SUMMARY
Operant conditioning is a ubiquitous but mechanistically poorly understood form of associative learning in which an animal learns the consequences of its behavior. Using a single-cell analogue of operant conditioning in neuron B51 of Aplysia, we examined second-messenger pathways engaged by activity and reward and how they may provide a biochemical association underlying operant learning. Conditioning was blocked by Rp-cAMP, a peptide inhibitor of PKA, a PKC inhibitor and by expressing a dominant negative isoform of Ca2+-dependent PKC (apl-I). Thus, both PKA and PKC were necessary for operant conditioning. Injection of cAMP into B51 mimicked the effects of operant conditioning. Activation of PKC also mimicked conditioning, but was dependent on both cAMP and PKA, suggesting that PKC acted at some point upstream of PKA activation. Our results demonstrate how these molecules can interact to mediate operant conditioning in an individual neuron important for the expression of the conditioned behavior.
INTRODUCTION
Operant conditioning (Thorndike, 1911; Skinner, 1938) and classical conditioning (Pavlov, 1927) are two major forms of associative learning, and they are exhibited by all animals including humans. Operant conditioning involves an association between a behavior and its consequence. Classical conditioning involves an association between a typically neutral stimulus and a subsequent more salient stimulus. The cellular and molecular mechanisms of classical conditioning have been explored in great depth (Lechner and Byrne, 1998; Schafe et al., 2001; Christian and Thompson, 2003). In contrast, although operant conditioning has been studied extensively in many different animal model systems (Hoyle, 1979; Cook and Carew, 1989; Botzer et al., 1998; Reynolds et al., 2001; Kelley, 2004), little is known about the molecular mechanisms that underlie this form of learning even in the invertebrate model systems, which have proven so useful for molecular analyses of sensitization and classical conditioning (e.g., Kandel, 2001). Some progress has been made in analyzing mechanisms of appetitive operant conditioning in rodents. For example, appetitive instrumental learning is impaired by blocking D1-type dopamine receptors (Smith-Roe and Kelley, 2000) or inhibition of cAMP-dependent protein kinase within the nucleus accumbens (Baldwin et al., 2002). However, the complete signaling pathways have not been elucidated nor is it known how these signaling cascades mediate the associative convergence between the behavior and reward signals. A better understanding of the molecular mechanisms of appetitive operant conditioning is essential for a deeper understanding of reward-related learning and may provide insight into related disorders, such as addiction. Furthermore, a mechanistic understanding of operant conditioning allows for a comparison between the molecular mechanisms of these two major forms of associative learning.
Feeding behavior of the marine mollusk Aplysia can be operantly conditioned with an appetitive protocol. The neural circuitry that underlies feeding is well characterized and amenable to biophysical and biochemical analyses. Appetitive operant conditioning of ingestive feeding behavior leads to an increase in the expression of this behavior and that increase is associated with changes in the biophysical properties of neuron B51 (Brembs et al., 2002). B51 is a “decision making” neuron that displays an all-or-nothing several second level of activity, which is denoted as a plateau potential (Plummer and Kirk, 1990). The threshold for eliciting the plateau potential is decreased and the input resistance is increased following in vivo operant training (Brembs et al., 2002). B51 is critical for the expression of ingestive behavior (Nargeot el al., 1999a; Nargeot et al., 1999b) and both changes to the membrane properties of B51 make the cell more likely to generate a plateau potential, which can contribute to the conditioned increase in the expression of ingestive behavior. Furthermore, the ganglia that contain the circuitry necessary for generating the feeding response can be removed from naïve animals and be successfully trained with an in vitro analogue of operant conditioning in which fictive ingestion is contingently reinforced with an esophageal nerve shock (Nargeot et al., 1997). The esophageal nerve likely mediates the food reward signal (Lechner et al., 2000; Brembs et al., 2002) and is rich with dopamine-containing fibers (Kabotyanski et al., 1998). This in vitro training protocol produces an increase in fictive ingestion and, as was also the case with the in vivo conditioning, an increase in the input resistance of B51 and a decrease in its burst threshold (Nargeot et al., 1999a). Burst activity in B51 is pivotal for the expression of the reinforced behavior, so it can be used as a substitute for the behavior in the training protocol. Indeed, if plateau potentials generated in B51 are contingently reinforced with esophageal nerve shock, then the input resistance and the burst threshold of B51 are altered in the same way as with the previous training protocols and fictive feeding is increased (Nargeot et al., 1999b). Also, in vitro operant conditioning can be blocked by using a dopamine receptor antagonist (Nargeot et al., 1999c), indicating that the reward signal is likely mediated by dopamine.
Because the behavior can be represented by activity in B51 and the reinforcement can be represented with dopamine, the in vitro analogue can be reduced to the level of a single cell. B51 can be isolated and placed in cell culture. Plateau potentials can be used as the analogue of behavior and dopamine can be iontophoretically applied to B51 as the analogue of reinforcement (Fig. 1A). This single-cell analogue of operant conditioning produces the same increase in the input resistance of B51 and the same decrease in the burst threshold, as observed with the previous operant training methods (Brembs et al., 2002). Moreover, applying dopamine in a non-contingent manner does not alter the membrane properties of B51 (Brembs et al., 2002). From the behaving animal, to the neural network, to the level of the single cell, the same changes in the membrane properties of B51 are consistently observed following each operant protocol, justifying the reductionist approach. In the present report, we exploited the advantages of the single-cell analogue to investigate the molecular mechanisms underlying operant conditioning.
Fig. 1.
The role of dopamine in the single-cell analogue of operant conditioning. (A) Light micrograph of B51 after 5 days in primary cell culture. Using conventional current-clamp techniques, it was possible to record and stimulate plateau potentials in the cell while iontophoresing dopamine onto the cell. (B) Single-cell PCR was conducted using individual B51 neurons. PCR primers were designed to detect the Aplysia D1-dopamine receptor. This receptor was expressed in B51, as the band of DNA detected was the expected size based on the primer design. Also, a negative control with RNase treatment of the cellular contents prior to the reverse transcriptase reaction showed only a faint band. (C) Distribution of D1-like dopamine receptors in B51. Confocal imaging of a cultured B51 neuron incubated with an Aplysia D1-like dopamine receptor antibody showed that the receptor localized to the membrane of B51 near the axon hillock (arrow head; n = 4). (D) Both dopamine and the D1 dopamine receptor agonist chloro-APB produced a transient depolarization in the membrane potential of B51. Dopamine or chloro-APB were applied extracellularly to the axon hillock region of a cultured B51 neuron. The dopamine or chloro-APB was delivered with iontophoresis in the same way as with the single-cell analogue of operant conditioning (200 mM dopamine or 100 mM chloro-APB in iontophoretic electrode; 35 nA amplitude; 6 s duration). (E–F) Contingent Reinforcement with a D1 agonist increased the excitability of B51. (E1–E2) Burst threshold. Representative intracellular recordings from B51 illustrating the measurement of the burst threshold before and after contingent reinforcement with the D1 agonist chloro-APB (E1) and the non-contingent control (E2). (E3) Summary data illustrating that the decrease in B51 burst threshold was significantly greater in neurons contingently reinforced with the D1 agonist (n = 4) as compared to the non-contingent control (n = 4; Mann-Whitney, p < 0.05). (F1–F2) Input resistance. Representative intracellular recordings from B51 illustrating the measurement of the input resistance before and after contingent reinforcement with the D1 agonist chloro-APB (F1) and the non-contingent control (F2). (F3) Summary data illustrating that the increase in B51 input resistance was significantly greater in neurons contingently reinforced with the D1 agonist (n = 4) as compared to the non-contingent control (n = 4; Mann-Whitney, p < 0.05). The bar graphs display means ± SEM in this and subsequent figures.
RESULTS
Contingent reinforcement with a D1 dopamine receptor agonist mimics conditioning
Dopamine appears to mediate the reward signal in this example of operant reward learning similar to its role in many other model systems (Schultz, 2002). Single-cell PCR was used to examine whether the Aplysia D1-like dopamine receptor was expressed in B51. As illustrated by the band in the left lane of Fig. 1B, the mRNA for the D1-like receptor was detected in B51, which was confirmed by a negative control (right lane, Fig. 1B) with RNase treatment of the cellular contents prior to the reverse transcriptase reaction, which showed only a faint band remaining. Furthermore, a D1-dopamine receptor antibody revealed that the receptors were localized to the axon hillock region of B51 (Fig. 1C). This localization is consistent with an observation that during conditioning, the tip of the dopamine-containing iontophoretic electrode needed to be positioned near the axon hillock region of B51 to elicit a response in the membrane potential (Fig. 1D). A similar response in the membrane potential of B51 was observed if the D1 receptor agonist chloro-APB (Barbas et al., 2006) was iontophoresed onto B51 (Fig. 1D). To further test the hypothesis that a D1-like dopamine receptor mediates reinforcement, we replicated the original findings of the single-cell analogue of operant conditioning (Brembs et al., 2002) by iontophoresing chloro-APB onto B51 in place of dopamine. Two experimental groups were examined. In one group, 7 plateau potentials were each contingently reinforced with brief iontophoretic puffs of chloro-APB. The second group received the same amount of chloro-APB, but it was not contingent on the expression of a plateau potential. Contingently reinforcing activity with chloro-APB produced an increase in the input resistance and a decrease in the burst threshold of B51 as compared to the non-contingent control (Fig. 1E, 1F) and these results are similar to previous results obtained when dopamine is used as the reinforcement (Brembs et al., 2002; See also Fig. 2, 7, 8).
cAMP is necessary for the single-cell analogue of operant conditioning
The D1-like dopamine receptors in B51 are likely coupled to adenylyl cyclase and increase the production of cAMP when dopamine is bound (Barbas et al., 2006). Therefore, cAMP and PKA are likely to be important elements of the intracellular signaling cascade underlying reinforcement. To test the hypothesis that cAMP is necessary for the conditioning, Rp-8-Br-cAMPS (Rp-cAMP) was used to block the cAMP pathway during conditioning. Three experimental groups were examined. In one group, 7 plateau potentials were each contingently reinforced with dopamine delivery. A second group received the same contingent reinforcement protocol, but in the presence of 2 mM bath-applied Rp-cAMP. The third group received the same application of Rp-cAMP but in the absence of plateau potentials or reinforcement. Contingent reinforcement decreased the burst threshold (Fig. 2A1, 2A3) and increased the input resistance of B51 (Fig. 2B1, 2B3), similar to previous observations (Nargeot et al., 1999a; Nargeot et al., 1999b; Brembs et al., 2002). Rp-cAMP by itself did not appear to appreciably alter the membrane properties of B51. However, the effects of conditioning were blocked in the presence of Rp-cAMP (Fig. 2A2, 2A3, 2B2, 2B3). Thus, the cAMP cascade appeared to be necessary for the conditioning and cAMP appears to act via PKA because a peptide inhibitor for PKA also blocked the conditioning (see Fig. 7).
In order to monitor the activation of PKA by the single-cell analogue of operant conditioning, we used an antibody against Aplysia CREB1 peptide with the target residue for PKA, Ser85, phosphorylated (Mohamed et al. 2005). Immediately after training with the single-cell analogue, cells were processed to measure the immunoreactivity for phospho-CREB1. Cells that received contingent reinforcement had significantly higher levels of phospho-CREB1 as compared to cells that received non-contingent reinforcement (Fig. 2C). Because the PKA site on CREB1 could have been phosphorylated by a kinase other than PKA, we repeated the experiment in the presence of Rp-cAMP to validate the use of the phospho-CREB1 assay as an indicator of activation of the cAMP/PKA pathway. In the presence of Rp-cAMP the increase in the level of phospho-CREB1 normally seen following contingent reinforcement was blocked (Fig. 2C). These results indicate that the single-cell analogue induced a rapid phosphorylation of CREB1 at a PKA site, which suggests that PKA was activated by contingent reinforcement.
Fig. 2.
Both PKA and PKC were necessary for the single-cell analogue of operant conditioning. (A) Burst threshold. (A1–A2) Representative intracellular recordings from a cell that received contingent reinforcement (CR) alone and from a cell that received CR in the presence of bath-applied Rp-cAMP. Depolarizing current pulses were injected into B51 until the cell produced a plateau potential. (A3) Summary data for Rp-cAMP. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the burst threshold was significantly greater in the CR alone group (n = 4) compared to either the CR plus Rp-cAMP group (n = 4; Student-Newman-Keuls, p < 0.05) or the Rp-cAMP alone group (n = 4; Student-Newman-Keuls, p < 0.05), indicating that the decrease in the burst threshold produced by CR was blocked by the presence of Rp-cAMP. (A4) Summary data for Bis. The PKC inhibitor Bis was applied using the same protocol. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the burst threshold was significantly greater in the CR alone group (n = 4) compared to either the CR plus Bis group (n = 4; Student-Newman-Keuls, p < 0.05) or the Bis alone group (n = 4; Student-Newman-Keuls, p < 0.05), indicating that the decrease in the burst threshold produced by CR was blocked by the presence of Bis. (B) Input resistance. (B1–B2) Intracellular recordings from the same two cells displayed in (A). Hyperpolarizing current pulses were injected into B51 to measure the input resistance. (B3) Summary data for Rp-cAMP. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the input resistance was significantly greater in the CR alone group (n = 4) compared to either the CR plus Rp-cAMP group (n = 4; Student-Newman-Keuls, p < 0.05) or the Rp-cAMP alone group (n = 4; Student-Newman-Keuls, p < 0.05), indicating that the increase in the input resistance produced by CR was blocked by the presence of Rp-cAMP. (B4) Summary data for Bis. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the input resistance was significantly greater in the CR alone group (n = 4) compared to either the CR plus Bis group (n = 4; Student-Newman-Keuls, p < 0.05) or the Bis alone group (n = 4; Student-Newman-Keuls, p < 0.05), indicating that the increase in the input resistance produced by CR was blocked by the presence of Bis. (C) Elevated levels of phospho-CREB1 in B51 produced by the single-cell analogue of operant conditioning. (C1) Representative confocal images from B51 neurons immediately after training with contingent reinforcement, the non-contingent control, or contingent reinforcement in the presence of Rp-cAMP. Phospho-CREB1 was localized to the nucleus. (C2) Summary data for phospho-CREB1 levels. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that that phospho-CREB1 levels were higher immediately after training with contingent reinforcement (n = 4) as compared to either the non-contingent control (n = 4; Student-Newman-Keuls, p < 0.05) or contingent reinforcement in the presence of Rp-cAMP (n = 4; Student-Newman-Keuls, p < 0.05).
PKC is necessary for the single-cell analogue of operant conditioning
The next step was to determine the second messenger cascade elicited by the analogue of the behavior. One possibility is that Ca2+ entry during the plateau potential (Plummer and Kirk, 1990) activates PKC similar to how burst firing in an Aplysia sensory neuron activates PKC (Jones et al., 2001). In fact, the Ca2+-dependent PKC isoform apl-I can be translocated from the cytosol to the plasma membrane, where PKC becomes active, with Ca2+ influx alone (Zhao et al., 2006). This Ca2+-dependent isoform of PKC was detected in B51 (Fig. 3A). A fusion protein of PKC apl-I and EGFP was expressed in B51 and bath application of 1 μM of the Ca2+ ionophore ionomycin caused translocation of PKC apl-I from the cytosol of B51 to the plasma membrane (Fig. 3C). There are some regions of the cell depicted in Fig. 3C1 where the translocation appears to be internal of the plasma membrane. However, the majority of translocation appears to be at the plasma membrane, which is supported by the summary data in Fig. 3C2. Also, modest translocation of PKC from the cytosol to the plasma membrane was observed following a single plateau potential in B51 (Supplementary Fig. 1). However, iontophoresing dopamine with the same parameters as with the single-cell analogue did not appear to cause any translocation of PKC (data not shown). The Ca2+-independent isoform PKC apl-II was not detected in B51 (Fig. 3A), although our PCR primers were able to detect this isoform in sensory neurons of Aplysia (Fig. 3A). To test the hypothesis that PKC is involved in the conditioning, bisindolylmaleimide I (Bis) was used to inhibit PKC during conditioning. Three experimental groups were examined. One group received the contingent reinforcement protocol. Another group received the same contingent reinforcement protocol, but in the presence of 10 μM bath-applied Bis. The third group received the same application of Bis, but in the absence of plateau potentials or reinforcement. Contingent reinforcement again produced the typical decrease in burst threshold (Fig. 2A4) and increase in input resistance (Fig. 2B4). Although Bis by itself did not appear to appreciably alter the membrane properties of B51, the effects of conditioning were blocked in the presence of Bis (Fig. 2A4, 2B4). Thus, PKC appears to be necessary for the conditioning.
Fig. 3.
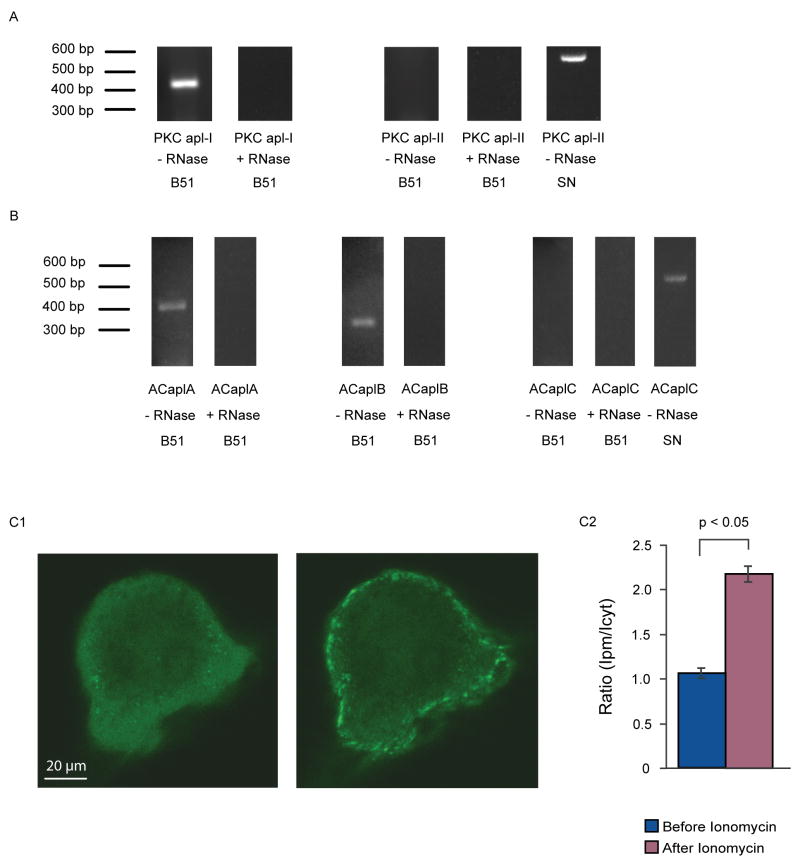
Single-cell PCR was used to determine which isoforms of PKC and adenylyl cyclase were detectable in B51. Single-cell PCR was conducted using individual B51 or sensory neurons. (A) PCR primers were designed to detect the apl-I and apl-II isoforms of PKC. The apl-I isoform was detected in B51, as the band of DNA shown in the first lane was the expected size based on the primer design. Also, a negative control with RNase treatment of the cellular contents prior to the reverse transcriptase reaction showed no bands. However, the apl-II isoform of PKC was not detected in B51, but we were able to detect it in sensory neurons. (B) PCR primers were designed to detect the three known Aplysia isoforms of adenylyl cyclase (ACaplA, ACaplB, and ACaplC). The A and the B isoforms were detected in B51, as the bands of DNA shown in the first and second lanes were the expected size based on the primer design. Also, a negative control with RNase treatment of the cellular contents prior to the reverse transcriptase reaction showed no bands. The C isoform was not detected in B51, but we were able to detect it in sensory neurons. (C) Ca2+ entry alone was sufficient for translocation to the B51 plasma membrane of the Ca2+-dependent apl-I isoform of PKC. (C1) Representative confocal images from B51 neurons expressing a fusion protein of PKC apl-I with EGFP both before (left panel) and 2 min after (right panel) the bath application of the Ca2+ ionophore ionomycin (1 μM). The Ca2+ entry induced the translocation of PKC apl-I to the plasma membrane, where it becomes activated. (C2) Summary data (n = 3) illustrating that the translocation of PKC was significantly greater 2 min after 1 μM ionomycin treatment as compared to immediately before the treatment (paired t-test, p < 0.05).
cAMP mimics the conditioning in a PKC-independent manner
Both PKC and PKA appear to be essential elements underlying operant conditioning. The two kinases could act either in series or in parallel. They could act in parallel if both kinases need to phosphorylate a common substrate at different sites, which would then lead to the modulation of B51 (Fig. 4A). Alternatively, the kinases could act in series if PKC phosphorylates some molecule upstream of the activation of PKA (e.g., adenylyl cyclase), causing an increased activation of PKA when the reward signal is given, which would be necessary to enact the changes in B51 (Fig. 4B). Another way for the kinases to act in series is if the PKC activation is involved somewhere downstream of PKA activation (Fig. 4C). To test these hypotheses, activators of PKA and PKC were used in concert with the inhibitors Rp-cAMP and Bis.
Fig. 4.
Putative models of the second-messenger cascade underlying operant conditioning. Three possible ways for PKA and PKC to interact are depicted. (A) The parallel pathway hypothesis. In this model, both PKA (activated by the reward pathway) and PKC (activated by the behavior) are required to jointly phosphorylate a common substrate (e.g., an ion channel protein) that would then cause the increase in the input resistance (Rin) and the decrease in the burst threshold (Tb). (B) The series pathway hypothesis with PKC acting upstream. If PKA and PKC are not acting in parallel, then they would be acting in series. In this model, a plateau potential in B51 produces an accumulation of Ca2+ in the cell, which can lead to the activation of PKC. The PKC then phosphorylates adenylyl cyclase and primes it for enhanced synthesis of cAMP. The reward signal is mediated by dopamine and likely acts through adenylyl cyclase to increase the production of cAMP. If the ingestive behavior had just preceded the delivery of the reward and adenylyl cyclase was phosphorylated by PKC, then there would be an even greater increase in the production of cAMP over that which would be seen after either behavior alone or dopamine alone. After a sufficient number of contingent reinforcements of behavior with the reward, the increased levels of cAMP would activate PKA sufficiently enough to produce the changes in the membrane properties. (C) The series pathway hypothesis with PKC acting downstream. In this model, the behavior and the reinforcement converge at adenylyl cyclase again with the Ca2+ influx from the plateau potential acting in place of the PKC to enhance the production of cAMP when the behavior is contingently reinforced. The increase in PKA would then initiate a cascade resulting in the downstream activation of PKC which would then lead to the changes in the membrane properties.
First, cAMP was injected into cultured B51 neurons. The cAMP injection elicited the same decrease in burst threshold (Fig. 5A1, 5A4) and increase in input resistance (Fig. 5B1, 5B4), as observed with conditioning previously (Nargeot et al., 1999a; Nargeot et al., 1999b; Brembs et al., 2002), whereas injecting a group of B51 neurons with vehicle did not appreciably alter the membrane properties (Fig. 5A2, 5A4, 5B2, 5B4). The cAMP injection alone was sufficient to mimic the conditioning, which likely rules out the parallel pathway hypothesis (Fig. 4A). A third group consisted of B51 neurons injected with cAMP while being bathed in 10 μM of the PKC inhibitor Bis. This PKC inhibitor, at the same concentration used to block the contingent reinforcement (Fig. 2A4, 2B4), did not block the changes in the membrane properties elicited by cAMP (Fig. 5A3, 5A4, 5B3, 5B4). Thus, the modulation of the membrane properties by cAMP appears to be independent of the activation of PKC, which likely excludes the pathway illustrated in Fig. 4C where PKC acts downstream of cAMP synthesis (Fig. 4B).
Fig. 5.
Activating PKA mimicked the conditioning in a PKC-independent manner. (A) Burst threshold. (A1-A3) Representative intracellular recordings from a cell that received an iontophoretic injection of cAMP, a cell that received vehicle injection, and from a cell that received cAMP injection in the presence of bath-applied Bis. Depolarizing current pulses were injected into B51 until the cell produced a plateau potential. (A4) Summary data. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the burst threshold was significantly greater in both the cAMP group (n = 4; Student-Newman-Keuls, p < 0.05) and the cAMP plus Bis group (n = 4; Student-Newman-Keuls, p < 0.05) compared to the vehicle injected group (n = 4). (B) Input resistance. (B1–B3) Intracellular recordings from the same cells displayed in (A). Hyperpolarizing current pulses were injected into B51 to measure the input resistance. (B4) Summary data. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the input resistance was significantly greater in both the cAMP group (n = 4; Student-Newman-Keuls, p < 0.05) and the cAMP plus Bis group (n = 4; Student-Newman-Keuls, p < 0.05) compared to the vehicle injected group (n = 4).
Activating PKC mimics the conditioning in a cAMP-dependent manner
If cAMP and PKC are acting in series during this form of operant reward learning and PKC acts upstream of cAMP synthesis, then it is possible that PKC phosphorylates adenylyl cyclase, increasing the production of cAMP and priming it for an enhanced activation if dopamine is delivered soon thereafter (Fig. 4B). Some isoforms of adenylyl cyclase exhibit this type of PKC sensitivity such as type II (Jacobowitz et al., 1993; Zimmermann and Taussig, 1996; Bol et al., 1997a; Bol et al., 1997b) and type V (Kawabe et al., 1994; Kawabe et al., 1996). Three isoforms of adenylyl cyclase (A, B, and C) have been identified in Aplysia (Sequence data available through accession numbers AY843025-7). Single-cell PCR was performed to determine which of these isoforms are expressed in B51 (Fig. 3B). The A and B isoforms of adenylyl cyclase were detected in B51, whereas C was not. The C isoform could be detected in sensory neurons of Aplysia. The A isoform is most closely related to the type I adenylyl cyclase, which is a Ca2+/calmodulin sensitive form, whereas the B isoform is most closely related to the type II adenylyl cyclase, which displays a high degree of PKC sensitivity (Jacobowitz et al., 1993; Zimmermann and Taussig, 1996; Bol et al., 1997a; Bol et al., 1997b). To test the model depicted in Fig. 4B, it is necessary to verify that PKC mimics the conditioning and that it engages the cAMP/PKA pathway in B51. To test this hypothesis, PDAc (2 μM) was bath applied to cultured B51 neurons. After 5 min, the PDAc application produced the same decrease in burst threshold (Fig. 6A1, 6A3) and increase in input resistance (Fig. 6B1, 6B3) as seen in previous conditioning experiments, indicating that a 5 min treatment with PDAc alone was sufficient to mimic the conditioning, whereas 2 min of PDAc treatment was insufficient to alter the membrane properties of B51 (data not shown). Another group of neurons received the same 2 μM treatment of PDAc while being bathed in 2 mM Rp-cAMP. This inhibitor blocked the changes in the membrane properties elicited by PDAc (Fig. 6A2, 6A3, 6B2, 6B3). Thus, the ability of PKC to mimic the conditioning was dependent on cAMP, which is consistent with a model (Fig. 4B) in which PKC acts upstream of cAMP synthesis.
Fig. 6.
Activating PKC mimicked the conditioning in a PKA-dependent manner. (A) Burst threshold. (A1–A2) Representative intracellular recordings from a cell that received PDAc and from a cell that received PDAc in the presence of bath-applied Rp-cAMP. Depolarizing current pulses were injected into B51 until the cell produced a plateau potential. (A3) Summary data. The burst threshold was decreased following 2 μM PDAc application (n = 4) and this effect was blocked by the presence of 2 mM Rp-cAMP (n = 4; Mann-Whitney, p < 0.05). (B) Input resistance. (B1–B2) Intracellular recordings from the same two cells displayed in (A). Hyperpolarizing current pulses were injected into B51 to measure the input resistance. (B3) Summary data. The input resistance was increased following 2 μM PDAc application (n = 4) and this effect was blocked by the presence of 2 mM Rp-cAMP (n = 4; Mann-Whitney, p < 0.05). (C) Elevated levels of phospho-CREB1 in B51 produced by PDAc treatment. (C1–C2) Representative confocal images from B51 neurons immediately after PDAc treatment (2 μM, 5 min) in the presence of 2 mM Rp-cAMP (n = 4; C2) or vehicle (n = 4; C1). Phospho-CREB1 was localized to the nucleus. (C3) Summary data illustrating that phospho-CREB1 levels were higher immediately after PDAc treatment and that this effect is blocked by Rp-cAMP (Mann-Whitney, p < 0.05). (D) Effects of sub-threshold application of PDAc (500 nM) and DA. (D1) Burst threshold. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the burst threshold was significantly decreased in the PDAc + DA group (n = 4) compared to either the PDAc alone group (n = 4; Student-Newman-Keuls, p < 0.05) or to the DA alone group (n = 4; Student-Newman-Keuls, p < 0.05). (D2) Input resistance. A significant difference was observed among the three groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the the input resistance was significantly increased in the PDAc + DA group (n = 4) compared to either the PDAc alone group (n = 4; Student-Newman-Keuls, p < 0.05) or to the DA alone group (n = 4; Student-Newman-Keuls, p < 0.05).
To further examine the possibility that PKC increases the level of cAMP and thereby, mimics conditioning, the level of phosphorylated CREB1 (phospho-CREB1) was again monitored as an indicator of the activation of PKA following PDAc treatment. One group of B51 neurons was bathed in 2 mM Rp-cAMP and another group was bathed in vehicle. Immediately after treatment with PDAc (2 μM, 5 min) the cells were processed to measure the immunoreactivity for phospho-CREB1. PDAc treated cells that were bathed in vehicle had significantly higher levels of phospho-CREB1 (Fig. 6C1, 6C3) and this effect was blocked by Rp-cAMP (Fig. 6C2, 6C3). These results indicate that PDAc induced a rapid phosphorylation of CREB1 at a PKA site. This effect was blocked by Rp-cAMP, which suggests that the ability of the PDAc treatment to phosphorylate the PKA site is cAMP-dependent. Interestingly, both the single-cell analogue (Fig. 1G2C) and PDAc treatment (Fig. 6C) appeared to increase the levels of phospho-CREB1 to a similar degree.
Sub-threshold doses of phorbol and dopamine can combine to mimic conditioning
As a further test of the synergistic actions of the PKC and dopamine pathways, we examined the combinatorial effects of sub-threshold application of phorbol with dopamine. The modulation of the membrane properties of B51 was examined under three conditions. First, to activate the reward pathway, dopamine was applied (7 evenly spaced iontophoretic puffs of dopamine during a 10 min period) in the same manner as during the single-cell analogue, but in the absence of the burst activity in B51). Next, sub-threshold doses of PDAc (500 nM, 10 min) were applied to mimic activation of the behavior/activity pathway. Lastly, the two manipulations were applied together on an additional group of cultured B51 neurons. Neither the sub-threshold phorbol treatment nor the dopamine treatment alone was able to modulate the membrane properties of B51 (Fig. 6D). However, when sub-threshold phorbol and DA were applied together, the input resistance of B51 increased and the burst threshold decreased (Fig. 6D), which is an effect identical to that produced by the single-cell analogue of operant conditioning (Fig. 1E, 1F, 2A, 2B).
A peptide inhibitor of PKA also blocks the conditioning
The previous experiments indicate that cAMP is necessary for the conditioning. To further examine whether PKA is necessary for the conditioning we used the specific peptide inhibitor PKI-A (6–22; Bao et al., 1998). B51 neurons were injected either with PKI-A or vehicle and then subjected to the single-cell analogue (SCA) of operant conditioning. After performing the single-cell analogue, the neurons were treated with the same phorbol treatment that mimicked the conditioning (Fig. 6), to test whether PKI-A could also block the PDAc treatment effect. Also, because the single-cell analogue was performed just prior to PDAc treatment in the vehicle group, it was possible to examine whether the single-cell analogue could occlude the effect of PDAc treatment. The PKI-A treatment blocked the changes in the membrane properties of B51 normally induced by either the single-cell analogue or PDAc (Fig. 7), indicating that cAMP mediates its effects on input resistance and burst threshold through PKA rather than through a PKA-independent pathway. Also, in the vehicle group, the prior treatment of B51 with the single-cell analogue occluded the effects of PDAc treatment (Fig. 7), indicating that the phorbol treatment acts on input resistance and burst threshold through the same mechanism as the single-cell analogue.
Fig. 7.
Inhibiting PKA with the peptide inhibitor PKI-A blocked the conditioned changes in B51. (A–B) Representative intracellular recordings from B51 illustrating the measurement of the burst threshold (A) and the input resistance (B) before and after both the single-cell analogue (SCA) and subsequent PDAc treatment (2 μM) of cells injected with either PKI-A (A1, B1) or vehicle (A2, B2). In this example, the burst threshold decreased in the vehicle treated group from 0.7 nA to 0.3 nA following contingent reinforcement. However, when the same cell was subsequently treated with PDAc, the burst threshold did not change (0.3 nA before treatment and 0.3 nA after treatment) illustrating the occlusion effect. A similar occlusion effect was also observed with the input resistance. (C) Summary data. (C1) Burst threshold. A significant difference was observed among the four groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the burst threshold was significantly greater in the single-cell analogue/vehicle group (n = 4) as compared to the PDAc/vehicle group (n = 4; Student-Newman-Keuls, p < 0.05), the single-cell analogue/PKI-A group (n = 4; Student-Newman-Keuls, p < 0.05), or the PDAc/PKI-A group (n = 4; Student-Newman-Keuls, p < 0.05). (C2) Input Resistance. A significant difference was observed among the four groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the input resistance was significantly greater in the single-cell analogue/vehicle group (n = 4) as compared to the PDAc/vehicle group (n = 4; Student-Newman-Keuls, p < 0.05), the single-cell analogue/PKI-A group (n = 4; Student-Newman-Keuls, p < 0.05), or the PDAc/PKI-A group (n = 4; Student-Newman-Keuls, p < 0.05). Thus, the PKI-A peptide blocked the conditioned changes in B51 normally resulting from the both the single-cell analogue and PDAc treatment. Also, the single-cell analogue appeared to occlude the effects of PDAc treatment.
The Ca2+-dependent isoform of PKC is necessary for conditioning, but not the Ca2+-independent isoform
To determine which isoform of PKC is mediating the conditioned changes in B51, we used dominant-negative isoforms of both PKC apl-I and PKC apl-II (Manseau et al., 2001). B51 neurons were injected with vectors encoding for either dominant-negative PKC apl-I or PKC apl-II and then subjected to the single-cell analogue of operant conditioning. After performing the single-cell analogue, the B51 neurons were treated with the same phorbol treatment that mimicked the conditioning (Fig. 6), to test whether dominant negative PKC could also block the effect of PDAc treatment. The dominant-negative isoform of the Ca2+-dependent PKC (apl-I) blocked the conditioned changes in B51 normally induced by either the single-cell analogue or PDAc (Fig. 8), indicating that this isoform was necessary for the conditioning. In contrast, the dominant-negative isoform of the Ca2+-independent PKC (apl-II) did not block the conditioning in B51 by the single-cell analogue. This negative result is unlikely to be due to ineffictiveness of the dominant-negative isoform of PKC apl-II. It has been previously shown to inhibit PKC-dependent facilitation of sensorimotor synapses in Aplysia (Manseau et al., 2001). Also, the PDAc treatment effect was blocked in this group, likely because the single-cell analogue occluded it in the same way as in Fig. 7. The alternative explanation that the PDAc effect was blocked by the dominant negative PKC apl-II is unlikely because PKC apl-II is not detected in B51 (Fig. 3A).
Fig. 8.
Inhibiting PKC with dominant negative PKC apl-I blocked conditioned changes in B51, but dominant negative PKC apl-II did not. (A–B) Representative intracellular recordings from B51 illustrating the measurement of the burst threshold (A) and the input resistance (B) before and after both the single-cell analogue (SCA) and subsequent PDAc treatment (2 μM) of cells expressing either the dominant negative isoform for PKC apl-I (A1, B1) or PKC apl-II (A2, B2). (C) Summary data. (C1) Burst threshold. A significant difference was observed among the four groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the burst threshold was significantly greater in the single-cell analogue/dominant negative PKC apl-II group (n = 4) as compared to the PDAc/dominant negative PKC apl-II group (n = 4; Student-Newman-Keuls, p < 0.05), the single-cell analogue/dominant negative PKC apl-I group (n = 4; Student-Newman-Keuls, p < 0.05), or the PDAc/dominant negative PKC apl-I group (n = 4; Student-Newman-Keuls, p < 0.05). (C2) Input Resistance. A significant difference was observed among the four groups (Kruskal-Wallis, p < 0.05). A post-hoc analysis revealed that the change in the input resistance was significantly greater in the single-cell analogue/dominant negative PKC apl-II group (n = 4) as compared to the PDAc/dominant negative PKC apl-II group (n = 4; Student-Newman-Keuls, p < 0.05), the single-cell analogue/dominant negative PKC apl-I group (n = 4; Student-Newman-Keuls, p < 0.05), or the PDAc/dominant negative PKC apl-I group (n = 4; Student-Newman-Keuls, p < 0.05). Thus, the expression of the dominant negative PKC apl-I isoform blocked the conditioned changes in B51 normally resulting from the both the single-cell analogue and PDAc treatment. Also, the single-cell analogue again appeared to occlude the effects of PDAc treatment.
DISCUSSION
During operant conditioning of feeding behavior, an Aplysia learns the consequences of its behavior. Because the behavior can be represented by activity in B51 and the reinforcement can be represented by dopamine, the in vitro analogue can be reduced to the level of a single cell. Using this reduced system, we found that both cAMP and PKC were necessary and sufficient for the conditioning. Our data are consistent with a model (Fig. 4B; see also Supplementary Fig. 2) of the molecular mechanisms of operant conditioning in which there is a convergence (at some point upstream of cAMP production) of an activity-induced signaling cascade involving Ca2+-dependent PKC apl-I and a D1 receptor-induced signaling cascade. One likely point of convergence is the adenylyl cyclase complex. Contingent activation of the two pathways would lead to larger production of cAMP than that produced by either pathway alone. Under normal conditions, the cAMP produced by activity alone or DA alone is insufficient to raise the level of cAMP past some threshold for inducing the cellular changes in B51. In contrast, repeated contingent reinforcements raise the level of cAMP greater than that produced by either pathway alone so that the cAMP level exceeds the threshold for producing the changes in B51. Although convergence is normally required to produce sufficient cAMP to induce the cellular changes, direct intracellular injection of cAMP (Fig. 5) presumably raised the levels of cAMP past the threshold necessary to induce the cellular changes in B51. Similarly, application of PDAc (2 μM, 5 min), used to mimic the activity pathway, would produce the cellular changes in B51 (Fig. 6) because it would lead to a much greater increase in cAMP levels than that normally produced by activity alone (B51 plateau potentials have a duration of only 5–15 s).
The model was corroborated by experimental evidence showing that phospho-CREB1 levels were significantly greater following contingent training as compared to the non-contingent control group (Fig. 2C). Immediately after training, cells that received contingent reinforcement had significantly higher levels of phospho-CREB1 as compared to cells that received non-contingent reinforcement or to cells that received contingent reinforcement in the presence of Rp-cAMP. These results indicate that the single-cell analogue induced a rapid phosphorylation of CREB1 at a PKA site, which suggests that PKA was activated by contingent reinforcement. This result is consistent with the model of Fig. 4B in which contingent training produces a greater increase in cAMP than non-contingent training. With a parallel model (see Fig. 4A), the level of activation of the cAMP/PKA pathway would be similar with either contingent or non-contingent reinforcements of plateau potentials with DA. However, this is clearly not the case, because phospho-CREB1 levels are significantly higher with contingent reinforcement, than with the non-contingent control (Fig. 2C).
Increased training-induced phosphorylation of CREB1 was mimicked by phorbol treatment and this effect was blocked by Rp-cAMP (Fig. 6), which suggested that PKC activated the cAMP/PKA pathway. Phorbol treatment alone has been shown to raise the levels of cAMP in cells containing type II-like adenylyl cyclase (Jacobowitz et al., 1993; Zimmermann and Taussig, 1996; Bol et al., 1997a; Bol et al., 1997b). For example, Jacobowitz et al. (1993) found that phorbol treatment alone induced a ~3-fold increase in cAMP production, forskolin treatment alone produced a ~6-fold increase in cAMP and the two together produced a ~12-fold increase. These results indicate that converging interaction between PKC and Gs can synergistically activate the adenylyl cyclase to produce levels of cAMP that are much higher than with either treatment alone. In addition, a direct assay of cAMP production in Aplysia sensory neuron clusters (Sugita et al., 1997) showed a significant increase in cAMP following phorbol treatment, indicating that a type II-like adenylyl cyclase is present in Aplysia. B51 expresses an isoform of adenylyl cyclase with homology to the type II isoform (Fig. 3B). Moreover, phorbol treatment in B51 mimicked the conditioning after 5 min of bath application (Fig. 6), whereas 2 min of treatment was insufficient. The effects of PDAc take time to develop and were blocked by blocking the actions of cAMP and PKA, suggesting that PDAc application led to slowly developing activation of the cAMP/PKA pathway.
Additional support for the model of Fig. 4B was provided by experiments showing that the changes produced by either PDAc application or the single-cell analogue of operant conditioning were blocked by Rp-cAMP (Fig. 2, 6) or by intracellular injection of a highly specific peptide inhibitor of PKA (PKI-A) (Fig. 7). Moreover, the changes produced by either PDAc application or the single-cell analogue were blocked by expressing a dominant-negative isoform of the Ca2+-dependent PKC but not by a dominant-negative isoform of the Ca2+-independent PKC (Fig. 8). The observation that cAMP alone is sufficient to produce the changes in the membrane (Fig. 5) excludes a parallel model (e.g., Fig. 4A) where PKA and PKC would be required to jointly phosphorylate distinct sites on their putative common substrate molecule. However, this observation does not rule out a model where PKA and PKC can both phosphorylate the same site on their putative common substrate molecule. In this single-site parallel model, either PKA or PKC could activate the substrate sufficiently alone, if they were at a high enough level or they could combine to provide the necessary level of phosphorylation. The observation that the phorbol-induced modulation of the membrane properties can be suppressed by blocking cAMP/PKA (Fig. 6) is supportive of a model in which the actions of cAMP are downstream of PKC (Fig. 4B) excluding the model depicted in Fig. 4C and also excluding the single-site parallel model (Fig. 4A). In that parallel model, the phorbol treatment would still activate PKC, which would still be able to phosphorylate and fully activate the putative common substrate, even if the cAMP/PKA pathway is blocked, but this does not occur. Rather, the observation that blocking cAMP/PKA blocks the phorbol-induced changes in the membrane, likely indicates that phorbol/PKC modulates the membrane properties by activating cAMP/PKA (i.e., the model depicted in Fig. 4B, not the parallel model). The notion that phorbol/PKC activates cAMP/PKA is also supported by the observation that phorbol treatment increases the levels of phospho-CREB1 (Fig. 6C) similar to the single-cell analogue (Fig. 2C) and the increase in phospho-CREB1 levels by either phorbol treatment or the single-cell analogue is blocked by Rp-cAMP (Fig. 2C, 6C) indicating that the phosphorylation is cAMP-dependent. Also, the phorbol effect was occluded by prior conditioning with the single-cell analogue (Fig. 7), indicating that they likely operate through a common pathway.
Interestingly, the mechanisms of activity-dependent neuromodulation for this appetitive form of operant conditioning appear to be very similar to a form of aversive classical conditioning observed in sensory neurons of Aplysia, which mediate withdrawal reflexes (Lechner and Byrne, 1998). In the sensory neuron, the coincidence detection involves, at least in part, a synergistic interaction between a Ca2+/calmodulin-sensitive adenylyl cyclase (Type I) and a serotonin-activated cAMP cascade (Ocorr et al., 1985; Yovell et al., 1992). Although the specific isoform of adenylyl cyclase appears to differ (Type I for classical conditioning and Type II for operant conditioning), adenylyl cyclase appears to serve as the molecule of convergence in both forms of learning. This finding is striking, but does not imply that adenylyl cyclase is a universal coincidence detector for all examples of classical and operant conditioning. Indeed, the membrane properties of B51 were also modulated by classical conditioning (Lorenzetti et al., 2006). However, following classical conditioning, the burst threshold of B51 increases rather than decreases as it does following operant conditioning and the input resistance does not change, indicating that the mechanisms underlying the modulation of B51 by classical conditioning involve a different as yet unidentified coincidence detector from that for operant conditioning. Because dopamine likely mediates both the unconditioned stimulus (US) of classical conditioning and the reinforcement of operant conditioning (Kabotyanski et al., 1998; Nargeot et al., 1999c; Brembs et al., 2002; Reyes et al., 2005), a key problem is to elucidate the mechanisms that lead to the induction of opposite effects on the burst threshold. One possibility is that the coincidence detector for classical conditioning involves an association between a transmitter released by the conditioned stimulus (CS) and the dopamine-mediated reward pathway, whereas for operant conditioning, the coincidence detector, as shown in the present study, likely involves an association between PKC and the dopamine-mediated reward pathway. Exploring these pathways and their interactions will provide important insights into the molecular logic of operant and classical conditioning.
Growing evidence indicates that many of the same molecular pathways that mediate operant conditioning in Aplysia are also involved in vertebrate reward learning in the striatum. For example, in vivo operant conditioning was blocked by infusions into the nucleus accumbens of antagonists for D1 dopamine receptors and NMDA receptors (Smith-Roe and Kelley, 2000) and also by inhibitors of cAMP/PKA (Baldwin et al., 2002). Furthermore, D1 dopamine receptors are necessary for potentiation of cortico-striatal synapses in an analogue of reward learning where induced electrical activity in striatal spiny neurons was paired with reward inducing stimulation of the substantia nigra (Reynolds et al. 2001). In addition to synaptic plasticity, striatal spiny neurons can display an increased level of intrinsic excitability known as the up-state. Increasing CREB levels in the nucleus accumbens increased the intrinsic excitability of spiny neurons mimicking the up-state and blocking CREB decreased the excitability (Dong et al., 2006). The Aplysia feeding system, however, offers several distinct advantages for investigating the molecular mechanisms of reward learning. Many of the circuit elements have been identified in the neuronal network responsible for Aplysia feeding behavior. This work has established a functional role for B51 during feeding and demonstrates how the modulation of the membrane properties of B51 following conditioning can modulate the behavioral response. Furthermore, knowing how B51 is activated during feeding behavior and the nature of the reinforcement has enabled the construction of the single-cell analogue of operant conditioning. The analogue, in turn, has provided access to the cellular and molecular processes underlying the learning and has enabled the elucidation of the how these molecular pathways interact and converge to mediate operant conditioning in an individual neuron important for the expression of the conditioned behavior.
EXPERIMENTAL PROCEDURES
Animals
Aplysia californica (80–130 g) were obtained from Alacrity Marine Biological Specimens (Redondo Beach, CA) and Marinus Scientific (Long Beach, CA). Animals were housed in perforated plastic cages floating in aerated seawater tanks at a temperature of 15 °C. Animals were fed ~1 g of dried seaweed three times per week.
Cell culture
Culturing procedures followed those described in (Schacher and Proshansky, 1983; Rayport and Schacher, 1986; Chin et al., 1999; Brembs et al., 2002). Buccal ganglia from adult Aplysia were incubated in 1% protease type IX (Sigma, St. Louis, MO) at room temperature for 24 h and then desheathed. B51 neurons were identified based on neurite morphology coupled with the size and the relative position of the cell in the ganglia. Fine-tipped glass microelectrodes were used to remove the cells from the ganglia, which were then plated on poly-L-lysine coated petri dishes with culture medium containing 50% hemolymph and 50% isotonic L15 (Sigma). The cells were allowed to grow for 4–5 days. The medium was changed on the third day. Culture medium was exchanged for a solution containing 50% artificial sea water (ASW) and 50% isotonic L15 prior to recording. The composition of the ASW was: 450 mM NaCl, 10 mM KCl, 30 mM MgCl2(6H2O), 20 mM MgSO4, 10 mM CaCl2(2H2O), 10 mM HEPES, with pH adjusted to 7.4.
Immunohistochemistry
An affinity-purified rabbit polyclonal antibody for the Aplysia D1-like dopamine receptor (Barbas et al., 2006) was raised in rabbits against a peptide fragment 12 amino acids long from the third extracellular loop of the protein (Genemed Synthesis, San Francisco, CA). Also, an affinity-purified rabbit polyclonal antibody for Aplysia phospho-CREB1 (Mohamed et al., 2005) was raised in rabbits against the peptide sequence KKRREILTRRPSYRK with Ser85 (underlined) phosphorylated (Genemed Synthesis, San Francisco, CA). D1 receptor antibody specificity was verified by Western blot and pre-absorption techniques. The D1 receptor antibody recognizes only one band out of all the proteins present in Aplysia CNS extract and the binding can be prevented by pre-incubation with the D1 receptor fragment (Barbas et al., 2006). Both the phospho-CREB1 antibody and a total CREB1 antibody (Mohamed et al., 2005) recognize only 2 bands from Aplysia ganglia extract, one at 33 kDa and another at 66 kDa. The lighter band migrates the same as CREB1 protein and the heavier band is likely a dimer, as pre-incubation with CREB1 protein prevents the total CREB1 antibody from binding with both bands (Mohamed et al., 2005). Also, phosphatase pre-treatment of CREB1 protein diminished the immunoreactivity of the phospho-CREB1 antibody, demonstrating its specificity for phosphorylated CREB1 (Mohamed et al., 2005).
Cultured B51 neurons were fixed in a solution of 4% paraformaldehyde in PBS containing 20% sucrose to maintain osmolarity. After four rinses in PBS, the fixed cells were blocked for 1 hr at room temperature in PBS containing 5% normal goat serum and subsequently incubated overnight at 4°C with either the D1-like dopamine receptor antibody (1:1000) or the phospho-CREB1 antibody (1:500) diluted in blocking solution. Secondary antibody (Alexa 594-conjugated goat anti-rabbit IgG at 1:100 dilution) was applied in blocking solution for 1 hr at room temperature. Slides were then mounted using Prolong anti-fade medium (Molecular Probes, Eugene, OR). Images were obtained with a Bio-Rad 1024 MP confocal microscope using a 60X oil immersion lens and consisted of 10–15 μm optical sections. The section through the middle of the nucleus was used for analysis of mean nuclear fluorescence intensities by Metamorph imaging software (Molecular Devices Corp., Sunnyvale, CA). The nuclear intensity measurements in the experimental groups were compared to nuclear intensity measurements from untreated B51 neurons that were processed in the same way as the experimental B51s.
Single-cell analogue
The procedures for the single-cell analogue have been described previously (Brembs et al., 2002). Conventional current-clamp techniques were used for the intracellular recordings (Axoclamp-2A, Molecular Devices, Downingtown, PA). Fine-tipped glass microelectrodes (resistance 10–15 MΩ) were filled with 2 M potassium acetate. The cells were current-clamped at −80 mV for the duration of the experiment. Five minutes after impalement, input resistance and burst threshold were determined. Input resistance was measured by injecting a hyperpolarizing current pulse of 0.5 nA for 5 s. The burst threshold of B51 was defined as the minimum amount of depolarizing current necessary to elicit a plateau potential. The burst threshold was tested by a series of successively higher amplitude depolarizing current pulses (in 0.1 nA increments) with a duration of 5 s. The series was spaced with 10 s between the end of one pulse and the start of another. In the training phase, plateau potentials were generated by a 5-s duration depolarizing current pulse with an amplitude 0.1 nA greater than the previously determined threshold. The cells received 7 evenly spaced supra-threshold depolarizing current pulses in a ten minute training period. The plateau potentials were contingently reinforced with a 6-s iontophoretic pulse of dopamine (Sigma, St. Louis, MO) immediately after the cessation of the plateau potential. Dopamine was iontophoresed through a fine-tipped glass microelectrode (resistance 10–15 MΩ). A retaining current of −1 nA was used during the course of the experiment. A square wave current pulse of 35 nA for 6 s was used to eject the dopamine. The concentration of dopamine in the electrode was 200 mM. An equimolar concentration of ascorbic acid was added to the electrode to reduce the oxidation of dopamine. Immediately after the training phase, the membrane properties were measured again and compared to the pre-test levels.
The single-cell analogue was also performed by iontophoresing the D1 dopamine receptor agonist chloro-APB (Sigma, St. Louis, MO), instead of dopamine. The cells were divided into either a contingent reinforcement group or a non-contingent control group. Both groups received 7 evenly spaced supra-threshold depolarizing current pulses in a ten-minute training period. The cells in the contingent reinforcement group received a 6 s iontophoretic pulse of chloro-APB immediately after the plateau potential, whereas iontophoresis was delayed by 40 s in the non-contingent control group. The iontophoretic parameters were the same as with dopamine and the concentration of chloro-APB in the electrode was 100 mM.
To test the role of PKA, the membrane permeable inhibitor Rp-8-Br-cAMPS (Rp-cAMP, Biolog, Bremen, Germany) was bath applied to cultured B51 neurons to a final concentration of 2 mM, similar to what has been used previously in Aplysia (Liao et al., 1999). The Rp-cAMP was applied for 30 min prior to the start of the single-cell analogue as described above and the inhibitor was maintained in the bath throughout the experiment. After the 30 min incubation, the membrane properties were measured, the single-cell analogue was performed, after which the membrane properties were retested. To examine the role of PKC, the membrane permeable inhibitor bisindolylmaleimide I (Bis, Calbiochem, La Jolla, CA) was bath applied to cultured B51 neurons to a final concentration of 10 μM, similar to what has been used previously in Aplysia (Kabir et al., 2001). The Bis was applied for 30 min prior to the start of the single-cell analogue as described above and the inhibitor was maintained in the bath throughout the experiment. After the 30 min incubation, the membrane properties were measured, the single-cell analogue was performed, after which the membrane properties were retested. The experimenter was blind to whether the inhibitors were present or not. All membrane property measurements are displayed as the percent difference between the post-test and pre-test values normalized to the pre-test. All recordings were performed at room temperature (approximately 20–22 °C).
cAMP injection
Cultured B51 neurons were current-clamped at −80 mV for the duration of the experiment. Five minutes after impalement, input resistance and burst threshold were determined. Next, cAMP (Sigma, St. Louis, MO) was iontophoresed directly into the soma of B51. The cAMP was iontophoresed with a fine-tipped double-barrel glass microelectrode. One barrel contained the standard recording solution of 2 M potassium acetate, whereas the other barrel was used for iontophoresis and contained 150 mM cAMP, 200 mM KOH, 20 mM Tris HCl, with pH adjusted to 7.4. The concentration of cAMP in the iontophoretic electrode was similar to what has been used previously in Aplysia (Walsh and Byrne, 1989). A square wave current pulse of −10 nA for 10 s was used to eject the cAMP. These iontophoretic parameters were chosen because they elicited a transient depolarization of the membrane potential, indicating that cAMP was entering the cell. The input resistance and burst threshold were measured again 5 minutes after cAMP delivery. Another group of cells received the same treatment as above, except instead of cAMP, a vehicle solution containing 200 mM KOH, 20 mM Tris HCl, with pH adjusted to 7.4 was in the iontophoretic barrel of the electrode. A third group of cells was bathed in 10 μM Bis for 30 min and then was current clamped to −80 mV and the input resistance and the burst threshold were measured 5 min after impalement. Next, cAMP was iontophoresed into the cell, as above, and the membrane properties were measured again 5 min after cAMP delivery. The experimenter was blind to whether cAMP or vehicle was present in the iontophoresing electrode.
Phorbol esters
Cultured B51 neurons were current-clamped at −80 mV for the duration of the experiment. Five minutes after impalement, input resistance and burst threshold were measured. Next, phorbol diacetate (PDAc, Sigma, St. Louis, MO), a membrane permeable activator of PKC, was bath applied to a final concentration of 2 μM, similar to what has been used previously in Aplysia (Sugita et al., 1997). The input resistance and the burst threshold were measured again after 5 min of PDAc application. Another group of cells was bathed in 2 mM Rp-cAMP for 30 min and then given the same protocol as described above, with the membrane properties being measured both before and after PDAc application. The experimenter was blind to whether the Rp-cAMP was present or not.
In a separate experiment, 7 evenly spaced iontophoretic puffs of dopamine were delivered to cultured B51 neurons during a 10 min phase, with the same parameters as with the single-cell analogue of operant conditioning (but with no plateau potentials). The membrane properties of B51 were measured both before and after the 10 min phase of dopamine application. Next, sub-threshold doses of PDAc (500 nM) were applied to these cultured B51 neurons. The membrane properties were tested before application and both 5 and 10 min after the application. Neither 5 nor 10 min of PDAc (500 nM) application appreciably altered the membrane properties of B51. A separate group of cultured B51 neurons were then simultaneously given the sub-threshold PDAc treatment along with the 7 iontophoretic puffs of dopamine in 10 min. The membrane properties were measured both before and after this 10 min phase.
PKC translocation
Plasmids encoding for the Ca2+-dependent PKC apl-I fused with EGFP (Zhao et al., 2006) were generously provided by Dr. Wayne Sossin (McGill University). The plasmids (1.5 μg/μL) were pressure injected into the nucleus of cultured B51 neurons 1 day prior to imaging to allow time for the proteins to express. The plasmids were co-injected with TRITC dye to monitor the success of the injection and protein expression was verified by EGFP fluorescence. Images were obtained with a Bio-Rad 1024 MP confocal microscope using a 60X oil immersion lens and consisted of 10–15 μm optical sections. The section through the middle of the nucleus was used for analysis of mean fluorescence intensities by Metamorph imaging software (Molecular Devices Corp., Sunnyvale, CA). Translocation of PKC was monitored following either bath application of the Ca2+ ionophore ionomycin (1μM; Sigma, St. Louis, MO), dopamine iontophoresis (same parameters as with the single-cell analogue), or a plateau potential in B51. The translocation observed with ionomycin application was quantified by calculating the ratio of the average intensity at the plasma membrane over the average intensity in the cytoplasm. Average intensity measurements for the plasma membrane and the cytoplasm were calculated by measuring the average intensity from 3 randomly placed rectangles in each region.
Dominant negative PKC
Plasmids encoding for dominant negative isoforms of the Ca2+-dependent and Ca2+-independent PKC apl-I and apl-II fused with EGFP (Zhao et al., 2006) were generously provided by Dr. Wayne Sossin (McGill University). The plasmids (1.5 μg/μL) were pressure injected into the nucleus of cultured B51 neurons 1 day prior to training to allow time for the proteins to express. The plasmids were co-injected with TRITC dye to monitor the success of the injection and protein expression was verified by EGFP fluorescence. The cultured B51 neurons expressing either dominant negative PKC apl-I or apl-II were then subjected to the single-cell analogue of operant conditioning as described previously, which was then followed by 5 min of 2 μM PDAc treatment. The experimenter was blind to whether the neuron was injected with dominant negative PKC apl-I or apl-II.
PKI-A
The PKA peptide inhibitor PKI-A (6–22, Sigma, St. Louis, MO) was pressure injected into the cytoplasm of cultured B51 neurons just prior to conditioning. The concentration of PKA in the electrode was 500 μM and the peptide was co-injected with TRITC dye to monitor the success of the injection. Half of the B51 neurons received PKI-A and the other half were injected with vehicle. The cultured B51 neurons were then subjected to the single-cell analogue of operant conditioning as described previously, which was then followed by 5 min of 2 μM PDAc treatment. The experimenter was blind to whether the neuron was injected with PKI-A or vehicle.
PCR
Single-cell PCR was performed using cultured individual B51 neurons or sensory neurons (positive control) as the source of the template RNA. Cultured B51 neurons were briefly fixed in ethanol prior to their removal from the dish with a sharp glass microelectrode and individual sensory neurons were removed from desheathed pleural ganglia just prior to being placed in the PCR tube. A reverse transcriptase reaction was performed with the mRNA from each cell. The resulting cDNA was then used for PCR. A negative control was also performed with the template RNA digested by ribonuclease A (Sigma, St. Louis, MO) prior to the reverse transcriptase reaction. The PCR primers were designed from the published sequences of the Aplysia D1 dopamine receptor, Aplysia PKC isoforms apl-I and apl-II, and the three known Aplysia isoforms of adenylyl cyclase (Sequence data available through accession numbers AY918891, M94883, M94884, AY843025, AY843026, and AY843027). The primers were checked for homology with other published sequences so that they would not amplify products other than the specific isoforms they were designed to amplify. For the D1 dopamine receptor, the forward primer was 5′-TCTTGGTAGCCTGTGCAGTCAT-3′ and the reverse primer was 5′-CGTTTGTACCAACCCAAGTGAA-3′. The PCR product generated by these primers will be 289 bp. For the apl-I isoform of PKC, the forward primer was 5′-GCGTGGACGTATACTCATCAAAG-3′ and the reverse primer was 5′-TGCATCTTGCTCTTGATCTCCT-3′. The PCR product generated by these primers will be 444 bp. For the apl-II isoform of PKC, the forward primer was 5′-AATGCTAGCAATGAGCATCAGG-3′ and the reverse primer was 5′-GCCACAGAATGTCTGCGTTAAT-3′. The PCR product generated by these primers will be 576 bp. For the A isoform of adenylyl cyclase, the forward primer was 5′-TTGGGGTTCAGACATTCTTCA-3′ and the reverse primer was 5′-TTGTTCACTCGATCAACAGGG-3′. The PCR product generated by these primers will be 409 bp. For the B isoform of adenylyl cyclase, the forward primer was 5′-GGACATGCGCATAGGAGTACA-3′ and the reverse primer was 5′-ACTTCCTCATGCCATGGTCAT-3′. The PCR product generated by these primers will be 326 bp. For the C isoform of adenylyl cyclase, the forward primer was 5′-CCAACTTCAATGTTTCGCAG-3′ and the reverse primer was 5′-CATCTCCACAGCGCAGTGA-3′. The PCR product generated by these primers will be 524 bp. The resulting PCR products were then sequenced to provide further verification of the process.
Statistical Analysis
All values were expressed as means ± SEM. Statistical significance was set at p < 0.05 and all statistical tests were two-tailed. Comparisons between two paired groups were made using paired t-tests. Comparisons between two unpaired groups were made using the Mann-Whitney test. Comparisons between either three groups or four groups were made using the Kruskal-Wallis test and post-hoc pairwise multiple comparisons were made using the Student-Newman-Keuls test. Statistics were performed using SigmaStat 2.0 (Jandel Scientific, San Rafael, CA).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH 58321) and the National Institute of Health (DE 015355). We thank Drs. L. Cleary, D. Fioravante, R. Mozzachiodi and G. Phares for their comments on an earlier draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1998;18:458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Mohamed HA, Byrne JH, Castellucci VF, DesGroseillers L. An Aplysia dopamine1-like receptor: molecular and functional characterization. J Neurochem. 2006;96:414–427. doi: 10.1111/j.1471-4159.2005.03561.x. [DOI] [PubMed] [Google Scholar]
- Bol GF, Gros C, Hulster A, Bosel A, Pfeuffer T. Phorbol ester-induced sensitisation of adenylyl cyclase type II is related to phosphorylation of threonine 1057. Biochem Biophys Res Commun. 1997a;237:251–256. doi: 10.1006/bbrc.1997.7123. [DOI] [PubMed] [Google Scholar]
- Bol GF, Hulster A, Pfeuffer T. Adenylyl cyclase type II is stimulated by PKC via C-terminal phosphorylation. Biochim Biophys Acta. 1997b;1358:307–313. doi: 10.1016/s0167-4889(97)00073-6. [DOI] [PubMed] [Google Scholar]
- Botzer D, Markovich S, Susswein AJ. Multiple memory processes following training that a food is inedible in Aplysia. Learn Mem. 1998;5:204–219. [PMC free article] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Science. 2002;296:1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH. TGF-beta1 in Aplysia: role in long-term changes in the excitability of sensory neurons and distribution of TbetaR-II-like immunoreactivity. Learn Mem. 1999;6:317–330. [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Cook DG, Carew TJ. Operant conditioning of head-waving in Aplysia. III. Cellular analysis of possible reinforcement pathways. J Neurosci. 1989;9:3115–3122. doi: 10.1523/JNEUROSCI.09-09-03115.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Mechanisms of simple motor learning. Trends Neurosci. 1979;2:153–155. [Google Scholar]
- Jacobowitz O, Chen J, Premont RT, Iyengar R. Stimulation of specific types of Gs-stimulated adenylyl cyclases by phorbol ester treatment. J Biol Chem. 1993;268:3829–3832. [PubMed] [Google Scholar]
- Jones LM, Izu LT, Abrams TW. PKC activation during burst-dependent protection in Aplysia sensory neurons: modeling the calcium dependence. Paper presented at the 31st Annual Meeting of the Society for Neuroscience; San Diego, CA. 13 November 2001. [Google Scholar]
- Kabir N, Schaefer AW, Nakhost A, Sossin WS, Forscher P. Protein kinase C activation promotes microtubule advance in neuronal growth cones by increasing average microtubule growth lifetimes. J Cell Biol. 2001;152:1033–1044. doi: 10.1083/jcb.152.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol. 1998;79:605–621. doi: 10.1152/jn.1998.79.2.605. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kawabe J, Ebina T, Toya Y, Oka N, Schwencke C, Duzic E, Ishikawa Y. Regulation of type V adenylyl cyclase by PMA-sensitive and -insensitive protein kinase C isoenzymes in intact cells. FEBS Lett. 1996;384:273–276. doi: 10.1016/0014-5793(96)00331-6. [DOI] [PubMed] [Google Scholar]
- Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem. 1994;269:16554–16558. [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Byrne JH. New perspectives on classical conditioning: a synthesis of Hebbian and non-Hebbian mechanisms. Neuron. 1998;20:355–358. doi: 10.1016/s0896-6273(00)80977-0. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci. 2000;20:3369–3376. doi: 10.1523/JNEUROSCI.20-09-03369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Gunstream JD, Lewin MR, Ambron RT, Walters ET. Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons. J Neurosci. 1999;19:1247–1256. doi: 10.1523/JNEUROSCI.19-04-01247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti FD, Mozzachiodi R, Baxter DA, Byrne JH. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat Neurosci. 2006;9:17–19. doi: 10.1038/nn1593. [DOI] [PubMed] [Google Scholar]
- Manseau F, Fan X, Hueftlein T, Sossin W, Castellucci VF. Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed aplysia sensorimotor synapses. J Neurosci. 2001;21:1247–1256. doi: 10.1523/JNEUROSCI.21-04-01247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HA, Yao W, Fioravante D, Smolen PD, Byrne JH. cAMP-response elements in Aplysia creb1, creb2, and Ap-uch promoters: implications for feedback loops modulating long term memory. J Biol Chem. 2005;280:27035–27043. doi: 10.1074/jbc.M502541200. [DOI] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci. 1997;17:8093–8105. doi: 10.1523/JNEUROSCI.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J Neurosci. 1999a;19:2247–2260. doi: 10.1523/JNEUROSCI.19-06-02247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J Neurosci. 1999b;19:2261–2272. doi: 10.1523/JNEUROSCI.19-06-02261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Patterson GW, Byrne JH. Dopaminergic synapses mediate neuronal changes in an analogue of operant conditioning. J Neurophysiol. 1999c;81:1983–1987. doi: 10.1152/jn.1999.81.4.1983. [DOI] [PubMed] [Google Scholar]
- Ocorr K, Walters ET, Byrne JH. Associative conditioning analog selectively increases cAMP levels of tail sensory neruons in Aplysia. Proc Natl Acad Sci USA. 1985;82:2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford: Oxford University Press; 1927. [Google Scholar]
- Plummer MR, Kirk MD. Premotor neurons B51 and B52 in the buccal ganglia of Aplysia californica: synaptic connections, effects on ongoing motor rhythms, and peptide modulation. J Neurophysiol. 1990;63:539–558. doi: 10.1152/jn.1990.63.3.539. [DOI] [PubMed] [Google Scholar]
- Rayport SG, Schacher S. Synaptic plasticity in vitro: cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci. 1986;6:759–763. doi: 10.1523/JNEUROSCI.06-03-00759.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes FD, Mozzachiodi R, Baxter DA, Byrne JH. Reinforcement in an in vitro analog of appetitive classical conditioning of feeding behavior in Aplysia: blockade by a dopamine antagonist. Learn Mem. 2005;12:216–220. doi: 10.1101/lm.92905. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The Behavior of Organisms: An Experimental Analysis. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Baxter DA, Byrne JH. Modulation of a cAMP/protein kinase A cascade by protein kinase C in sensory neurons of Aplysia. J Neurosci. 1997;17:7237–7244. doi: 10.1523/JNEUROSCI.17-19-07237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL. Animal Intelligence: Experimental Studies. New York: Macmillan; 1911. [Google Scholar]
- Walsh JP, Byrne JH. Modulation of a steady-state Ca2+-activated, K+ current in tail sensory neurons of Aplysia: role of serotonin and cAMP. J Neurophysiol. 1989;61:32–44. doi: 10.1152/jn.1989.61.1.32. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Kandel ER, Dudai Y, Abrams TW. A quantitative study of the Ca2+/calmodulin sensitivity of adenylyl cyclase in Aplysia, Drosophila, and rat. J Neurochem. 1992;59:1736–1744. doi: 10.1111/j.1471-4159.1992.tb11005.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Leal K, Abi-Farah C, Martin KC, Sossin WS, Klein M. Isoform specificity of PKC translocation in living Aplysia sensory neurons and a role for Ca2+-dependent PKC APL I in the induction of intermediate-term facilitation. J Neurosci. 2006;26:8847–8856. doi: 10.1523/JNEUROSCI.1919-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G, Taussig R. Protein kinase C alters the responsiveness of adenylyl cyclases to G protein alpha and beta gamma subunits. J Biol Chem. 1996;271:27161–27166. doi: 10.1074/jbc.271.43.27161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.