and keratohyalin define a morphologically distinct subset of individuals with ichthyosis vulgaris. Exp. Dermatol. 2002;11:327–336. doi: 10.1034/j.1600-0625.2002.110406.x. [DOI] [PubMed] [Google Scholar]
- 121.Gan SQ, McBride OW, Idler WW, Markova N, Steinert PM. Organization, structure, and polymorphisms of the human profilaggrin gene. Biochemistry. 1990;29:9432–9440. doi: 10.1021/bi00492a018. [DOI] [PubMed] [Google Scholar]
- 122.Kuechle MK, Thulin CD, Presland RB, Dale BA. Profilaggrin requires both linker and filaggrin peptide sequences to form granules: implications for profilaggrin processing in vivo. J Invest Dermatol. 1999;112:843–852. doi: 10.1046/j.1523-1747.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi M, Tezuka T, Katunuma N. Filaggrin linker segment peptide and cystatin alpha are parts of a complex of the cornified envelope of epidermis. Arch Biochem Biophys. 1996;329:123–126. doi: 10.1006/abbi.1996.0199. [DOI] [PubMed] [Google Scholar]
- 124.Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–1081. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 125.Scott IR, Harding CR, Barrett JG. Histidine-rich protein of the keratohyalin granules. Source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim Biophys Acta. 1982;719:110–117. doi: 10.1016/0304-4165(82)90314-2. [DOI] [PubMed] [Google Scholar]
- 126.Nachat R, Mechin MC, Takahara H, Chavanas S, Charveron M, Serre G, Simon M. Peptidylarginine deiminase isoforms 1–3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005;124:384–393. doi: 10.1111/j.0022-202X.2004.23568.x. [DOI] [PubMed] [Google Scholar]
- 127.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115:84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- 128.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731–741. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 129.Pearton DJ, Dale BA, Presland RB. Functional analysis of the profilaggrin N-terminal peptide: identification of domains that regulate nuclear and cytoplasmic distribution. J Invest Dermatol. 2002;119:661–669. doi: 10.1046/j.1523-1747.2002.01831.x. [DOI] [PubMed] [Google Scholar]
- 130.Gruber R, Terron-Kwiatkowski A, Sandilands A, Utermann G, Fritsch P, Janecke A, McLean WH, M S. Filaggrin mutations in ichthyosis vulgaris. J Invest Dermatol. 2006;126:s40. doi: 10.1038/sj.jid.5700459. [DOI] [PubMed] [Google Scholar]
- 131.Anton-Lamprecht I, Hofbauer M. Ultrastructural distinction of autosomal dominant ichthyosis vulgaris and X-linked recessive ichthyosis. Humangenetik. 1972;15:261–264. doi: 10.1007/BF00702363. [DOI] [PubMed] [Google Scholar]
- 132.Fleckman P, Holbrook KA, Dale BA, Sybert VP. Keratinocytes cultured from subjects with ichthyosis vulgaris are phenotypically abnormal. J Invest Dermatol. 1987;88:640–645. doi: 10.1111/1523-1747.ep12470251. [DOI] [PubMed] [Google Scholar]
- 133.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 134.Katagiri C, Sato J, Nomura J, Denda M. Changes in environmental humidity affect the water-holding property of the stratum corneum and its free amino acid content, and the expression of filaggrin in the epidermis of hairless mice. J Dermatol Sci. 2003;31:29–35. doi: 10.1016/s0923-1811(02)00137-8. [DOI] [PubMed] [Google Scholar]
- 135.Schmuth M, Crumrine D, Presland RB, Fleckman P, Fritsch PO, Elias PM. Basis for the epidermal functional abnormalities in granular layer-absent (AGL) vs. -present (PGL) ichthyosis vulgaris. J Invest Dermatol. 2005;124:A72. [Google Scholar]
- 136.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, Bonafe JL, Wilkinson J, Taieb A, Barrandon Y, Harper JI, de Prost Y, Hovnanian A. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 137.Sprecher E, Chavanas S, DiGiovanna JJ, Amin S, Nielsen K, Prendiville JS, Silverman R, Esterly NB, Spraker MK, Guelig E, de Luna ML, Williams ML, Buehler B, Siegfried EC, Van Maldergem L, Pfendner E, Bale SJ, Uitto J, Hovnanian A, Richard G. The spectrum of pathogenic mutations in SPINK5 in 19 families with Netherton syndrome: implications for mutation detection and first case of prenatal diagnosis. J Invest Dermatol. 2001;117:179–187. doi: 10.1046/j.1523-1747.2001.01389.x. [DOI] [PubMed] [Google Scholar]
- 138.Bitoun E, Chavanas S, Irvine AD, Lonie L, Bodemer C, Paradisi M, Hamel-Teillac D, Ansai S, Mitsuhashi Y, Taieb A, de Prost Y, Zambruno G, Harper JI, Hovnanian A. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352–361. doi: 10.1046/j.1523-1747.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 139.Raghunath M, Tontsidou L, Oji V, Aufenvenne K, Schurmeyer-Horst F, Jayakumar A, Stander H, Smolle J, Clayman GL, Traupe H. SPINK5 and Netherton syndrome novel mutations demonstration of missing LEKTI and differential expression of transglutaminases. J Invest Dermatol. 2004;123:474–483. doi: 10.1111/j.0022-202X.2004.23220.x. [DOI] [PubMed] [Google Scholar]
- 140.Walden M, Kreutzmann P, Drogemuller KJ, John H, Forssmann WG, Hans-Jurgen M. Biochemical features, molecular biology and clinical relevance of the human 15-domain serine proteinase inhibitor LEKTI. Biol Chem. 2002;383:1139–1141. doi: 10.1515/BC.2002.124. [DOI] [PubMed] [Google Scholar]
- 141.Magert HJ, Standker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, Fritz H, Forssmann WG. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 142.Egelrud T, Brattsand M, Kreutzmann P, Walden M, Vitzithum K, Marx UC, Forssmann WG, Magert HJ. hK5 and hK7, two serine proteinases abundant in human skin are inhibited by LEKTI domain 6. Br J Dermatol. 2005;153:1200–1203. doi: 10.1111/j.1365-2133.2005.06834.x. [DOI] [PubMed] [Google Scholar]
- 143.Borgono CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, Sotiropoulou G, Diamandis EP. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282:3640–3652. doi: 10.1074/jbc.M607567200. [DOI] [PubMed] [Google Scholar]
- 144.Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D'Alessio M, Ishida-Yamamoto A, Bodemer C, Zambruno G, Hovnanian A. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622–1632. doi: 10.1038/sj.jid.5700284. [DOI] [PubMed] [Google Scholar]
- 145.Schechter NM, Choi EJ, Wang ZM, Hanakawa Y, Stanley JR, Kang Y, Clayman GL, Jayakumar A. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI) Biol Chem. 2005;386:1173–1184. doi: 10.1515/BC.2005.134. [DOI] [PubMed] [Google Scholar]
- 146.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, Wong K, Abecasis GR, Jones EY, Harper JI, Hovnanian A, Cookson WO. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–178. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 147.Thorne EG, Zelickson AS, Mottaz JH, Katz HI, Deaton BH. Netherton's syndrome: an electronmicroscopic study. Arch Dermatol Res. 1975;253:177–183. doi: 10.1007/BF00582069. [DOI] [PubMed] [Google Scholar]
- 148.Mevorah B, Frenk E, Brooke EM. Ichthyosis linearis circumflexa comel. A clinico-statistical approach to its relationship with Netherton's syndrome. Dermatologica. 1974;149:201–209. [PubMed] [Google Scholar]
- 149.Zina AM, Bundino S. Ichthyosis linearis circumflexa Comel and Netherton's syndrome; an ultrastructural study. Dermatologica. 1979;158:404–412. doi: 10.1159/000250790. [DOI] [PubMed] [Google Scholar]
- 150.Frenk E, Mevorah B. Ichthyosis linearis circumflexa Comel with Trichorrhexis invaginata (Netherton's Syndrom) an ultrastructural study of the skin changes. Arch Dermatol Forsch. 1972;245:42–49. doi: 10.1007/BF00596151. [DOI] [PubMed] [Google Scholar]
- 151.Hausser I, Anton-Lamprecht I, Hartschuh W, Petzoldt D. Netherton's syndrome ultrastructure of the active lesion under retinoid therapy. Arch Dermatol Res. 1989;281:165–172. doi: 10.1007/BF00456387. [DOI] [PubMed] [Google Scholar]
- 152.Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, Elias P, Barrandon Y, Zambruno G, Sonnenberg A, Hovnanian A. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- 153.Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O'Brien TJ, Wakamatsu K, Ohtsubo S, Takahashi H, Hashimoto Y, Dopping-Hepenstal PJ, McGrath JA, Iizuka H, Richard G, Hovnanian A. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124:360–366. doi: 10.1111/j.0022-202X.2004.23583.x. [DOI] [PubMed] [Google Scholar]
- 154.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 155.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 156.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 157.Saghari S, Woolery-Lloyd H, Nouri K. Squamous cell carcinoma in a patient with Netherton's syndrome. Int J Dermatol. 2002;41:415–416. doi: 10.1046/j.1365-4362.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 158.Kubler HC, Kuhn W, Rummel HH, Kaufmann I, Kaufmann M. [Development of cancer (vulvar cancer) in the Netherton syndrome (ichthyosis, hair anomalies, atopic diathesis)] Geburtshilfe Frauenheilkd. 1987;47:742–744. doi: 10.1055/s-2008-1036037. [DOI] [PubMed] [Google Scholar]
- 159.Hintner H, Jaschke E, Fritsch P. [Netherton syndrome: weakened immunity, generalized verrucosis and carcinogenesis] Hautarzt. 1980;31:428–432. [PubMed] [Google Scholar]
- 160.Krasagakis K, Ioannidou DJ, Stephanidou M, Manios A, Panayiotides JG, Tosca AD. Early development of multiple epithelial neoplasms in Netherton syndrome. Dermatology. 2003;207:182–184. doi: 10.1159/000071791. [DOI] [PubMed] [Google Scholar]
- 161.Williams ML, Elias PM. Enlightened therapy of the disorders of cornification. Clin Dermatol. 2003;21:269–273. doi: 10.1016/s0738-081x(03)00059-2. [DOI] [PubMed] [Google Scholar]
- 162.Williams ML, Schmuth M, Crumrine D, Hachem JP, Bruckner A, Demerjian M, Elias P. Pathogenesis of the Ichthyoses: Update and Therapeutic Implications. The Journal of Skin Barrier Research. 2005;7:122–133. [Google Scholar]