Abstract
The efficacy of fenofibrate (FEN), rosiglitazone (RSG), or a calorie-restricted diet (CRD) to reduce cardiovascular disease risk was compared in 37 overweight/obese, insulin resistant, nondiabetic individuals. Measurements were made of insulin sensitivity, fasting lipid and lipoprotein concentrations, and postprandial plasma glucose, insulin, free fatty acid, and triglyceride concentrations, before and after three months of treatment with FEN, RSG, or the CRD. Weight fell in the CRD group, but did not change significantly following treatment with either drug. Insulin sensitivity improved significantly in the CRD and RSG-treated groups, but to a greater extent in those given RSG, without a significant difference when comparing FEN treatment to the CRD. Total cholesterol concentrations were significantly lower following FEN and CRD. Fasting plasma triglyceride concentrations decreased significantly in FEN-treated and CRD groups, but postprandial concentrations decreased only in the FEN-treated subjects. Significant decreases in postprandial glucose and insulin concentrations were only seen in the RSG-treated and CRD groups. FEN administration improved the dyslipidemia in these subjects, without changing insulin sensitivity, whereas insulin sensitivity was enhanced in RSG-treated patients, without improvement in the dyslipidemia. Weight loss in the CRD group led to improvements in both insulin sensitivity and dyslipidemia, but the change in the former was less than in RSG-treated individuals, and the improvement in lipid metabolism not as great as with administration of FEN. In conclusion, there does not appear to be one therapeutic intervention that effectively treats all the metabolic abnormalities present in these patients at greatly increased risk of cardiovascular disease.
Keywords: dyslipidemia, insulin resistance, fenofibrate, rosiglitazone
Introduction
The report to be presented involved quantifying the effect on multiple cardiovascular disease (CVD) risk factors in overweight/obese, insulin resistant, dyslipidemic individuals, without known heart disease, randomized to treatment with either fenofibrate (FEN); a peroxisome proliferator-activated receptor (PPAR)-alpha agonist; rosiglitazone (RSG); a PPAR-gamma agonist, or a calorie-restricted diet (CRD).
Methods
The study sample consisted of 37 volunteers (17 women and 20 men), selected from 74 individuals who responded to newspaper advertisements describing our interest in evaluation of treatment approaches to prevent CVD risk in overweight, nondiabetic, insulin resistant individuals. In order to be eligible for this study volunteers had to be overweight, but in apparently good health, without known heart disease, and taking no medication known to affect carbohydrate or lipid metabolism.
Stanford University’s Human Subjects committee approved the study protocol, and all subjects gave written informed consent for participation in the study. Potential participants were screened at Stanford General Clinical Research Center (GCRC). Individuals with fasting glucose concentrations >125 mg/dL, hematocrit <32%, and abnormal alanine amino transferase concentrations were excluded from the study. Subjects with a body mass index (BMI) ≥ 25.0 and ≤ 38.0 kg/m2, a normal physical examination, and a plasma triglyceride/high-density lipoprotein cholesterol concentration ratio ≥3.0, a screening test for the presence of insulin resistance1, were admitted to the GCRC to undergo an insulin suppression test to see if they were sufficiently insulin resistant to enter the treatment portion of the study. For this purpose, insulin-mediated glucose uptake was quantified by a modified version2 of the insulin suppression test as described and validated by our research group3,4. Following an overnight fast, an intravenous catheter was placed in each arm, one for the simultaneous 3-hour infusion of octreotide (0.27 µg/m2/minute), insulin (32 mU/m2/minute), and glucose (267 mg/m2/minute), and the other for drawing blood. Plasma glucose and insulin concentrations were measured every 10 minutes during the 150 to 180-minute time period, and averaged to determine the steady-state plasma glucose (SSPG) and insulin (SSPI) concentrations. Since SSPI concentrations are comparable in all individuals, and glucose infusion is identical, the SSPG concentration is a direct measure of insulin-mediated glucose uptake i.e., the higher the SSPG concentration, the more insulin resistant the individual. Measurements of insulin-mediated glucose uptake with the insulin suppression test have been shown to be highly correlated (r-value >0.9) to those obtained via the hyperinsulinemic, euglycemic clamp method4. Insulin suppression test was performed on 74 volunteers. Of these volunteers, 52 had SSPG concentrations in the upper 50% of a previously studied group of 490 normal individuals5; therefore, they were operationally defined as being insulin resistant. Of the 52 insulin resistant individuals, 5 declined to participate further, and the remaining 47 were randomized.
Baseline measurements were made of daylong plasma glucose, insulin, free fatty acid (FFA), and triglyceride (TG) concentrations before and after test breakfast and lunch meals6,7. Each meal contained (as percent of daily calories) 15% protein, 42% carbohydrate, and 43% fat. Breakfast was given at 8:00 AM (20% of estimated daily caloric intake), and lunch was given at noon (40% of estimated daily caloric intake). Blood was drawn at hourly intervals before and after the test meals from 8 AM to 4 PM. In addition, on the mornings of the insulin suppression test and the daylong meal profile, fasting blood was drawn for measurement of plasma lipid and lipoprotein concentrations by the core clinical laboratory of Stanford Medical Center. After baseline studies were completed, subjects were randomized to treatment with FEN, RSG, or the CRD. FEN was given at a dose of 160 mg once daily for 12 weeks, while RSG was given at a dose of 4 mg once daily for 4 weeks, followed by 4 mg twice daily for 8 weeks. Subjects in the FEN and RSG groups were instructed to maintain their usual diet and were seen bimonthly. On each visit, a member of the research team interviewed the participants and checked medication compliance. Alanine aminotransferase levels were checked every 4 weeks during the study. After 3 months of treatment, all baseline measurements were repeated. Individuals randomized to the CRD arm were seen by a registered dietitian and were instructed on a calorie-restricted diet to lead to a weight loss of 0.5 kg per week. The period of weight loss was 3 months, during which subjects were seen weekly to be weighed and receive dietary advice. At the completion of the weight loss phase, subjects were instructed on a weight maintenance diet. After 2 weeks of stable weight, all baseline measurements were repeated. International Physical Activity Questionnaire8 was used to quantify the amount of habitual physical activity. All study participants were instructed to maintain their baseline physical activity level for 12 weeks. At the completion of the study, the reported physical activity level of the subjects was not significantly different from the baseline.
SPSS version 15.0 (SPSS Point Richmond, CA, USA) was used for data analyses. Summary data are expressed as mean ± SD or number of subjects. Plasma insulin and TG concentrations were log-transformed to improve normality for statistical analyses. Among the three treatment groups, differences in gender and race distribution were compared by Fisher’s exact test, and means of baseline variables were compared by one-way analysis of variance (ANOVA). Daylong glucose, insulin, FFA, and TG responses to meals were evaluated by calculating their area-under-the-curve (AUC) using the trapezoidal method. Within each group, before and after metabolic variables were compared by Student’s paired t test. Treatment-associated changes (post minus baseline) among the three groups were compared by one-way ANOVA and Tukey’s pairwise comparison test.
Results
Ten of the 47 patients randomized did not finish the study; 7 dropped-out within a few weeks for unclear reasons (4 assigned to FEN and 3 to weight loss), whereas the 3 patients in the FEN group who developed alanine aminotransferase levels >1.5-times the upper limit of normal were withdrawn. Baseline demographic and metabolic characteristics of the 37 subjects who completed the study are shown in Table 1. There were no significant differences in the age and gender distribution of the three groups, and the majority of subjects were Caucasian. The BMI values were elevated, but there were no significant differences between the three groups in either BMI or waist circumference. Finally, there were no differences in fasting plasma glucose or insulin concentrations.
Table 1.
Baseline Characteristics (mean ± SD and range) of the Experimental Groups
| Variable | Fenofibrate (n=12) | Rosiglitazone (n=12) | Calorie-Restricted Diet (n=13) | p |
|---|---|---|---|---|
| Age (years) | 55 ± 7 (38–62) |
52 ± 6 (39–64) |
53 ± 7 (39–61) |
0.71 |
| Men | 9 (75%) | 7 (58%) | 7 (54%) | 0.58 |
| Caucasian | 8 (67%) | 8 (67%) | 9 (69%) | 1.0 |
| Body mass index (kg/m2) | 32.8 ± 3.6 (28.3–38.0) |
30.4 ± 3.2 (26.1–36.1) |
30.8 ± 3.2 (25.7–36.7) |
0.19 |
| Waist circumference (cm) | 109 ± 9 (101–122) |
105 ± 14 (85–134) |
102 ± 10 (84–112) |
0.41 |
| Fasting glucose (mg/dl) | 97 ± 7 (85–111) |
99 ± 12 (75–119) |
103 ± 10 (91–124) |
0.27 |
| Fasting insulin (µU/ml) | 10.8 ± 4.7 (6.2–22.4) |
9.6 ± 4.9 (3.8–18.7) |
9.4 ± 2.3 (6.8–15.2) |
0.58 |
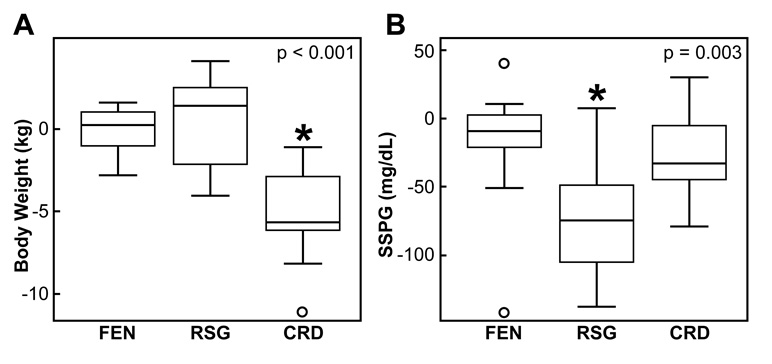
Weight did not change significantly in either the FEN- or RSG-treated groups, whereas it fell by a mean of 5.3 kg in the CRD group (p<0.001). There was no change in the SSPG concentration in the FEN-treated group, whereas SSPG concentrations fell significantly in the RSG-treated (p<0.001) and CRD (p<0.01) groups. The changes in these three groups are compared in Figure 1A and 1B, where box-and-whisker plots depict treatment-associated changes in weight and SSPG concentration among the three treatment groups. In each plot, the box extends from the 25th to the 75th percentile, and the solid horizontal line represents the 50th percentile (median). The whiskers extend from the ends of the box to the smallest and the largest values within 1.5-times interquartile range distance, and values falling outside the whiskers (outliers) are shown by open circles. More weight was lost in the CRD group than in those treated with FEN (p<0.001) or RSG (p<0.001). The change in weight in the RSG-treated group was not statistically different from that in the FEN-treated group (p=0.79). Although SSPG concentrations fell in all three groups, the decrease in the RSG-treated group was significantly greater as compared to the other two groups (p≤0.02), and there was no significant difference in the improvement in SSPG concentration between the FEN-treated and CRD groups (p=0.76).
Figure 1.
Comparison of treatment-associated changes (post minus baseline) in body weight and SSPG concentrations among the fenofibrate (FEN), rosiglitazone (RSG), and calorie-restricted diet (CRD) groups.
p value indicates significance of difference among the 3 groups by one-way ANOVA. *Tukey’s pairwise comparisons: A) CRD vs. RSG (p<0.001) and CRD vs. FEN (p<0.001); and B) RSG vs. FEN (p=0.004) and RSG vs. CRD (p=0.02).
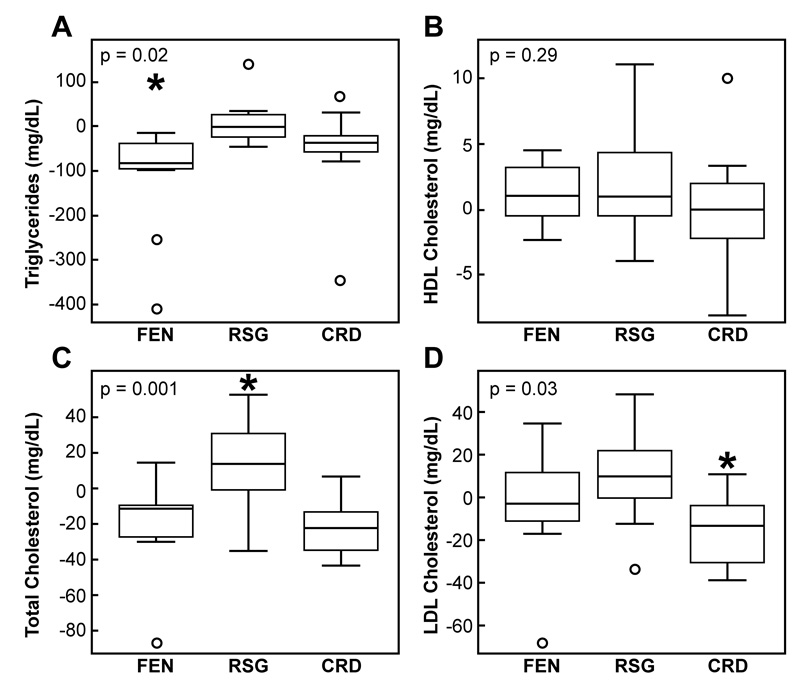
Changes in fasting plasma lipid and lipoprotein concentrations are shown in Table 2. Plasma TG concentrations decreased significantly in both the FEN- and CRD-treated groups. High-density lipoprotein cholesterol (HDL-C) concentrations were somewhat higher on average in the FEN and CRD-treated groups, but these increases were not statistically significant. Total cholesterol was significantly lower following both FEN and the CRD. Low-density lipoprotein cholesterol (LDL-C) concentration was also lower in the FEN-treated and CRD groups, but the decrease was only significant in the CRD group. In contrast, it should be noted that both total and LDL-C concentrations increased somewhat, albeit not significantly, in RSG-treated individuals.
Table 2.
Treatment-associated Changes (mean ± SD) in Fasting Lipid and Lipoprotein Concentrations Within Each Experimental Group
| Variable | Fenofibrate (n=12) | Rosiglitazone (n=12) | Calorie-Restricted Diet (n=13) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | |
| Triglycerides (mg/dl) | 231 ± 117 | 140 ± 47 | 0.001 | 209 ± 67 | 232 ± 72 | 0.63 | 201 ± 121 | 143 ± 49 | 0.01 |
| High-density lipoprotein cholesterol (mg/dl) | 35 ± 7 | 36 ± 8 | 0.10 | 40 ± 11 | 42 ± 13 | 0.10 | 38 ± 9 | 38 ± 7 | 0.91 |
| Total cholesterol (mg/dl) | 191 ± 33 | 173 ± 23 | 0.03 | 203 ± 43 | 217 ± 41 | 0.07 | 216 ± 39 | 194 ± 44 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dl) | 114 ± 34 | 111 ± 22 | 0.72 | 119 ± 41 | 129 ± 37 | 0.18 | 144 ± 34 | 128 ± 34 | 0.01 |
Within each experimental group, before and after means were compared by Student’s paired t-test.
Box-and-whisker plots, similar to those shown in Figure 1, compare the effect of the three treatments in Figure 2. These data indicate that fasting TG (2A) concentrations improved the most in the FEN-treated group and were significantly lower as compared to the RSG-treated group (p=0.01), whereas the triglyceride levels in the CRD group did not significantly differ from those treated with FEN (p=0.26) or RSG (p=0.29). HDL-C concentrations (2B) were not significantly different among the three groups after treatment. Total cholesterol levels (2C) actually significantly increased in the RSG-treated individuals as compared to the other two groups (p≤0.03), and the fall in cholesterol levels in the FEN-treated group was not significantly different from that in the CRD group (p=0.93). The only significant difference in the treatment-associated change in LDL-C concentrations (2D) among the three groups was a decrease in the CRD group as compared to those treated with RSG (p=0.03).
Figure 2.
Comparison of treatment-associated changes (post minus baseline) in fasting lipid and lipoprotein concentrations among the fenofibrate (FEN), rosiglitazone (RSG), or a calorie-restricted diet (CRD) groups.
p value indicates significance of difference among the 3 groups by one-way ANOVA. *Tukey’s pairwise comparisons: A) FEN vs. RSG (p=0.01); C) RSG vs. FEN (p=0.003) and RSG vs. CRD (p=0.001); and D) CRD vs. RSG (p=0.03).
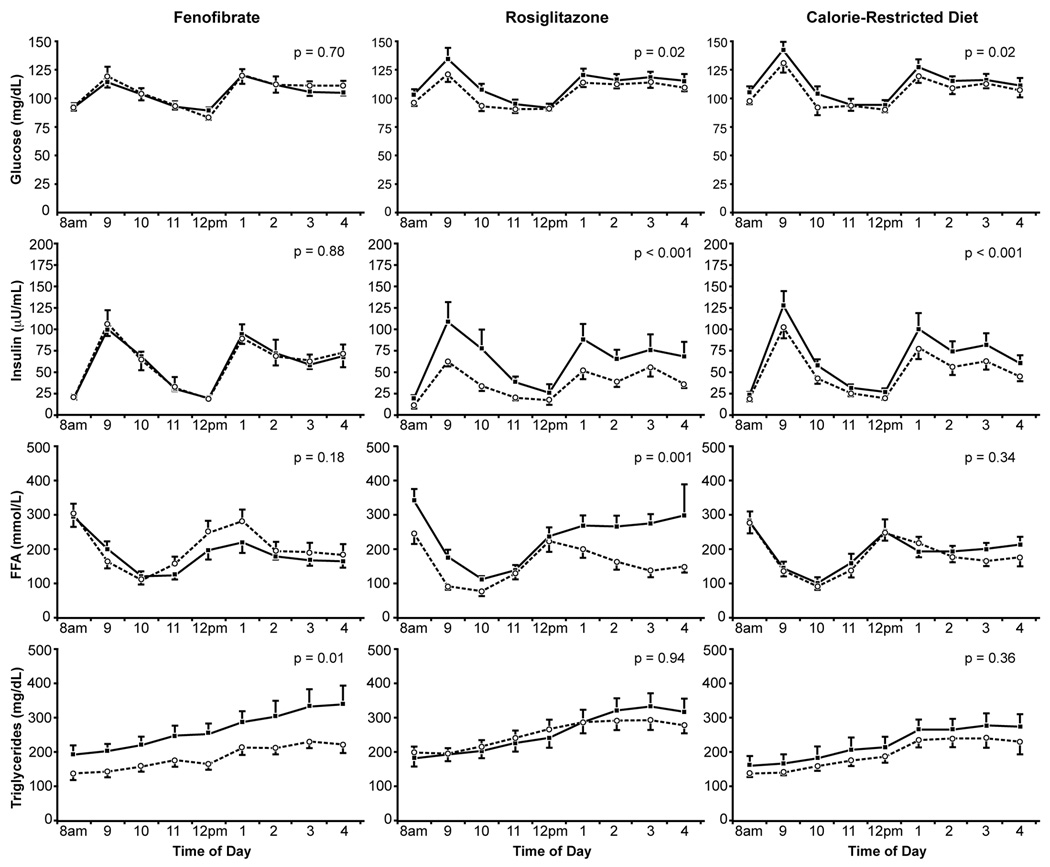
The effects of the three experimental interventions on daylong total integrated plasma glucose, insulin, FFA, and TG responses to the test meals are given in Table 3 and depicted in Figure 3. Daylong glucose and insulin responses decreased significantly in the RSG-treated (p<0.001) and CRD (p=0.02) groups, but did not significantly change in the FEN-treated subjects. Daylong FFA concentrations significantly decreased after RSG treatment (p=0.001), but did not significantly change in those treated with FEN or CRD. Postprandial TG concentrations decreased in both the FEN-treated and CRD groups, but the improvement in TG concentrations was statistically significant only in the FEN-treated subjects (p=0.01). Finally, despite lower daylong glucose, insulin, and FFA levels, postprandial TG concentrations did not significantly change in the RSG-treated subjects.
Table 3.
Effect of Three Experimental Interventions on Daylong Integrated Plasma Glucose, Insulin, Free Fatty Acid, and Triglyceride Responses to Test Meals
| Variable (Area-under -the curve) | Fenofibrate (n=12) | Rosiglitazone (n=12) | Calorie-Restricted Diet (n=12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | p | Before | After | Change | p | Before | After | Change | p | |
| Glucose (mg/dl.8hr) | 838 ± 103 | 845 ± 108 | 1% | 0.70 | 890 ± 101 | 836 ± 85 | −6% | 0.02 | 899 ± 129 | 850 ± 126 | −6% | 0.02 |
| Insulin (µU/ml.8hr) | 499 ± 164 | 501 ± 163 | 0.4% | 0.88 | 520 ± 379 | 301 ± 154 | −42% | <0.001 | 540 ± 219 | 419 ± 179 | −22% | <0.001 |
| Free Fatty Acids (mmol/l.8hr) | 1455 ± 334 | 1608 ± 428 | 9.5% | 0.18 | 1793 ± 511 | 1221 ± 393 | −32% | 0.001 | 1489 ± 362 | 1402 ± 334 | −6% | 0.34 |
| Triglycerides (mg/dl.8hr) | 2120 ± 887 | 1492 ± 473 | −30% | 0.01 | 2067 ± 787 | 2034 ± 639 | −2% | 0.94 | 1796 ± 859 | 1565 ± 494 | −13% | 0.36 |
Within each experimental group, before and after means were compared by Student’s paired t-test
Figure 3.
Mean (SEM) daylong plasma glucose, insulin, free fatty acid, and triglyceride concentrations before ( ) and after (
) and after ( ) treatment.
) treatment.
Within each group, the effect of treatment was evaluated by comparing baseline and after-treatment area-under-the-curves by Student’s paired t test.
Discussion
The results of this study do not identify one simple approach to most effectively reduce CVD risk in a high risk group of overweight, insulin resistant, dyslipidemic individuals; a dangerous, and not uncommon, phenotype. However, the manuscript contains useful clinical information as to the pros and cons of the individual approaches evaluated. Given the current controversy concerning the relationship between RSG treatment and CVD risk9,10, perhaps the most interesting comparison is between the RSG-treated and the other two interventions. The differences between RSG and FEN are quite clear: RSG-treated subjects became more insulin sensitive, with lower daylong glucose and insulin concentrations. However, plasma lipid and lipoprotein concentrations, if anything worsened following treatment with RSG, whereas the atherogenic lipoprotein profile improved considerably in FEN-treated patients. The changes in overweight, insulin resistant, dyslipidemic patients treated with FEN that we observed are similar to what has been described in studies in which gemfibrozil treatment has been shown to reduce CVD11–13, with evidence that the benefits were particularly significant in patients similar to those enrolled in this study: insulin resistant and dyslipidemic13. The relevance of our finding that total and LDL-C concentrations did not improve, but tended to increase in RSG-treated individuals, to the suggestion that RSG may increase CVD risk9,10, can only be speculated upon.
Comparison of the clinical benefits of a CRD with FEN treatment is more complicated. When considered by itself, the CRD seemed to address every CVD risk in these patients, with the group demonstrating significant weight loss, enhanced insulin sensitivity, lower daylong glucose and insulin levels, and lower fasting TG and LDL-C concentrations, whereas metabolic improvement in subjects receiving FEN was limited to significantly lower fasting cholesterol and TG concentrations, as well as a decrease in daylong TG concentrations. However, in view of recent reports of the atherogenicity of nonfasting TG concentrations14,15, it is of clinical interest that an improvement in this variable was only seen in FEN-treated patients.
In light of these comparisons of the clinical benefits of a CRD or FEN treatment, an argument can be made in support of both. The fundamental question related to use of a CRD is how effectively patients assigned to this program would follow dietary instruction, actually lose weight, and gain the expected clinical benefit. In this study, although all 13 patients lost some weight, the amount varied from 1.2 to 11.1 kg, with 5 out of 13 of losing >7 % of initial body weight. It was of interest that the SSPG concentration in these individuals decreased by a mean of 59 mg/dl, as compared to a fall of 11 mg/dl in those that lost <7% of body weight. Thus, it appears that the more weight that is lost, the greater the metabolic improvement, similar to the recent results reported by Dixon, et al16 of the benefits of adjustable gastric banding in patients with type 2 diabetes. In that context, 8 of the patients assigned to CRD in our study would have met the inclusion criteria for gastric banding in the study of Dixon and associates16. Given the dramatic clinical benefits they observed following gastric banding, and the apparent lack of serious complications, it may not be unreasonable to think of using that approach in obese, insulin resistant, dyslipidemic individuals, who are unable to lose weight effectively.
Since no single intervention effectively treated all of the CVD risk factors in overweight/obese, insulin resistant, dyslipidemic individuals, the current results suggest that an attempt at weight loss is a reasonable first approach. If weight loss is not managed and/or maintained, and if dyslipidemia persists irrespective of dietary changes, the addition of FEN to the treatment program seems to be a reasonable next step in light of evidence that the lipid phenotype of a high TG and a low HDL-C concentration is closely linked to increased risk of CVD, and treatment with compounds that improve these changes has been shown in prospective studies to decrease CVD11–13. Finally, the possibility that gastric banding might eventually be the treatment of choice for many individuals with this clinical syndrome cannot be excluded.
Acknowledgments
Supported in part by research grants from the National Institutes of Health (RR-000070) and Abbott Laboratories, Abbott Park, Illinois, USA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors have no potential conflicts of interest. The funding sources played no role in the study design; collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication.
References
- 1.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 2.Pei D, Jones CN, Bhargava R, Chen YD, Reaven GM. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia. 1994;37:843–845. doi: 10.1007/BF00404344. [DOI] [PubMed] [Google Scholar]
- 3.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–2160. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 5.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23:171–175. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- 6.Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism. 2004;53:159–164. doi: 10.1016/j.metabol.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Lamendola C, Abbasi F, Chu JW, Hutchinson H, Cain V, Leary E, McLaughlin T, Stein E, Reaven G. Comparative effects of rosuvastatin and gemfibrozil on glucose, insulin, and lipid metabolism in insulin-resistant, nondiabetic patients with combined dyslipidemia. Am J Cardiol. 2005;95:189–193. doi: 10.1016/j.amjcard.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 10.Psaty BM, Furberg CD. Rosiglitazone and cardiovascular risk. N Engl J Med. 2007;356:2522–2524. doi: 10.1056/NEJMe078099. [DOI] [PubMed] [Google Scholar]
- 11.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, Frick MH. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 13.Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, Collins D. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 14.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 15.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Jama. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Jama. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]