Abstract
Oral premalignant lesions (OPLs) are early genetic events en route to oral cancer. To identify individuals susceptible to OPL is critical to the prevention of oral cancer. In a case-control study consisting of 147 patients with histologically confirmed OPL and 147 matched controls, we evaluated the associations of 10 genetic variants in nine genes of the double-strand break (DSB) DNA repair pathway with OPL risk. The most notable finding was an intronic polymorphism (A17893G) of the XRCC3 gene. Compared with the homozygous wild-type AA genotype, the odds ratios [OR] (95% confidence interval [CI]) for the heterozygous AG and homozygous variant GG genotype were 0.85 (0.49–1.48) and 0.18 (0.07–0.47), respectively (P for trend=0.002). In addition, compared with the most common A-C haplotype of XRCC3 (in the order of A17893G-T241M), the G-C haplotype was associated with a significantly decreased risk of OPL (OR=0.40, 95% CI 0.23–0.68). Moreover, compared with individuals without the G-C haplotype, the ORs were 1.04 (0.56–1.95) and 0.20 (0.08–0.51) for subjects with one copy and two copies of the G-C haplotype, respectively (P for trend=0.005). Classification and regression tree (CART) analysis further revealed potential high-order gene-gene and gene-environmental interactions and categorized subjects into different risk groups according to their specific polymorphic signatures. Overall, our study provides the first epidemiological evidence supporting a connection between DSB gene variants and OPL development. Our data also suggest that the effects of high-order interactions should be taken into consideration when evaluating OPL predisposition.
Keywords: Double-strand break, Polymorphism, Haplotype, Oral premalignant lesion
INTRODUCTION
Cancers of the oral cavity accounted for 274,000 new cases in 2002 worldwide (1). Western and southern Europe areas have the highest incidence of oral cancer in men (1). There was a steady increase in the mortality rate of oral cancer in most European countries and United States from 1950’s to 1980’s, although a trend of decline was observed after late 1980’s, mostly due to the reduced tobacco and alcohol exposures (2). However, this recent decline in mortality rate in Europe was not homogeneous, as evidenced by the persistent rising number of oral cancer-related deaths in some central and eastern European countries such as Hungary, Slovakia, and Russia (2). Most oral cancers are preceded by the development of oral premalignant lesions (OPLs), which mainly include oral leukoplakia and erythroplakia (3). The rate at which OPLs transform into oral cancers varied worldwide, dependent upon the geographical location and length of follow-up (4, 5). A prospective follow-up study of a hospital-based population of 166 patients with OPLs from the Netherland reported a 2.9% annual malignant transformation rate (6). Both genetic and environmental factors contribute to OPL development. Epidemiological studies have identified environmental risk factors such as tobacco smoking, tobacco chewing, alcohol drinking, and virus infection (3, 7, 8). However, the genetic components responsible for OPL formation are largely unknown.
DNA repair pathways are important in the defense against genetic insults, the maintenance of genome integrity, and the prevention of tumorigenesis. Of the four major DNA repair pathways in mammals, the double-strand break (DSB) repair pathway is mainly involved in the repair of DSBs arising during many pivotal cellular processes such as DNA replication, meiosis, telomere metabolism, and V(D)J recombination (9). Exogenous challenges, such as ionizing radiation and certain chemotherapy drugs, can also induce DSBs. DSBs are sensed by the MRN (MRE11, RAD50, and NBS1) complex, after which the signals are transmitted to the ATM protein. One of two distinct mechanisms may next be induced, depending on the source of the DSB damage—the homologous recombination (HR) or the non-homologous end joining (NHEJ) pathway. The HR pathway repairs DSBs with high fidelity, as it uses one sister DNA strand as the repair template. The direct interaction of RAD51 and RAD52 proteins is an essential step in the HR pathway, and this interaction is facilitated by other important proteins such as BRCA1, BRCA2, XRCC2, and XRCC3 (10, 11). In the NHEJ pathway, the heterodimer of KU70 and KU80 binds to the end of DSBs and activates the DNA-dependent protein kinase catalytic subunit—DNAPKcs. LIG4 and XRCC4 proteins are then recruited to ligate and fill the gap in the broken DNA strands (12). Deficiencies in these DSB repair genes have been extensively reported to be associated with high incidence of chromosome aberrations, elevated radiation sensitivity, and tumorigenesis (13–16).
DNA polymorphisms of DSB pathway genes have also been found to be significantly associated with the etiology of many malignancies, including breast, lung, skin, and epithelial ovarian cancers (17–22). We previously reported our finding of functional variants in the nucleotide excision repair genes that affect OPL risk individually and jointly (23). We have also found that a single nucleotide polymorphism (SNP) influencing the splicing of CCND1, a key regulator of mammalian cell cycle progression, is associated with a significantly altered smoking-dependent risk of OPL (24). However, to the best of our knowledge, no studies have been done that examine an association of DSB gene variants with the risk of OPL. In this study, we conducted a case-control analysis to assess the individual and joint effects on OPL predisposition of 10 potential functional SNPs in nine DSB genes. We further evaluated the high-order gene-gene and gene-environment interactions between these factors using Classification and Regression Tree (CART) methodology.
MATERIALS AND METHODS
Study population
A total of 147 OPL patients were identified at the University of Texas M.D. Anderson Cancer Center for the years 1997 to 2006. All cases were histologically confirmed by the finding of leukoplakia and/or erythroplakia. Patients less than 18 years old and patients with a prior history of cancer (except non-melanoma skin cancer) that had been treated within the preceding two years were excluded. Patients with acute intercurrent illnesses or infections were also excluded. A self-administered questionnaire was used to gather epidemiological data from the patients. Healthy controls (N = 147) were identified from a control database used for ongoing case-control studies. The controls in our study were recruited in collaboration with Kelsey–Seybold Clinic, the largest multi-specialty medical organization in Houston, Texas. The controls were identified by reviewing short survey forms distributed to individuals coming to Kelsey-Seybold Clinic for annual health check-ups or for addressing health concerns. A Kelsey-Seybold staff member provided the form to each potential control subject during clinical registration. They were subsequently contacted by telephone at a later date to confirm their willingness to participate and to schedule an interview appointment at a Kelsey-Seybold clinic convenient to the participant. On the day of the interview, the controls visited the clinic specifically for the purpose of participating in this study but not for any treatment purposes. Controls had no cancer history (except non-melanoma skin cancer) and were matched to cases with regard to age (± 5 yr), gender, and ethnicity (Caucasian, African-American, and Hispanic). This control selection strategy has been well described and proved to be feasible and effective for molecular epidemiological studies when population-based control selection poses a practical challenge (25). Epidemiological questionnaire data were obtained through in-person interviews. After the interview, 40 ml of blood was collected from each participant into a heparinized tube and sent to the lab for immediate molecular analysis. Approval for the use of human subjects was obtained from the institutional review boards of both M. D. Anderson and Kelsey-Seybold. Informed consent was also obtained from each participant.
Selection of DSB polymorphisms and genotyping
A total of 10 potential functional SNPs in nine DSB genes were genotyped in this study. All polymorphisms have been reported to affect either the expression or function of their host genes or to be associated with the risk of malignancy (26–34). These SNPs consisted of six nonsynonymous SNPs (nsSNPs) with an amino acid substitution (ATM D1853N, NBS1 E185Q, BRCA2 N372H, XRCC3 T241M, RAG1 K820R, and LIG4 T91I), one intronic SNP (XRCC3 A17893G), a splicing site SNP (XRCC4 IV7-1), one SNP in the 3’ untranslated region (UTR) of KU80 gene, and one SNP in the 3’ region of XRCC2. Genotyping was performed using a 5’nuclease assay-based TaqMan assay. Probes and primers for the genotyping were either acquired from the SNP500Cancer database or designed using PrimerExpress 2.0 software (Applied Biosystems, Foster, CA) and were available upon request. The probes were labeled fluorescently with either 6-FAM or VIC on the 5’ end and a nonfluorescent minor groove binder quencher on the 3’ end. The genotyping procedure was done exactly as described in a previous study (35). Briefly, genomic DNA was extracted from peripheral blood lymphocytes using the Human Whole Blood Genomic DNA Extraction Kit (Qiagen, Valencia, CA). The PCR amplification mix (5 µl) included sample DNA (5 ng), 1 X TaqMan buffer A, 200 µM deoxynucleotide triphosphates, 5 mM MgCl2, 0.65 units of AmpliTaq Gold, 900 nM each primer, and 200 nM each probe. The PCR conditions consisted of one cycle for 10 min at 95 °C and 40 cycles for 15 sec at 95 °C and 1 min at 60 °C. PCR was performed using the ABI PRISM® 7900HT sequence detection system (Applied Biosystems). SDS 2.1 software (Applied Biosystems) was used to analyze the end-point genotyping data. Internal quality controls and negative controls were used to ensure genotyping accuracy. Laboratory personnel performing the genotyping were blinded to the case-control status.
Statistical analysis
Statistical analyses were performed using the Intercooled Stata 8.0 statistical software package (Stata Corp., College Station, TX). χ2 and student’s t tests were used to assess patient characteristics. The Hardy-Weinberg equilibrium (HWE) was tested using a goodness-of-fit χ2 test. The OPL risks were estimated as odds ratios (OR) and 95% confidence intervals (95% CI) using multivariate logistic regression adjusted by age, gender, ethnicity, smoking status, and alcohol consumption, where appropriate. To account for the use of multiple comparisons, the Bonferroni correction was used to adjust P-values while setting the family wise significance level at 0.05. Definition of smoking status was as previously described (24). Never drinkers are defined as those who never consumed alcohol or consumed less than or equal to one drink per month. One drink is defined as one bottle or can (12 ounce) of beer, one medium glass of wine (4 ounce), one mixed drink, or one straight shot. Ever drinkers are those who consumed more than one drink per month. Haplotype frequencies were estimated using the Expectation-Maximization algorithm implemented in the HelixTree program (Golden Helix Inc., Bozeman, MT). The adjusted OR and 95% CI for each haplotype were assessed using multivariate logistic regression. High-order gene-gene and gene-environment interactions were evaluated using CART analysis implemented in the HelixTree software. CART is a non-parametric decision tree-based data mining approach that can identify specific combinations of contributing factors associated with disease risk. The recursive-partitioning algorithm uses a statistical hypothesis-testing method — formal inference-based recursive modeling — to determine the first locally optimal split and each subsequent split of the data set, with multiplicity-adjusted P values generated to control tree growth (P<0.05). This process continues until the terminal nodes have no subsequent statistically significant splits or the terminal nodes reach a pre-specified minimum size. Subgroups of individuals with differential risk patterns were then identified in the different order of nodes of the tree structure, indicating the presence of gene-gene and gene-environment interactions. Logistic regression was used to calculate the OR and 95% CI in each terminal node of the tree. P ≤0.05 was considered as the threshold of significance in this study. All statistical analyses were two-sided.
RESULTS
Characteristics of the study population
Table 1 lists the selected characteristics of the study population. There were 147 OPL patients and 147 cancer-free controls adequately matched on age (cases versus controls [mean±standard deviation]: 57.5±13.6 years versus 59.1±11.0 years, P=0.26), gender (P=1.00), and ethnicity (P=1.00). As expected, significant differences in smoking status existed between cases and controls. The percentage of never and ever smokers was 31.3% and 68.7%, respectively, in the cases, and 55.1% and 44.9%, respectively, in the controls (P<0.001). Higher percentage of subjects in controls were ever drinkers compared to cases (Never drinkers versus ever drinkers: 46.3% versus 53.7% in cases; 32.0% versus 68.0% in controls; P=0.01).
Table 1.
Distribution of Selected Host Characteristics by Case–Control Status
| Variable | Case (n=147) | Control (n=147) | P valuea |
|---|---|---|---|
| Age, Mean(SDb) | 57.5 (13.6) | 59.1 (11.0) | 0.26 |
| Gender, n (%) | |||
| Male | 82 (55.8) | 82 (55.8) | |
| Female | 65 (44.2) | 65 (44.2) | 1.00 |
| Ethnicity, n (%) | |||
| Caucasian | 129 (87.8) | 129 (87.8) | |
| Hispanic | 11 (7.4) | 11 (7.4) | |
| African American | 7 (4.8) | 7 (4.8) | 1.00 |
| Smoking status, n (%) | |||
| Never | 46 (31.3) | 81 (55.1) | |
| Ever | 101 (68.7) | 66 (44.9) | <0.001 |
| Alcohol consumption, n (%) | |||
| Never | 68 (46.3) | 47 (32.0) | |
| Ever | 79 (53.7) | 100 (68.0) | 0.01 |
P values were derived from the χ2 test for categorical variables (gender, ethnicity, and smoking status) and t test for continuous variable (age)
SD, standard deviation
Individual DSB polymorphisms and OPL risk
Table 2 summarizes the name, reference number, genetic position, and genotype distribution of each SNP and its association with OPL risk. None of the 10 SNPs departed from the HWE among controls, indicating that the chance of selection bias or genotyping errors was small. The most notable finding was an intronic SNP in the XRCC3 gene (A17893G), which showed a protective effect on OPL risk. Compared with the wild-type genotype (AA), the heterozygous genotype (AG) was associated with a non-significantly reduced OPL risk (OR=0.85, 95% CI 0.49–1.48, P = 0.57) (remained significant after Bonferroni adjustment for multiple comparisons) while the homozygous variant genotype (GG) was associated with a significantly decreased risk (OR=0.18, 95% CI 0.07–0.47) and a significant gene-dosage effect (P for trend=0.002). The risk of the variant-containing genotypes (AG plus GG) was 0.63 (95% CI 0.38–1.07) compared with the risk associated with the homozygous wild-type genotype, which showed a borderline statistical significance (P=0.09). In an exploratory analysis, we further stratified the risk associations of XRCC3 A17893G with various host characteristics. This showed that the reduction in risk was only significant in males (OR=0.13, 95% CI 0.03–0.52, P=0.004) (remained significant after Bonferroni adjustment for multiple comparisons) and in ever smokers (OR=0.22, 95% CI 0.07–0.69, P=0.01) (Table 3). The protective effect of the homozygous variant genotype were significant in both young subjects (OR=0.18, 95% CI 0.04–0.73, P=0.02) and old subjects (OR=0.14, 95% CI 0.02–0.78, P=0.02), and in both ever drinkers (OR=0.12, 95% CI 0.02–0.63, P=0.01) and never drinkers (OR=0.06, 95% CI 0.11–0.33, P=0.001) (remained significant after Bonferroni adjustment for multiple comparisons) (Table 3). No significant associations with OPL risk were identified for the other SNPs examined in this study (Table 2).
Table 2.
Case-Control Distribution of Genotypes of DSB Polymorphisms.
| Polymorphism | Position | Genotype | Control/Case (N) | OR (95% CI)a | P value | HWEb |
|---|---|---|---|---|---|---|
| (rs number) | P value | |||||
| ATM D1853N | nsSNPc | GG | 114/102 | Reference | 0.41 | |
| rs1801516 | GA | 28/30 | 1.40 (0.74–2.62) | 0.30 | ||
| AA | 3/5 | 2.85 (0.62–13.11) | 0.18 | |||
| GA/AA | 33/35 | 1.52 (0.84–2.78) | 0.17 | |||
| P trend=0.11 | ||||||
| NBS1 E185Q | nsSNP | CC | 67/67 | Reference | 0.45 | |
| rs1805794 | CG | 59/59 | 1.06 (0.62–1.81) | 0.83 | ||
| GG | 18/11 | 0.54 (0.22–1.30) | 0.17 | |||
| CG/GG | 77/70 | 0.93 (0.56–1.54) | 0.77 | |||
| P trend=0.36 | ||||||
| BRCA2 N372H | nsSNP | TT | 76/70 | Reference | 0.53 | |
| rs144848 | TG | 54/58 | 1.01 (0.59–1.72) | 0.98 | ||
| GG | 13/8 | 0.64 (0.23–1.80) | 0.40 | |||
| TG/GG | 67/66 | 0.94 (0.56–1.58) | 0.82 | |||
| P trend=0.59 | ||||||
| XRCC2 C41657T | 3’ region | CC | 131/119 | Reference | 1.00 | |
| rs718282 | CT | 11/16 | 1.59 (0.67–3.77) | 0.29 | ||
| TT | 0/1 | NAd | NA | |||
| CT/TT | 11/17 | 1.01 (0.59–1.71) | 0.98 | |||
| P trend=0.16 | ||||||
| XRCC3 T241M | nsSNP | CC | 66/63 | Reference | 1.00 | |
| rs861539 | CT | 65/63 | 1.15 (0.68–1.95) | 0.60 | ||
| TT | 16/21 | 1.41 (0.64–3.11) | 0.40 | |||
| CT/TT | 81/84 | 1.21 (0.73–1.98) | 0.46 | |||
| P trend=0.38 | ||||||
| XRCC3 A17893G | Intron | AA | 54/63 | Reference | 0.30 | |
| rs1799796 | AG | 63/66 | 0.85 (0.49–1.48) | 0.57 | ||
| GG | 27/7 | 0.18 (0.07–0.47) | 0.0005e | |||
| AG/GG | 90/73 | 0.63 (0.38–1.07) | 0.09 | |||
| P trend=0.002 | ||||||
| RAG1 K820R | nsSNP | AA | 116/107 | Reference | 0.09 | |
| rs2227973 | AG | 25/28 | 1.13 (0.59–2.14) | 0.72 | ||
| GG | 4/2 | 0.47 (0.08–2.82) | 0.41 | |||
| AG/GG | 29/30 | 1.03 (0.56–1.91) | 0.92 | |||
| P trend=0.85 | ||||||
| XRCC4 IV7-1G>A | splicing site | GG | 109/98 | Reference | 0.09 | |
| rs1805377 | GA | 31/32 | 1.13 (0.61–2.06) | 0.70 | ||
| AA | 6/5 | 0.61 (0.14–2.64) | 0.51 | |||
| GA/AA | 37/37 | 1.05 (0.59–1.88) | 0.87 | |||
| P trend=0.92 | ||||||
| KU80 Exon21+466A>G | 3’ UTR | AA | 108/103 | Reference | 0.17 | |
| rs1051685 | AG | 31/31 | 1.13 (0.62–2.09) | 0.69 | ||
| GG | 5/3 | 0.87 (0.19–4.12) | 0.86 | |||
| AG/GG | 36/34 | 1.10 (0.61–1.98) | 0.74 | |||
| P trend=0.84 | ||||||
| LIG4 T91I | nsSNP | CC | 93/93 | Reference | 0.29 | |
| rs1805388 | CT | 49/40 | 0.74 (0.43–1.29) | 0.29 | ||
| TT | 3/3 | 1.34 (0.23–7.69) | 0.74 | |||
| CT/TT | 52/43 | 0.77 (0.45–1.32) | 0.34 | |||
| P trend=0.46 |
Adjusted by age, gender, ethnicity, smoking status, and alcohol consumption
HWE: Hardy-Weinberg equilibrium
nsSNP: nonsynonymous SNP
NA, not available
Remained significant after Bonferroni adjustment for multiple comparisons
Table 3.
Association of XRCC3 A17893G Polymorphism with OPL Risk Stratified by Host Characteristics
| Host Characteristic | Control/Case | OR (95% CI)aP value | Control/Case | OR (95% CI) P value |
|---|---|---|---|---|
| Ageb | Old | Young | ||
| AA | 22/23 | Reference | 32/40 | Reference |
| AG | 29/24 | 0.96 (0.38–2.40) P=0.93 | 34/42 | 0.93 (0.45–1.90) P=0.84 |
| GG | 14/4 | 0.14 (0.02–0.78) P=0.02 | 13/3 | 0.18 (0.04–0.73) P=0.02 |
| AG/GG | 43/28 | 0.66 (0.28–1.58) P=0.35 | 47/45 | 0.71 (0.36–1.41) P=0.33 |
| Gender | Female | Male | ||
| AA | 29/29 | Reference | 25/34 | Reference |
| AG | 25/25 | 1.02 (0.45–2.35) P=0.96 | 38/41 | 0.75 (0.34–1.64) P=0.47 |
| GG | 10/3 | 0.28 (0.06–1.30) P=0.10 | 17/4 | 0.13 (0.03–0.52) P=0.004c |
| AG/GG | 35/28 | 0.82 (0.37–1.81) P=0.62 | 55/45 | 0.56 (0.26–1.18) P=0.13 |
| Smoking status | Never | Ever | ||
| AA | 30/21 | Reference | 24/42 | Reference |
| AG | 33/22 | 0.93 (0.40–2.13) P=0.86 | 30/44 | 0.66 (0.30–1.42) P=0.28 |
| GG | 15/0 | NAd | 12/7 | 0.22 (0.07–0.69) P=0.01 |
| AG/GG | 48/22 | 0.64 (0.29–1.44) P=0.28 | 42/51 | 0.52 (0.25–1.08) P=0.08 |
| Alcohol consumption | Never | Ever | ||
| AA | 16/32 | Reference | 38/31 | Reference |
| AG | 18/26 | 0.39 (0.13–1.19) P=0.10 | 45/40 | 1.17 (0.57–2.43) P=0.67 |
| GG | 12/4 | 0.06 (0.11–0.33) P=0.001c | 15/3 | 0.12 (0.02–0.63) P=0.01 |
| AG/GG | 30/30 | 0.26 (0.09–0.75) P=0.01 | 60/43 | 0.90 (0.45–1.81) P=0.76 |
Adjusted for age, gender, ethnicity, smoking status, and alcohol consumption, where appropriate
Used median age in controls as cut-off point
Remained significant after Bonferroni adjustment for multiple comparisons
NA, not available
Associations of XRCC3 haplotypes with OPL risk
Table 4 shows the associations of OPL risk with XRCC3 haplotypes constructed in the order of A17893G-T241M. The A-C haplotype, which accounts for 38.9% and 46.3% of all haplotypes in cases and controls, respectively, was used as the referent. The G-C haplotype containing the wild-type allele of T241M and the variant allele of A17893G was associated with a significantly decreased OPL risk (OR=0.40, 95% CI 0.23–0.68, P=0.001) (remained significant after Bonferroni adjustment for multiple comparisons), which was consistent with the result from the single SNP analysis. The A-T haplotype was associated with a reduced OPL risk (OR=0.67, 95% CI 0.40–1.11, P=0.12) compared with the reference A-C haplotype. The global haplotype test indicated that the distribution pattern of the four haplotypes differed significantly between cases and controls (χ2=12.64, P3df=0.006).
Table 4.
Associations of XRCC3 Haplotypes with OPL Risk
| Haplotypea | Case, N (%) | Control, N (%) | OR (95% CI)b | P value |
|---|---|---|---|---|
| A-C | 112 (38.9%) | 126 (46.3%) | Reference | |
| G-C | 81 (28.1%) | 49 (18.0%) | 0.40 (0.23–0.68) | 0.001b |
| A-T | 59 (20.5%) | 66 (24.3%) | 0.67 (0.40–1.11) | 0.12 |
| G-T | 36 (12.5%) | 31 (11.4%) | 0.45 (0.20–1.01) | 0.05 |
| Global test P valuec | χ2=12.64, P3df=0.006 |
In the order of A17893G-T241M with nucleotide A and C as the common allele, respectively.
Remained significant after Bonferroni adjustment for multiple comparisons
Adjusted for age, gender, ethnicity, smoking status, and alcohol consumption
Combined XRCC3 diplotypes and OPL risk
To further investigate the effect of the G-C haplotype of XRCC3 on OPL risk, we performed a trend analysis using the study subjects without this haplotype as the reference group. A statistically significant trend toward a reduction in OPL risk was noted for subjects with increasing number of the G-C haplotypes. That is, the ORs were 1.04 (0.56–1.95, P=0.90) and 0.20 (0.08–0.51, P=0.001) for subjects with one copy and two copies of the G-C haplotype, respectively (P for trend=0.005).
CART analysis
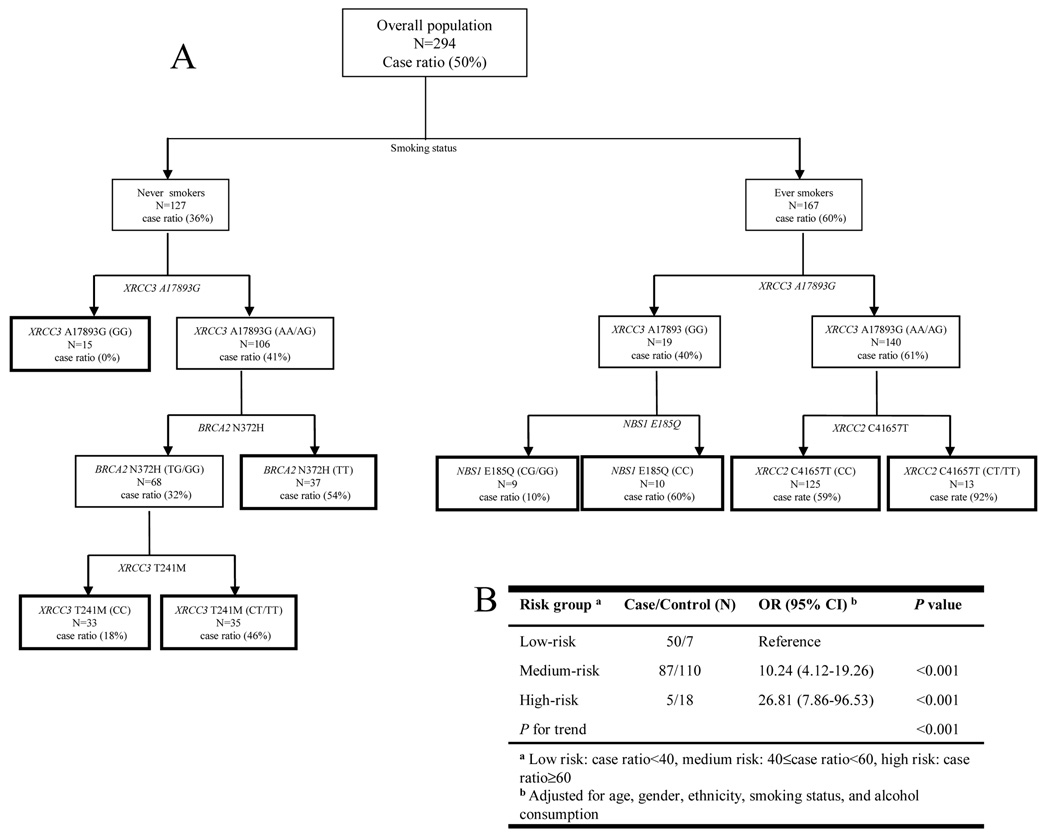
Figure 1A depicts the tree structure generated by the CART analysis, which included all investigated genetic variants and the smoking status variable. Smoking status was singled out in the first splitting node (well-matched variables such as age, gender, and ethnicity were excluded from the CART analysis), confirming that smoking is the predominant risk factor in OPL development. XRCC3 A17893G was important in the tree generation in both never and ever smokers. Never smokers with the XRCC3 A17893G GG genotype exhibited the lowest case ratio (no cases in the 15 subjects). For never smokers with the XRCC3 A17893 AA/AG genotypes, BRCA2 N372H and XRCC3 T241M were the determining factors for downstream tree structures. The case ratio for subjects with the BRCA2 N372H TG/GG-XRCC3 T241M CC, BRCA2 N372H TG/GG-XRCC3 T241M CT/TT, and BRCA2 N372H TT genotype combinations were 18%, 46%, and 54%, respectively. The key predictors downstream of XRCC3 A17893G SNP in ever smokers were NBS1 E185Q and XRCC2 C41657T. Individuals with the GG genotype of XRCC3 A17893G and the CG/GG genotypes of NBS1 E185Q had the lowest OPL risk, with a 10% case ratio. In comparison, subjects with the AA/AG genotypes of XRCC3 A17893G and the CT/TT genotypes of XRCC2 C41657T exhibited the highest OPL risk, with a 92% case ratio. Figure 1B shows the OR estimates generated for the three different risk groups determined on the basis of the case ratio of each CART terminal node. Compared with the low-risk group combining terminal nodes with a case ratio less than 40%, the medium-risk (case ratio between 40% and 60%) and high-risk group (case ratio more than 60%) were both associated with a significantly increased OPL risk, with ORs of 10.24 (95% CI 4.12–19.26) and 26.81 (95% CI 7.86–96.53), respectively (P for trend<0.001).
Figure 1.
(A) CART analysis of genetic variants in the DSB repair pathway and smoking in modulating OPL risk. (B) Combined analysis of the effects of genetic variants in the DSB repair pathway on OPL risk based on the results of the CART analysis.
DISCUSSION
Given that patients with OPLs are at a dramatically elevated risk of oral cancer, identification of subjects susceptible to OPL development is critical for oral cancer prevention. In this study, we assessed the effects of 10 potential functional polymorphisms in the DSB DNA repair pathway on OPL susceptibility. We found that the variant allele of the XRCC3 A17893G SNP exhibited a significant protective effect on OPL risk which remained robust after conservative Bonferroni adjustment for multiple comparisons. The effect was further confirmed in the haplotype and diplotype analyses of XRCC3. Remarkably, through the CART analysis we were also able to identify distinctive polymorphic signatures in the DSB repair pathway that differentiated the study participants into different risk subsets, indicating the potential presence of high-order gene-gene and gene-environment interactions.
XRCC3 is a key member of the RecA/Rad51-related protein family that is essential for HR DNA repair (9). The A17893G SNP is located in the intronic region, and its functional significance has not been evaluated. Nonetheless, multiple studies have shown an association between this SNP and cancer risk. For example, Kuschel and colleagues noted a significant protective effect of the variant allele of XRCC3 A17893G SNP against breast cancer in a UK population (30). This was later confirmed by two population-based studies, one conducted in the United States and the other in Poland (36). This variant allele has also been reported to confer a significantly decreased susceptibility to bladder (35), invasive ovarian (21), and upper aerodigestive (37) cancers. However, a few studies have shown no such association with cancer (37–40). Factors such as study design, sample size, cancer sites, population heterogeneity, and the biological complexity of low-penetrance cancer susceptibility genes may explain the observed discrepancies. In keeping with our results showing a protective role for the variant allele of XRCC3 A17893G in OPL development, a meta-analysis of a total of 24,795 cancer patients and 34,209 controls reported a significantly reduced cancer risk associated with this allele under a dominant genetic model with an OR of 0.92 (95% CI 0.87–0.96, P=0.0004) (41). Whether this intronic SNP has any functional impact on the splicing of the XRCC3 transcript needs to be further evaluated through the assessment of XRCC3 mRNA levels in subjects with different genotypes. Until that is done, we cannot rule out the possibility that the decrease in OPL risk is due to other variants that are in linkage disequilibrium with this SNP.
The T241M SNP, which is located in exon 8 of the XRCC3 gene, has been investigated in many studies, with conflicting results. For example, some studies have shown the variant allele to be associated with an increased risk for bladder, breast, and lung cancers (30, 35, 36, 40), and these results were in agreement with the results from various functional assays showing that the variant allele was associated with an increased DNA adduct level and compromised capacity to repair X-ray-induced chromosome aberrations (42, 43). In contrast, other groups found a lack of association of the T241M SNP with a risk for ovarian, skin, and endometrial cancers (20, 21, 39). The findings from these studies were supported by an in vitro study conducted by Araujo and colleagues, who showed that the XRCC3 T241M SNP did not affect intrinsic DSB repair capacity (44). In further contrast, in our study, the variant T allele was associated with a non-significantly increased OPL risk in the main analysis. However, the haplotype analysis comparing the A-T haplotype with the A-C haplotype (Table 4) showed that, in conjunction with the A17893G polymorphism, the variant allele of T241M might be associated with a reduced OPL risk. This indicates the presence of a potential interaction between these two variants. However, a statistical interaction analysis did not yield any significant finding (data not shown). Further studies using tagged SNPs performed in a larger study are warranted to provide additional insight into these observations.
OPL is a multifactorial and multistep disease with complex interactions among these factors. We therefore performed an exploratory CART analysis to further elucidate the high-order gene-gene and gene-environment interactions of the DSB repair pathway in OPL development, and the results were informative. For example, the XRCC3 A17893G SNP was identified to be the best factor for discriminating between cases and controls among both never and ever smokers, which is consistent with the finding from the main effect analysis. We then identified distinctive polymorphic signatures that grouped the subjects according to different risk levels. This latter finding indicates that the development of OPL involves complex genetic interactions and follows different pathways depending on the specific genetic background and environmental exposures of the subjects. By combining risk groups with comparable case ratios, we found a statistically significant dose effect on OPL risk in groups with different case ratios, suggesting a good discriminating ability of the CART analysis. However, it should be noted that in this study we focused on those well-established DSB genes and SNPs with reported functional roles in tumorigenesis. A collection based on an unbiased selection of all DSB-related genes/SNPs would be necessary to provide more in-depth insights. Furthermore, our results should also be interpreted with a certain amount of caution because of the post hoc data-mining approach and the limited number of subjects in the stratified analyses and some CART terminal nodes. Independent validations done in a larger study population may shed further light on the mechanisms underlying the complex high-order interactions.
We calculated the power of our study (45). We found that with our current sample size, assuming a conservative 0.09% prevalence of OPL in the general population (46), an additive genetic model and a two-sided significance level of 0.05, the power to detect an increased OR of 1.6 ranged from 60% to 80% given minor allele frequency from 0.15 to 0.45. The power to detect an increased OR of 1.8 was from 80% to 94% under the same model and the power to detect an increased OR of 2.0 was even higher, ranging from 91% to 98%. In addition, the major finding of our study on XRCC3 A17893G remained significant after the conservative Bonferroni correction for multiple comparisons. However, the possibility of chance finding cannot be completely ruled out and further larger epidemiologic and functional studies are needed to validate the results.
In summary, we used strict matching criteria to eliminate the potential confounding effects of age, gender, and ethnicity. In addition, we have complete information on smoking status and alcohol consumption data for each subject. This study presents the first epidemiological evidence supporting the associations between the genotypes and haplotypes of genetic variants in the DSB DNA repair pathway and OPL risk. The XRCC3 gene was identified as a possible culprit in OPL development. Furthermore, we demonstrated the presence of complex interactions between DSB gene variations and smoking that influenced OPL susceptibility.
Table 5.
Combined XRCC3 Diplotypes and OPL Risk
| Haplotype Paira | Case, N (%) | Control, N (%) | OR (95% CI)b | P value |
|---|---|---|---|---|
| Otherc : Other | 89 (61.8%) | 94 (69.1%) | Reference | |
| Other : G-C | 29 (20.1%) | 35 (25.7%) | 1.04 (0.56–1.95) | 0.90 |
| G-C : G-C | 26 (18.1%) | 7 (5.1%) | 0.20 (0.08–0.51) | 0.001 |
| P for trend | 0.005 |
In the order of A17893G-T241M
Adjusted for age, gender, ethnicity, smoking status, and alcohol consumption
A-C, A-T, or G-T haplotype
Acknowledgments
Supported by National Cancer Institute grants CA 106451 and CA 097007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
No authors of this study declared conflict of interest.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Lucchini F, Negri E, Levi F. Trends in oral cancer mortality in Europe. Oral Oncol. 2004;40(4):433–439. doi: 10.1016/j.oraloncology.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 4.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53(3):563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Lind PO. Malignant transformation in oral leukoplakia. Scand J Dent Res. 1987;95(6):449–455. doi: 10.1111/j.1600-0722.1987.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 6.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34(4):270–275. [PubMed] [Google Scholar]
- 7.Hashibe M, Sankaranarayanan R, Thomas G, Kuruvilla B, Mathew B, Somanathan T, et al. Alcohol drinking, body mass index and the risk of oral leukoplakia in an Indian population. Int J Cancer. 2000;88(1):129–134. [PubMed] [Google Scholar]
- 8.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29(8):779–792. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 9.Scott SP, Pandita TK. The cellular control of DNA double-strand breaks. J Cell Biochem. 2006;99(6):1463–1475. doi: 10.1002/jcb.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson JY, Tarsounas MC, Stasiak AZ, Stasiak A, Shah R, McIlwraith MJ, et al. Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev. 2001;15(24):3296–3307. doi: 10.1101/gad.947001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002;420(6913):287–293. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 12.Karran P. DNA double strand break repair in mammalian cells. Curr Opin Genet Dev. 2000;10(2):144–150. doi: 10.1016/s0959-437x(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 13.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13(20):2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8(25):1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LH, Schild D. Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutat Res. 2001;477(1–2):131–153. doi: 10.1016/s0027-5107(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 16.Deans B, Griffin CS, O'Regan P, Jasin M, Thacker J. Homologous recombination deficiency leads to profound genetic instability in cells derived from Xrcc2-knockout mice. Cancer Res. 2003;63(23):8181–8187. [PubMed] [Google Scholar]
- 17.Ralhan R, Kaur J, Kreienberg R, Wiesmuller L. Links between DNA double strand break repair and breast cancer: accumulating evidence from both familial and nonfamilial cases. Cancer Lett. 2007;248(1):1–17. doi: 10.1016/j.canlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, Stangeland L, et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis. 2006;27(3):560–567. doi: 10.1093/carcin/bgi232. [DOI] [PubMed] [Google Scholar]
- 19.Kadouri L, Kote-Jarai Z, Hubert A, Durocher F, Abeliovich D, Glaser B, et al. A single-nucleotide polymorphism in the RAD51 gene modifies breast cancer risk in BRCA2 carriers, but not in BRCA1 carriers or noncarriers. Br J Cancer. 2004;90(10):2002–2005. doi: 10.1038/sj.bjc.6601837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Colditz GA, Samson LD, Hunter DJ. Polymorphisms in DNA double-strand break repair genes and skin cancer risk. Cancer Res. 2004;64(9):3009–3013. doi: 10.1158/0008-5472.can-04-0246. [DOI] [PubMed] [Google Scholar]
- 21.Auranen A, Song H, Waterfall C, Dicioccio RA, Kuschel B, Kjaer SK, et al. Polymorphisms in DNA repair genes and epithelial ovarian cancer risk. Int J Cancer. 2005;117(4):611–618. doi: 10.1002/ijc.21047. [DOI] [PubMed] [Google Scholar]
- 22.Fu YP, Yu JC, Cheng TC, Lou MA, Hsu GC, Wu CY, et al. Breast cancer risk associated with genotypic polymorphism of the nonhomologous end-joining genes: a multigenic study on cancer susceptibility. Cancer Res. 2003;63(10):2440–2446. [PubMed] [Google Scholar]
- 23.Wang Y, Spitz MR, Lee JJ, Huang M, Lippman SM, Wu X. Nucleotide excision repair pathway genes and oral premalignant lesions. Clin Cancer Res. 2007;13(12):3753–3758. doi: 10.1158/1078-0432.CCR-06-1911. [DOI] [PubMed] [Google Scholar]
- 24.Huang M, Spitz MR, Gu J, Lee JJ, Lin J, Lippman SM, et al. Cyclin D1 gene polymorphism as a risk factor for oral premalignant lesions. Carcinogenesis. 2006;27(10):2034–2037. doi: 10.1093/carcin/bgl048. [DOI] [PubMed] [Google Scholar]
- 25.Hudmon KS, Honn SE, Jiang H, Chamberlain RM, Xiang W, Ferry G, et al. Identifying and recruiting healthy control subjects from a managed care organization: a methodology for molecular epidemiological case-control studies of cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):565–571. [PubMed] [Google Scholar]
- 26.Ryk C, Kumar R, Thirumaran RK, Hou SM. Polymorphisms in the DNA repair genes XRCC1, APEX1, XRCC3 and NBS1, and the risk for lung cancer in never- and ever-smokers. Lung Cancer. 2006;54(3):285–292. doi: 10.1016/j.lungcan.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Maillet P, Chappuis PO, Vaudan G, Dobbie Z, Muller H, Hutter P, et al. A polymorphism in the ATM gene modulates the penetrance of hereditary non-polyposis colorectal cancer. Int J Cancer. 2000;88(6):928–931. doi: 10.1002/1097-0215(20001215)88:6<928::aid-ijc14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Gu J, Grossman HB, Amos CI, Etzel C, Huang M, et al. Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am J Hum Genet. 2006;78(3):464–479. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roddam PL, Rollinson S, O'Driscoll M, Jeggo PA, Jack A, Morgan GJ. Genetic variants of NHEJ DNA ligase IV can affect the risk of developing multiple myeloma, a tumour characterised by aberrant class switch recombination. J Med Genet. 2002;39(12):900–905. doi: 10.1136/jmg.39.12.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuschel B, Auranen A, McBride S, Novik KL, Antoniou A, Lipscombe JM, et al. Variants in DNA double-strand break repair genes and breast cancer susceptibility. Hum Mol Genet. 2002;11(12):1399–1407. doi: 10.1093/hmg/11.12.1399. [DOI] [PubMed] [Google Scholar]
- 31.Figueroa JD, Malats N, Rothman N, Real FX, Silverman D, Kogevinas M, et al. Evaluation of genetic variation in the double-strand break repair pathway and bladder cancer risk. Carcinogenesis. 2007;28(8):1788–1793. doi: 10.1093/carcin/bgm132. [DOI] [PubMed] [Google Scholar]
- 32.Hayden PJ, Tewari P, Morris DW, Staines A, Crowley D, Nieters A, et al. Variation in DNA repair genes XRCC3, XRCC4, XRCC5 and susceptibility to myeloma. Hum Mol Genet. 2007;16(24):3117–3127. doi: 10.1093/hmg/ddm273. [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Dong XJ, Zhou RM, Guo W, Zhang XJ, Li Y. An investigation on the polymorphisms of two DNA repair genes and susceptibility to ESCC and GCA of high-incidence region in northern China. Mol Biol Rep. 2007 doi: 10.1007/s11033-007-9187-y. [DOI] [PubMed] [Google Scholar]
- 34.Palli D, Falchetti M, Masala G, Lupi R, Sera F, Saieva C, et al. Association between the BRCA2 N372H variant and male breast cancer risk: a population-based case-control study in Tuscany, Central Italy. BMC Cancer. 2007;7:170. doi: 10.1186/1471-2407-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matullo G, Guarrera S, Sacerdote C, Polidoro S, Davico L, Gamberini S, et al. Polymorphisms/haplotypes in DNA repair genes and smoking: a bladder cancer case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2569–2578. doi: 10.1158/1055-9965.EPI-05-0189. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Closas M, Egan KM, Newcomb PA, Brinton LA, Titus-Ernstoff L, Chanock S, et al. Polymorphisms in DNA double-strand break repair genes and risk of breast cancer: two population-based studies in USA and Poland, and meta-analyses. Hum Genet. 2006;119(4):376–388. doi: 10.1007/s00439-006-0135-z. [DOI] [PubMed] [Google Scholar]
- 37.Matullo G, Dunning AM, Guarrera S, Baynes C, Polidoro S, Garte S, et al. DNA repair polymorphisms and cancer risk in non-smokers in a cohort study. Carcinogenesis. 2006;27(5):997–1007. doi: 10.1093/carcin/bgi280. [DOI] [PubMed] [Google Scholar]
- 38.Han J, Hankinson SE, Ranu H, De Vivo I, Hunter DJ. Polymorphisms in DNA double-strand break repair genes and breast cancer risk in the Nurses' Health Study. Carcinogenesis. 2004;25(2):189–195. doi: 10.1093/carcin/bgh002. [DOI] [PubMed] [Google Scholar]
- 39.Han J, Hankinson SE, Hunter DJ, De Vivo I. Genetic variations in XRCC2 and XRCC3 are not associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13(2):330–331. doi: 10.1158/1055-9965.epi-03-0332. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen NR, Raaschou-Nielsen O, Nexo B, Wallin H, Overvad K, Tjonneland A, et al. XRCC3 polymorphisms and risk of lung cancer. Cancer Lett. 2004;213(1):67–72. doi: 10.1016/j.canlet.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Han S, Zhang HT, Wang Z, Xie Y, Tang R, Mao Y, et al. DNA repair gene XRCC3 polymorphisms and cancer risk: a meta-analysis of 48 case-control studies. Eur J Hum Genet. 2006;14(10):1136–1144. doi: 10.1038/sj.ejhg.5201681. [DOI] [PubMed] [Google Scholar]
- 42.Matullo G, Palli D, Peluso M, Guarrera S, Carturan S, Celentano E, et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001;22(9):1437–1445. doi: 10.1093/carcin/22.9.1437. [DOI] [PubMed] [Google Scholar]
- 43.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111(15):1843–1850. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araujo FD, Pierce AJ, Stark JM, Jasin M. Variant XRCC3 implicated in cancer is functional in homology-directed repair of double-strand breaks. Oncogene. 2002;21(26):4176–4180. doi: 10.1038/sj.onc.1205539. [DOI] [PubMed] [Google Scholar]
- 45.Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol. 2002;155(5):478–484. doi: 10.1093/aje/155.5.478. [DOI] [PubMed] [Google Scholar]
- 46.Hashibe M, Mathew B, Kuruvilla B, Thomas G, Sankaranarayanan R, Parkin DM, et al. Chewing tobacco, alcohol, and the risk of erythroplakia. Cancer Epidemiol Biomarkers Prev. 2000;9(7):639–645. [PubMed] [Google Scholar]