Abstract
Stress fibers are contractile bundles in the cytoskeleton that stabilize cell structure by exerting traction forces on extracellular matrix. Individual stress fibers are molecular bundles composed of parallel actin and myosin filaments linked by various actin-binding proteins, which are organized end-on-end in a sarcomere-like pattern within an elongated three-dimensional network. While measurements of single stress fibers in living cells show that they behave like tensed viscoelastic fibers, precisely how this mechanical behavior arises from this complex supramolecular arrangement of protein components remains unclear. Here we show that computationally modeling a stress fiber as a multi-modular tensegrity network can predict several key behaviors of stress fibers measured in living cells, including viscoelastic retraction, fiber splaying after severing, non-uniform contraction, and elliptical strain of a puncture wound within the fiber. The tensegrity model also can explain how they simultaneously experience passive tension and generate active contraction forces; in contrast, a tensed cable net model predicts some, but not all, of these properties. Thus, tensegrity models may provide a useful link between molecular and cellular scale mechanical behaviors, and represent a new handle on multi-scale modeling of living materials.
Keywords: Cell mechanics, cell structure, computer model, contractility, cytoskeleton, tensegrity
INTRODUCTION
Cultured cells exert traction on extracellular matrix (ECM) by assembling cytoskeletal stress fiber (SF) bundles that extend tens of micrometers in length and insert into adhesion complexes at the cell-ECM interface. SFs are linear bundles of parallel actin and myosin filaments that also contain other actin-binding linker proteins, including tropomyosin, troponin, α-actinin and caldesmon. These components self assemble into a periodic three dimensional (3D) lattice reminiscent of the sarcomeric arrangement of thin and thick filaments in muscle (Langanger, 1986). These multi-molecular assemblies have been shown to shorten and generate tension in intact living cells (Kumar, 2006), membrane-permeabilized cells (Kreis, 1980; Sims, 1992), and when isolated (Katoh, 1998). Cell-generated tensional forces drive changes in cell shape and ECM remodeling, and contribute to control of cell growth and function, as well as tissue patterning and mechanotransduction at the organ level (Ingber, 2003, 2006). Yet, little is known about how the supramolecular architecture of the SF contributes to its mechanical behavior.
Studies with isolated SFs reveal that they can shorten by ~20% (Katoh, 1998) in response to agonists that induce contraction in membrane-permeabilized cells (Sims, 1992). This observation, combined with the finding that SFs shorten ~15% within 1 sec after being dislodged from their ECM adhesions in lysed cells, has led to the conclusion that SFs are passively strained by ~20% (Kumar, 2006). When intact SFs were physically severed with femtosecond laser in living cells adherent to ECM, they retracted on the time scale of 15–20 sec with viscoelastic recoil dynamics, and most of this response could be inhibited by interfering with myosin-based contractility (Kumar, 2006). These results indicate that the tension experienced by the SF is primarily due to active tension generation; however, even under these conditions, there was a small contribution of passive tension to SF retraction. Thus, SFs are both passively distended through their ECM attachments and actively tensed by internal myosin motors.
Other experiments show that SFs buckle in cells adherent to tensed flexible substrates when tension is released and the substrate retracts rapidly (Costa, 2002). These findings suggest that SFs experienced a non-uniform distribution of ~0–20% pre-extension, and that they can resist compression even though they also actively contract and shorten. Moreover, SFs do not significantly change their width when they contract against fixed adhesions (Kumar, 2006), and hence some of their components must resist lateral compression exerted by neighboring actomyosin filaments. It is therefore difficult to envision how SFs can be organized at the molecular level to provide this unique combination of mechanical properties.
Individual SFs in living cells behave mechanically like tensed viscoelastic cables, and their recoil dynamics can be captured by a macroscopic, continuum model that incorporates springs and dashpots (Kumar, 2006). Yet, the reality is that each SF is organized as a discrete 3D network composed of multiple interacting fibrillar components. Thus, there is a need for physical models that incorporate the discrete nature of these molecular building components and quantitatively relate component properties to network behavior.
Discrete network models based on tensegrity architecture have been used to successfully predict the mechanical responses of whole cells (Ingber, 1993, 2003; Wang, 1993; Stamenovic, 1996, 2000, 2002; Coughlin, 1997;Volokh, 2000; Adasnwz, 2002; Sultan, 2004), the erythrocyte membrane (Vera, 2005) and viruses (Sitharam, 2006); they also have been studied for use as ‘intelligent’ materials and structures for commercial applications (Shea, 2002; Sultan, 2003). Here we describe a mechanical model of a SF based on tensegrity, and show that this model effectively predicts a diverse range of SF behaviors observed in living cells, whereas a tensed cable network model of similar architecture fails to predict all of these behaviors. These results suggest that SFs may be effectively described as tensegrity structures even though the precise molecular architectural details remain unknown.
METHOD AND MODEL
Tensegrity Model
Tensegrities are tensile network structures that require prestress in their members before external load is applied to self-stabilize and resist shape distortion (10). When a tensegrity is subjected to a mechanical load, or the prestress magnitudes in its members are varied such that the structure is out of equilibrium, it adjusts its configuration and the prestress in its members to reach a new equilibrium state (Tibert, 2003). Tensegrities exhibit many common mechanical behaviors regardless of their geometric arrangement (Sultan, 2003, 2004), and thus we modeled the SF using a generic, planar tensegrity mast composed of multiple similar structural modules (Fig. 1A) to simplify the mathematical analysis. To mimic the high slenderness ratio of the SF (~100 based on width and length of 100 nm and 10 µm, respectively), the tensegrity model is 6 modules wide and 251 modules long with a slenderness ratio of 42 (Fig. 1A). Each module is composed of 8 structural members organized in a prestressed tensegrity array in which tension cables form a continuous web that is balanced by compression struts (Fig. 1B,C); in each module, the relative dimensions of the structural element lengths, l1:l2:l3 (Fig. 1C) are 1:2:2. An additional member (number 9) is added to the modules located at the ends of the tensegrity model to maintain the entire structure in a state of prestress (De Jager, 2006). Cables are treated as viscoelastic Voight elements that support only tensile forces, whereas struts are modeled as linearly elastic elements under compression (see Supplementary Information for their constitutive characteristics).
Fig. 1. Structural configuration of the multi-modular, tensegrity, stress fiber model.
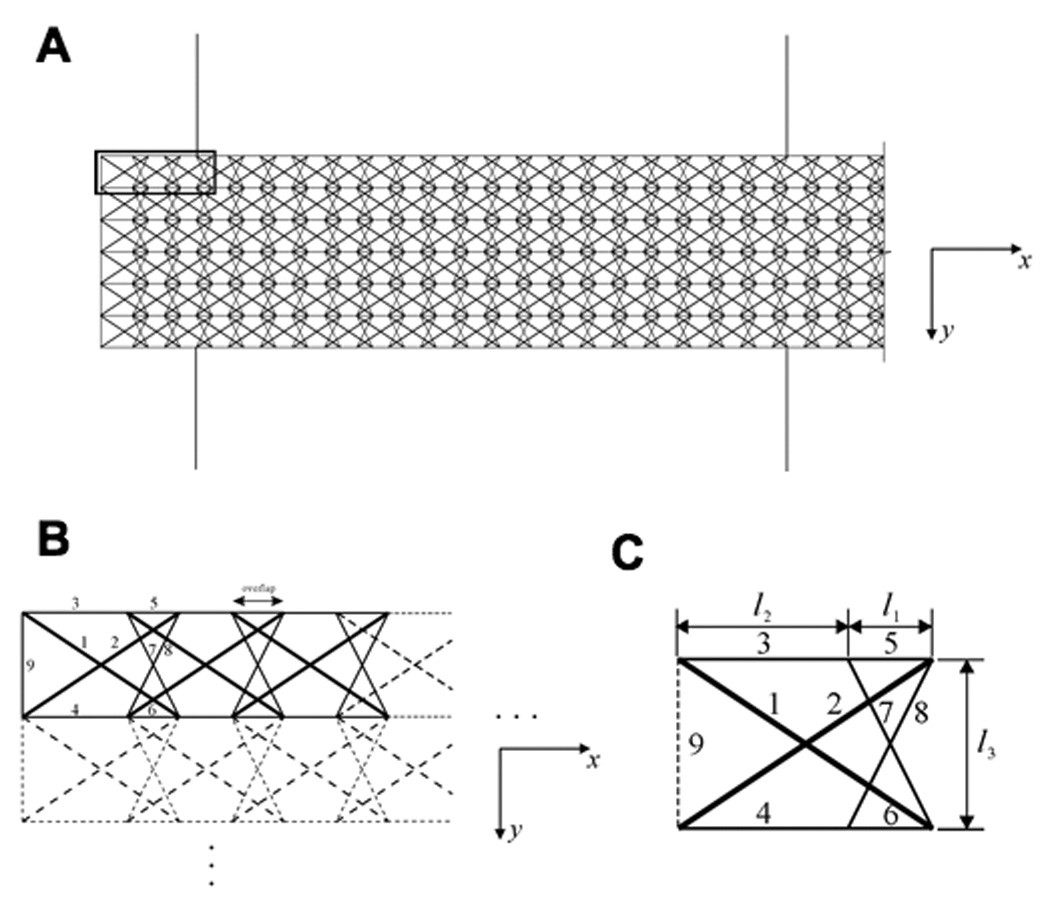
A) Organization of a large section of the planar multi-modular tensegrity; the bold rectangle at the upper left indicates the area which is shown in greater detail in B. B) Assembly of individual modules into a self-equilibrium tensegrity structure. C) A single tensegrity module from A and B, with elements labeled: elements 1 and 2 (bold lines) are struts; 3–8 are cables (thin lines), and 9 (dashed line) is an additional cable for end modules. The modules connect one by one in the x-direction by overlapping by a distance of l1, and they can be replicated in the y-direction by shifting a distance of l2. Element 9 is only added to the distal ends of the modules of the model to mimic fixed ends of the SF and thereby, maintain structural self-equilibrium .
At the reference (initial) state, tensile forces in the cables are balanced by the compression forces in the struts and by the model’s fixed ends. A single actin filament has a Young’s modulus E on the order of GPa and cross-sectional area A of ~10 nm2 (Gittes, 1993; Holmes, 1990; Kabsch, 1990; Kojima, 1994). While there are essentially no corresponding values available for other SF proteins proteins (e.g., myosin, tropomysion, α-actinin), the linear stiffness of the myosin-actin bridge and titin are ~2 pN/nm (Huxley, 1996) and 1–4 pN/nm (Jeffrey, 2004), respectively, in muscle, whereas that of an isolated actin filament is ~44 pN/nm (Gittes, 1993; Holmes, 1990; Kabsch, 1990; Kojima, 1994). Here we assume that the average cross-sectional areas and lengths of the cross-linking proteins have the same magnitude as those of actin filaments, and their cross-sectional stiffness therefore will be similarly one order smaller. To generalize the model while maintaining this ratio, we simplified calculations by assuming the sectional stiffness of the longitudinal cables (corresponding to F-actin) and the cross-linking proteins to be 1 and 0.1 pN, respectively.
Prestress in the tensegrity model
Given the geometry and dimensions of the tensegrity SF model (Fig. 1), the self-stressed state of the plane structure was determined by equilibrium analysis as 1: 2: 3: 4: 5: 6: 7: 8: 9 = −0.3605: −0.3605: +0.3000: +0.3000: +0.5000: +0.5000: +0.2236: +0.2236: +0.2000 (−, compression; +, tension). This represents the ratio of the internal stress of the various members shown in Fig. 1B, and it indicates that the whole structure will remain in equilibrium and self-stabilize itself under these internal stresses if the elements 1 to 9 all have internal forces proportional to these ratios.
The prestress introduced into the planar, multimodular tensegrity array (Fig. 1A) is equilibrated in part by the structure itself, and in part by reaction forces due to the end constraints. The former contribution (‘self-stress’) keeps the structure in a stable state without end constraints, which is consistent with the observation that SFs remain stable when they are isolated from living cells (Katoh, 1998). The magnitude of the self-stress used in this study (−0.0295553, −0.0295553, +0.0245915, +0.0245915, +0.0409859, +0.0409859, +0.0183294, +0.0183294 and +0.0163943 pN) is proportional to the self-stressed state values described above. We also introduced an additional prestress of 0.2 pN into the longitudinal cables of the tensegrity SF model because stress fibers are passively strained by about 20% in cells (Kumar, 2006). Thus, in the presence of the self-equilibrated prestress, the tensegrity structure will remain in a state of static equilibrium without end constraints. In contrast, with passive prestress, the tensegrity structure will shorten if the end constraints are removed or relaxed (see Supplementary Information for details).
RESULTS
While the precise molecular scale architecture of the SF is not known, we took advantage of the finding that tensegrity structures with different architectural configurations share common mechanical behaviors (Sultan, 2003, 2004), and used a generic tensegrity design in our model (Fig. 1). Our tensegrity SF model incorporates multiple linear filaments oriented in parallel along its main axis, with many lateral structural links that would correspond to actin-associated molecules (e.g., α-actinin, tropomyosin, troponin, caldesmon) that closely associate with actin and myosin filaments along their length (Burridge, 1988; Byers, 1984). We then used this simplified tensegrity model to explore whether it embodies features sufficient to predict mechanical behaviors previously observed in SFs in living cells.
Viscoelastic retraction and splaying of stress fibers
Past studies have demonstrated that individual SFs labeled with yellow fluorescent protein (YFP)-actin in living cells immediately retract when physically severed using a femtosecond laser and their cut ends splay (Fig. 2A)(Kumar, 2006). The dynamics of retraction closely match that exhibited by a tensed viscoelastic cable modeled using springs and dashpots (solid line, Fig. 2B). To explore whether the planar multi-modular tensegrity SF model exhibits similar viscoelastic retraction behavior, we modeled SF severing by deleting structural elements that span the width of the model at its center (Fig. 2C), and then carried out a structural transient analysis (ANSYS, 2004) that incorporates material damping of the cables. The predicted retraction kinetics of the tensegrity model were very sensitive to the viscoelastic characteristics of the cables, and matched experimentally observed kinetics for a material damping coefficient of 3 sec/nm (Fig. 2B); this value was chosen for the rest of the simulations. Reducing the passive prestress of the model SF from 0.2 to 0.1 and then to 0 pN resulted in a progressive decrease in the retraction response (Fig. 2D) that also closely paralleled the response exhibited by SFs in living cells when exposed to progressively more potent inhibitors of actomyosin-based tension generation (Y27632 or ML7, respectively; Kumar, 2006). Moreover, the cut ends of the tensegrity SF model also widened as the retraction distance increased, whereas a tensed cable model with identical structure to the tensegrity model (i.e., except lacking compression-resistant struts) did not exhibit any splaying response under identical loading conditions (Fig. 2C).
Fig. 2. Stress fiber retraction after surgical incision.
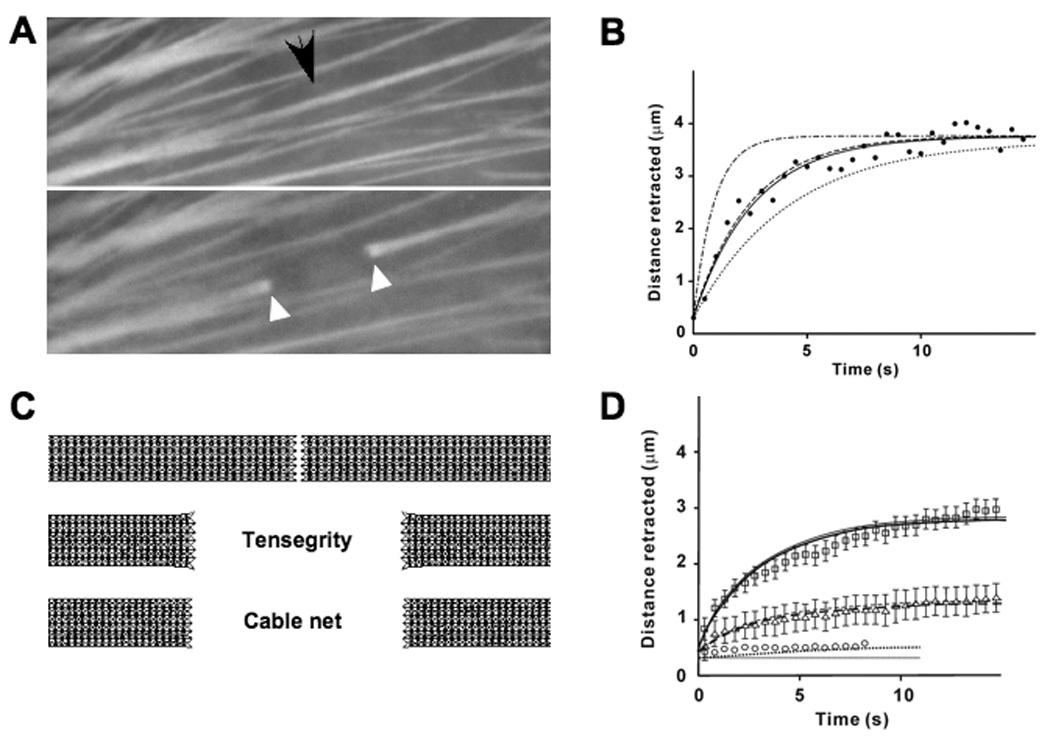
A) Fluorescence micrographs of a single YFP-labeled stress fiber (arrow) before (top) and 15 sec after (bottom) being surgically incised with a femtosecond laser(black arrow indicates site of laser irradiation; reprinted from (Kumar, 2006) with permission). Note that the cut ends of the SF (white arrowheads) physically retract and splay after cutting. B) Time course of SF retraction predicted by the tensegrity model simulations with cable material damping coefficients of 5 sec/nm, 3sec/nm and 1 sec/nm corresponding to time constants of 4.15 s (dashed-dotted line), 2.61 s (dashed line) and 0.9 s( dotted line) respectively. As can be seen, the simulation with a material damping coefficient of 3 sec/nm matches the experimental data (black circles), and the predictions of a spring-dashpot model with a time constant of 2.66 s (solid line, (Kumar, 2006)). C) Images showing results of simulations after the SF models are severed at their center (top). The tensegrity model both retracts and exhibits splayed ends (middle) like the living SF, whereas the purely tensed cable net model that lacks compression struts retracts but fails to produce similar splaying behavior (bottom). D) Time course of SF retraction predicted by the tensegrity model simulation for a passive prestress of 0.2pN (solid line), 0.1pN (dashed line) or no passive prestress (dotted line) fit well to data obtained from experiments in which SFs were incised in control cells (squares) or treated with the chemical inhibitors of cytoskeletal tension generation, Y27632 (10 µM for 1 hr)(triangles) or ML7 (67 µM for 30 min) (circles), respectively; error bars represent mean ± SEM.
Stress fiber response to puncturing
When the laser was used to create a 300 nm wide puncture wound in a single living SF, the circular hole progressively deformed along the main axis of the SF due to internal prestress, resulting in formation of an elliptical defect (Fig. 3A, top) (Kumar, 2006). In our simulations, when a hole was introduced into the tensegrity SF model, it also elongated formed into an elliptical shape, but the SF narrowed along the lateral borders of the hole (not shown), whereas these regions maintained relatively constant in width in living cells (Fig. 3A, top). This subtle difference can be explained, however, if SFs have lateral constraints in addition to their end-links to fixed focal adhesions (Costa, 2002), as demonstrated by electron microscopy (Marek, 1982). We therefore incorporated lateral guy wires connected to the sides of the SF model to mimic these lateral flexible constraints in this and all subsequent modeling studies. Importantly, this tethered SF model effectively predicted the shape changes in the puncture wound observed in past experiments (Fig. 3A, bottom vs. top). Normalized values of simulated elongation measured during a time course of 15 seconds also closely matched the dynamic structural rearrangements observed in punctured SFs in living cells (Fig. 3B).
Fig. 3. Response of a stress fiber to puncturing.
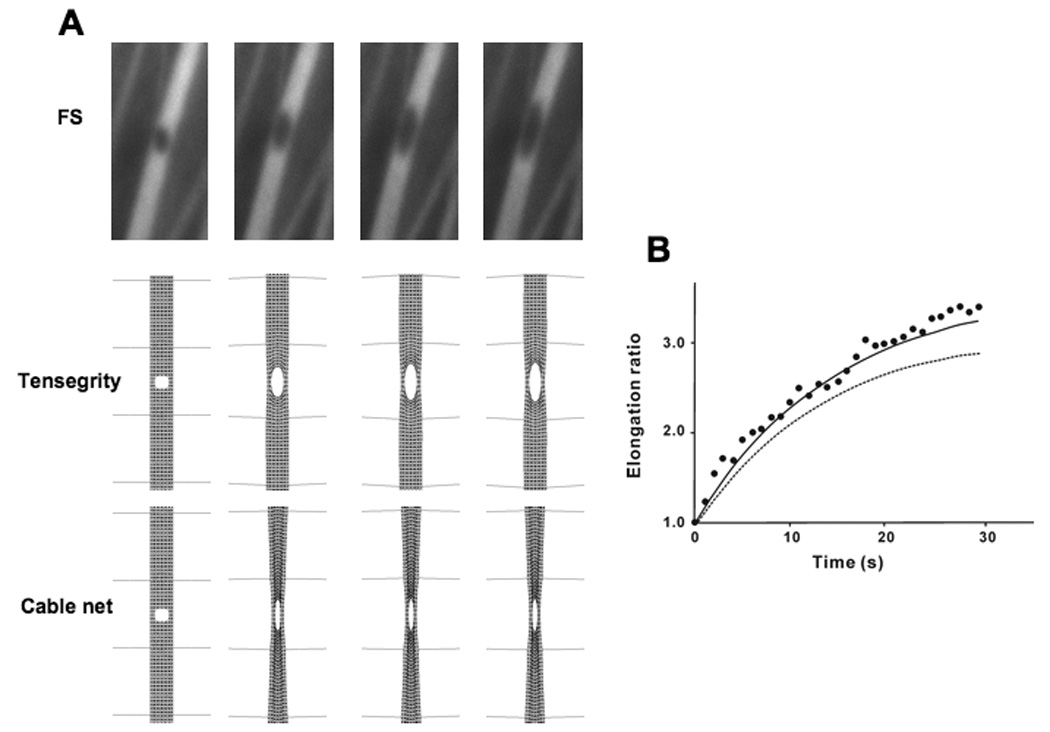
A) Progressive elongation of a puncture hole (300 nm diameter) produced in a YFP-labeled SF with a femtosecond laser (Kumar, 2006) over a period of 15 sec (left views), and similar responses depicted in a simulation of the tensegrity SF model (right views). B) Graphic depiction of the time course of hole elongation along the main axis of the SF showing that the results of the tensegrity simulation (black line) fit well with previously reported experimental results (black circles; 5). The elongation ratio is defined as the ratio of increased diameter along the main axis to the initial diameter. C) A tensegrity SF model that also incorporates lateral guy wire cables to model lateral filamentous constraints observed in past electron microscopic studies effectively predicts the lateral thickening of the SF puncture wound, as observed in the past experimental studies.
Stress fiber buckling
When tension is rapidly released in cells spread on stretched, flexible ECM substrates, the resulting cell retraction causes SFs aligned along the shortening direction to buckle when compressed rapidly beyond their unloaded slack length (Fig. 4A) (Costa, 2002). The pattern of buckling has a relatively short wavelength and varies along the length of the SF, which provides additional support for the existence of lateral constraints. A nonlinear buckling analysis was carried out to determine the tensegrity model’s response when one end was fixed and the other was subjected to a uniformly distributed compression force. These simulations revealed that the laterally tethered, multi-modular tensegrity SF also exhibited buckling behavior and short-wave wavelengths similar to that observed in living cells (Fig. 4B). Again, tethered tensile networks with the same architecture that lacked compression members failed to exhibit this behavior.
Fig. 4. Short-wave pattern of stress fiber buckling.
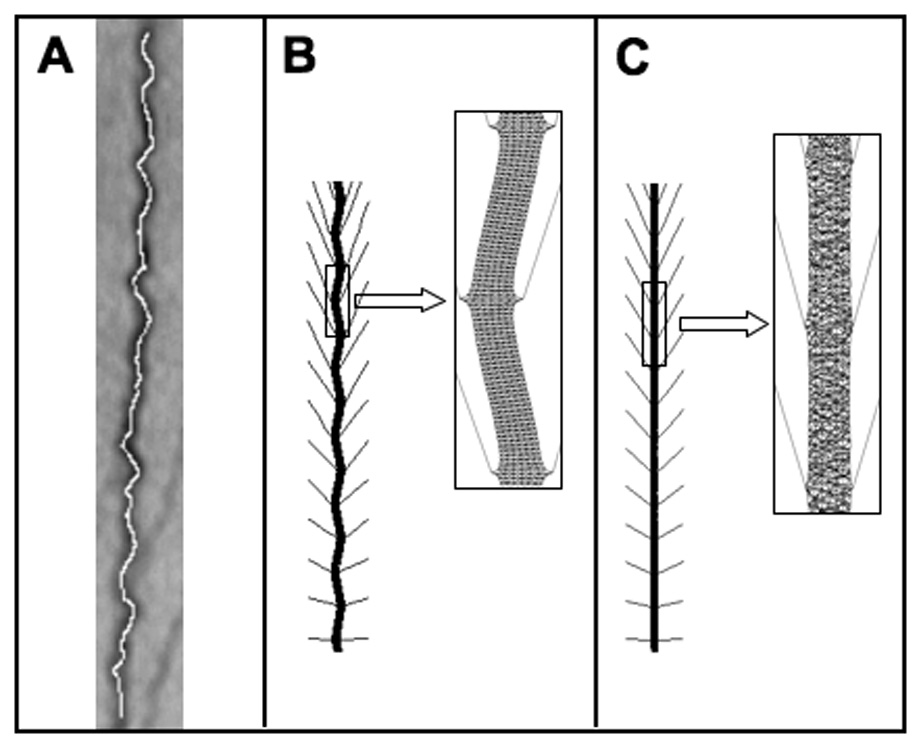
A) Buckling pattern of a SF in living cells produced by rapid release of tension within cells adherent to stretched ECM substrates (reprinted with permission from (Costa, 2002). B) Periodic short wavelength buckling pattern produced in the tensegrity SF model (inset shows a portion of the buckled configuration at higher magnification).
Non-uniform contraction of stress fibers
When cells containing SFs are treated with contractile agonists, many stress fibers do not contract uniformly along their lengths; instead, myosin activity preferentially concentrates at their ends, causing them to contract peripherally and stretch at their center (Peterson, 2004). SF contraction is driven by the relative motion of actin and myosin filaments along the main axis of the fiber, which increases internal isometric tension within the entire fiber to the point where the total applied stress overcomes the stiffness of the SF (or subportions of it), as well as the resisting forces exerted by its anchoring points to basal focal adhesions. We therefore modeled this contraction as resulting from increased prestress in the SF.
A tensegrity SF model initially in a stable state of uniform pre-extension and flexibly fixed at its ends was divided into three parts: a central region surrounded by equally-sized peripheral areas (Fig. 5A). Then the prestress of the longitudinal cables in the peripheral regions was increased to 0.8 pN to disturb the equilibrium. The structure rearranged its internal elements to find a new equilibrium state, resulting in contraction of the end portions of the tensegrity model, and stretching of its center (Figs. 5A, B). Thus, the tensegrity model qualitatively predicts the non-uniform contraction of SFs observed in living cells. In contrast, a cable net without compression struts did not capture this non-uniform retraction (Fig. 5C), and increasing the prestresses of all members resulted in uniform shortening of all elements in both models (data not shown).
Fig. 5. Nonuniform contraction of a tensegrity stress fiber model.
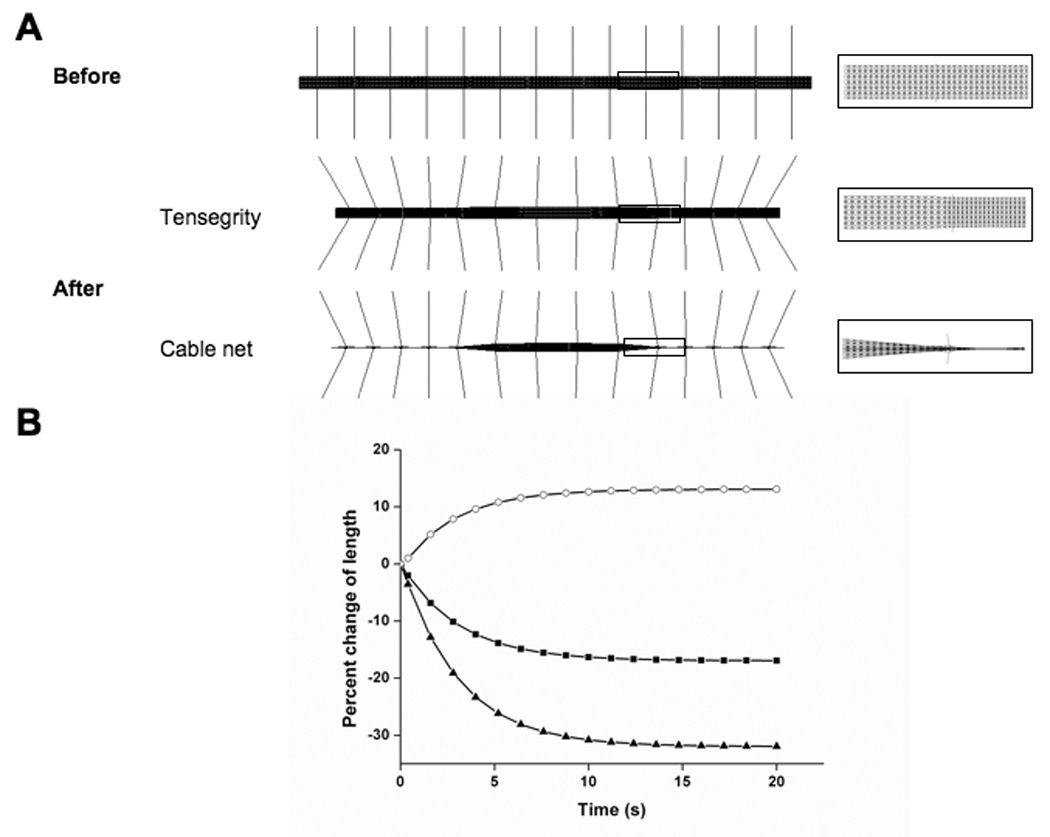
A) Configurations of the tensegrity SF model before (top) and after (bottom) contraction. Enlarged views of the junctions between the peripheral and central regions of the SF pre- and post-contraction are also shown (middle). B and C show percent change in length exhibited by the whole tensegrity structure (black squares) versus the peripheral (black triangles) and central regions (open circles) of the tensegrity model and a tensed cable net model with similar configuration that lacked compression struts, respectively.
Contraction of the tensegrity model resulted in 38 and 19% decreases in length of the peripheral regions and the whole SF, respectively (Fig. 5B), which are comparable to the magnitudes observed in living cells (Peterson, 2004). In contrast, the average elongation of the central region in the model was 18% (Fig. 5B), which is only one third of the reported elongation of central sarcomeres in living SFs (Peterson, 2004). Interestingly, in our study, the whole tensegrity SF model shortened when contraction was increased (Fig. 5B), indicating that some prestress was released through the inward movement of the flexible fixed ends. In contrast, this would not occur in cells where the ends are fixed to stable ECM adhesions, and thus, increased elongation of the central region would result instead, as is observed experimentally.
Active contraction-dependent stress fiber shortening
SF shortening is due to actomyosin-based contraction; however, it is difficult to understand how a structure that behaves like a tensed viscoelastic cable and buckles when compressed, can also actively contract and shorten. To investigate this further, we treated the compression struts in the tensegrity SF model as actuators whose lengths can be actively changed, as previously explored in studies of intelligent tensegrity structures for commercial applications (Shea, 2002; Sultan, 2003).
To computationally mimic the stress and boundary conditions of the isolated SF (Katoh, 1998), we first anchored both ends of the tensegrity to a substrate and introduced prestress along its length (corresponding to its living state in a cell). Then, one of the ends was freed to release part of the prestress, resulting in passive retraction (as occurs during SF isolation). Finally, the struts were shortened in the simulation, thus mimicking active SF contraction due to actomyosin filament sliding. This caused all of the interconnected structural elements of the tensegrity network to rearrange and change their positions relative to each other, which caused the entire tensegrity model to shorten (Fig. 6A).
Fig. 6. Contraction response of the tensegrity stress fiber model when struts are actively shortened.
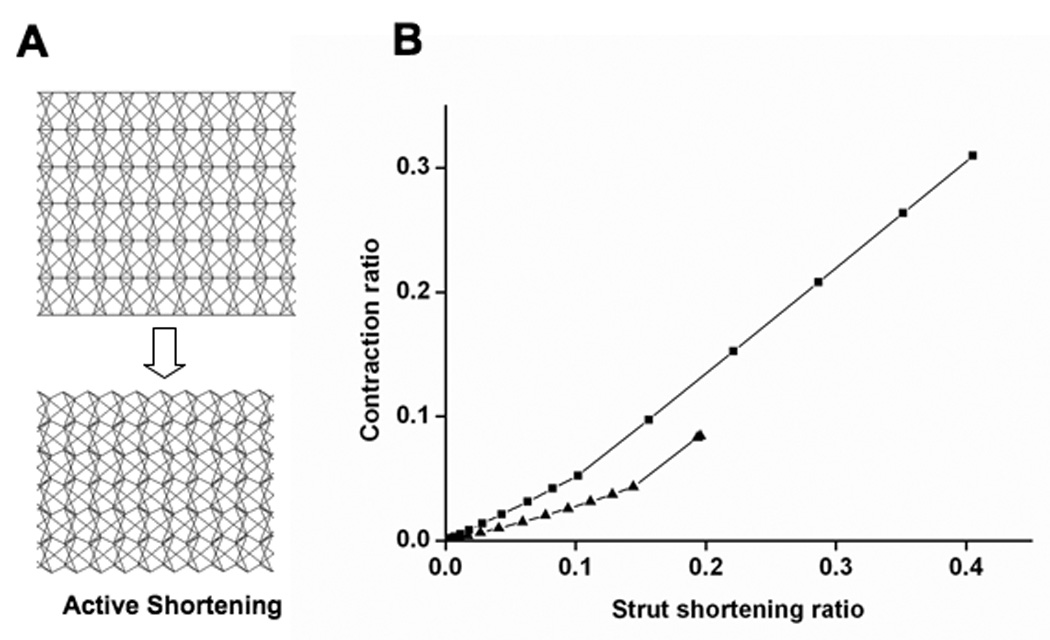
A) The configurations of the model before (left) and after (right) struts are actively shortened showed that whole fiber contracts. B) Graph showing SF contraction ratio as a function of strut shortening ratio for values of strut stiffness of 0.1 (triangle) or 0.2 (square); contraction and strut shortening were normalized by the initial length of the model and strut, respectively.
We quantified shortening of the tensegrity model by determining the ratio of the magnitude of the retraction to the initial length, as previously done with isolated SFs (Katoh, 1998). The stable configuration of the tensegrity SF before the struts were shortened was used as the reference state; convergent results were obtained when struts shortened to 20% of their initial length. In contrast to our studies on passive retraction, the width of the tensegrity SF model decreased with this active contraction (Fig. 6A), again predicting behavior of living SFs (Kumar, 2006). Due to the repeated multi-modular layout of the model, the normalized contraction scaled almost linearly with the normalized shortening of the struts (Fig. 6B). The corresponding contraction of the model only reached 10%, which was lower than the reported value of 23% (Katoh, 1998); however, by increasing strut stiffness from 0.1 to 0.2 pN (i.e., one fifth the stiffness of the longitudinal cables), we obtained convergent contraction of more than 30% with 40% strut shortening (Fig. 6B). This may indicate that cross-link elements in SFs are stiffer than we assumed, at least in the case of this particular tensegrity configuration.
DISCUSSION
Mechanical tension generated via actomyosin filament sliding within cytoskeletal SFs influences mammalian cell form and function, as well as tissue morphogenesis (Ingber, 2003, 2006). For this reason, the mechanical properties of SFs have been studied extensively both in vitro and in situ (Katoh, 1998; Kreis, 1980; Kumar, 2006; Sims, 1992). Yet, little is known about how the supramolecular architecture of the SF contributes to its wide range of novel mechanical behaviors, and a theoretical model to explain SF mechanics is lacking. Here, we developed and analyzed a generic, multi-modular tensegrity structure as a potential mechanical model of the SF, without attempting to mimic its precise molecular geometry at the nanoscale. Numerical simulations based on this multi-modular tensegrity effectively reproduced multiple physical behaviors of SFs that have been previously measured in living cells, including viscoelastic retraction and fiber splaying after severing, non-uniform contraction, buckling, and elliptical strain of a puncture hole created with a fine laser.
Importantly, the viscoelastic retraction of a SF also can be simulated by a single prestressed viscoelastic cable, but only the tensegrity model is capable of explaining all of these phenomena exhibited by SFs within living cells. In addition, tensed cable net structures with similar geometry, but lacking internal compression members, failed to mimic these behaviors. Without fixed ends (or a resisting compression strut), no prestress can be introduced into a cable net. As a result, if all of the elements of the SF are tensed, it will lose all prestress and become unstable after isolation, and exhibit no ability to contract, which is inconsistent with experimental observations (Katoh, 1998). In the tensegrity model, an increase in prestress also results in a more stable structure, and this result is consistent with the observation that highly phosphorylated myosin filaments, which are more contractile, are more rigid (Peterson, 2004). Thus, these observations suggest that the discrete network organization of the proteins in SFs should be taken into account in the investigation of its mechanical properties, and that at least a subset of these proteins may bear compression at the molecular scale in tensed SFs in living cells.
Although the precise organization of the SF remains to be determined, we can speculate that actin and myosin filaments function as tension cables, whereas some of the associated binding proteins (e.g., α-actinin, tropomyosin, troponin, caldesmon) act as lateral struts, either alone or in combination with actomyosin filaments, when the SF is in its tensionally prestressed state. These cross-linked filament bundles also resist compression and provide structural rigidity when tension is released, and this may contribute to SF buckling produced by rapid retraction of flexible substrates (Costa, 2002). Tropomyosin is a good candidate for a lateral strut as it significantly increases the stiffness of actomyosin filaments (Kojima, 1994), and it is absent from the more flexible hinge regions between sarcomeres and at the vertices of actin geodomes (Lazarides, 1976). The compression members in our tensegrity SF model are similarly distributed along the length of each repeating module, whereas they are relatively absent in the hinge regions between adjacent modules.
Regardless of the precise architecture, the use of a multimodular tensegrity arrangement provides a mechanism to maintain and control self-equilibrium within subregions of the SF (e.g., center vs. periphery). It also provides similar functions at higher hierarchical levels, including at level of the whole integrated SF, and the entire cell that contains multiple SFs that pull isometrically against fixed ECM adhesions. If all the prestress in the SF were to equilibrate itself, no force interactions between SFs and the focal adhesions would occur. Thus, given that traction forces can be visualized at focal adhesions (Beningo, 2001), the prestress of each SF does appear to be balanced in part by the ability of their fixed ends to bear these loads.
It was recently reported that the Young’s modulus of isolated SFs is ~1.45 MPa, which is some three orders of magnitude smaller than previously reported elastic moduli for single actin filaments (Deguchi, 2006). This difference may indicate that axial deformation of SFs is not simply caused by axial elongation/contraction of F-actins. Other mechanisms, such as tightening or relaxation of the mutual twisting of microfilament bundles, may also contribute the axial deformation of SFs. While the relatively simple tensegrity model presented here does not incorporate these structural details, more sophisticated multimodular tensegrity models may be able to do so. A key challenge in developing these models will be transitioning from planar 2D structures to more complex 3D geometries (along with more sophisticated algebric formalisms) that will be needed to capture complex interfilament rearrangements.
A multimodular tensegrity model of icosahedral shells has been used to explain the movement and self-assemblies of viral capsids (Sitharam, 2006), and viral self-assembly was also modeled based on icosahedral tensegrity using computational algebra (Caspar, 1980). Although virus assembly and SF contraction are two different physiological phenomena, their tensegrity models share a common mathematical basis, i.e. a ‘form-finding’ process in which the structure takes on a configuration with minimum potential energy under given constraints. In past studies, simple (single module) tensegrity models were shown to predict static and dynamic mechanical behaviors of living mammalian cells, including linear stiffening and instantaneous softening, elastic and viscoelastic characteristics, and cell shape modulation (Ingber, 1993, 2003; Wang, 1993; Stamenovic, 1996, 2000, 2002; Coughlin, 1997; Volokh, 2000; Adasnwz, 2002; Sultan, 2004). However, the tensegrity module used in most of those studies only has 6 compression members and 24 tension cables, and it fails to take into account the multi-modularity and structural hierarchies that are present in living cells (Ingber, 2003). For example, one SF may be severed in a cell adherent to a rigid substrate without fully compromising cell structure, whereas global structural rearrangements are observed throughout the cytoskeleton if these cells are attached to flexible ECM substrates (Kumar, 2006). The actin filaments that permeate the cytoplasm also display multiple levels of organization in that they can be organized as nets, geodesic dome-like structures, short bundles and long SFs, and actin-binding proteins also can organize into modular structures (Puius, 1998). Thus, the multi-modular tensegrity model presented here may offer more utility for simulating complex collective behaviors of these cellular components.
In summary, these studies suggest that the discrete network organization of the SF contributes greatly to its mechanical properties, and tensegrity principles convey multiple physical properties to these complex nanoscale structures in living cells. Although SFs are tensed, all of their subcomponents do not experience tension, and some of these elements must bear compressive forces at the molecular scale for the entire SF to exhibit its novel organic properties. Given that tensegrity appears to be utilized at multiple size scales in the hierarchy of life (Ingber, 2003, 2006); the multimodular tensegrity model described here may provide a useful link between molecular and cellular scale mechanical behaviors, and provide a new handle with which to develop novel multi-scale models of living materials.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from NIH (CA45548 and F32-NS048669), NSF (DMR-0213805 and MRSEC at Harvard University) and AHA 0735203N, and by a Pao Yu-Kong and Pao Zhao-Long Scholarship from Zhejiang University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adasnwz PC, Laurentz VM, Oddouw C, Isabeyz D, Wendlingw S. A Cellular Tensegrity Model to Analyse the Structural Viscoelasticity of the Cytoskeleton. J. Theor. Biol. 2002;218:155–173. doi: 10.1006/jtbi.2002.3064. [DOI] [PubMed] [Google Scholar]
- ANSYS. ANSYS Multiphysics. Canonsburg, PA: 2004. [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang Y. Nascent Focal Adhesions Are Responsible for the Generation of Strong Propulsive Forces in Migrating Fibroblasts. The Journal of Cell Biology. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly K, Nuckolls G, Turner C. Focal adhesions: transmembrane junction between the exracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Byers HR, White GE, Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Caspar DLD. Movement and self-control in protein assemblies. Biophysical Journal. 1980;32:103–108. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KD, Hucker WJ, Yin FC. Buckling of Actin Stress Fibers: A NewWrinkle in the Cytoskeletal Tapestry. Cell Motility and the Cytoskeleton. 2002;52:266–274. doi: 10.1002/cm.10056. [DOI] [PubMed] [Google Scholar]
- Coughlin MF, Stamenovic D. A tensegrity structure with buckling compression elements: application to cell mechanics. Journal of Applied Mechanics, ASME. 1997;64:480–486. [Google Scholar]
- Deguchi S, Ohashi T, Sato M. Tensile properties of single stress fibers isolated from vascular smooth muscle cells. J. Biomech. 2006;39:2603–2610. doi: 10.1016/j.jbiomech.2005.08.026. [DOI] [PubMed] [Google Scholar]
- De Jager B, Skelton RE. Stiffness of planar tensegrity truss topologies. International Journal of solids and structures. 2006;43:1308–1330. [Google Scholar]
- Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. Journal of Cell biology. 1993;120:932–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Tideswell S. Filament compliance and tensiontransients in muscle. J. Muscle Res. Cell Motil. 1996;17:507–511. doi: 10.1007/BF00123366. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. Journal of Cell Science. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Jeffrey GF, Wang K. Simultaneous dynamic stiffness and extension profiles of single titin molecules: Nanomechanical evidence for unfolding intermediates. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 2004;22:1439–1444. [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-Kinase-mediated Contraction of Isolated Stress Fibers. The Journal of Cell Biology. 2001a;153:569–583. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am J Physiol Cell Physiol. 2001b;280:1669–1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Masuda M, Onishi H, Fujiwara K. Isolation and Contraction of the Stress Fiber. Molecular Biology of the Cell. 1998;9:1919–1938. doi: 10.1091/mbc.9.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Ishijima A, Yanagida T. Direct Measurement of Stiffness of Single Actin Filaments with and Without Tropomyosin by in vitro Nanomanipulation. Proceedings of the National Academy of Sciences USA. 1994;91:2962–12966. doi: 10.1073/pnas.91.26.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TI, Birchmeier W. Stress fiber sarcomers of fibroblasts are contractile. Cell. 1980;22:555–561. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele T, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization and extracellular matrix mechanics. Biophysical Journal. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langanger G, Moeremans M, Daneels G, Sobieszek A, De Brabander M, De Mey J. The molecular organization of myosin in stress fibers of cultured cells. J Cell Biol. 1986;102:200–209. doi: 10.1083/jcb.102.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Actin, a-actinin, and tropomyosin interactions in the structural organization of actin filaments in nonmuscle cells. J. Cell Biol. 1976;68:202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek LF, Kelley RO, Perdue BD. Organization of the cytoskeleton in square fibroblasts. Cell Motility and the Cytoskeleton. 1982;2:115–130. doi: 10.1002/cm.970020204. [DOI] [PubMed] [Google Scholar]
- Peterson LJ, Rajfur Z, Maddox AS, Freel CD, Chen Y, Edlund M, Otey C, Burridge K. Simultaneous Stretching and Contraction of Stress Fibers In Vivo. Molecular Biology of the Cell. 2004;15:3497–3508. doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Current Opinion in Cell Biology. 1998;10:23–34. doi: 10.1016/s0955-0674(98)80083-5. [DOI] [PubMed] [Google Scholar]
- Shea K, Fest E, Smith IFC. Developing intelligent tensegrity structures with stochastic search. Advanced engineering informatics. 2002;16:21–40. [Google Scholar]
- Sims J, Karp S, Ingber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell. Sci. 1992;103:1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- Sitharam M, Agbandje-Mckenna M. Modeling Virus Self-Assembly Pathways: Avoiding Dynamics Using Geometric Constraint Decomposition. Journal of Computational Biology. 2006;13:1232–1265. doi: 10.1089/cmb.2006.13.1232. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Fredberg JJ, Wang N, Butler JP, Ingber DE. A Microstructural Approach to Cytoskeletal Mechanics based on Tensegrity. J. theor. Biol. 1996;181:125–136. doi: 10.1006/jtbi.1996.0120. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Ingber DE. Models of cytoskeletal mechanics of adherent cells. Biomechanics and Modeling in Mechanobiology. 2002;1:95–108. doi: 10.1007/s10237-002-0009-9. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Coughlin MF. A Quantitative Model of Cellular Elasticity Based on Tensegrity. Journal of Biomechanical Engineering. 2000;122:39–43. doi: 10.1115/1.429631. [DOI] [PubMed] [Google Scholar]
- Sultan C, Skelton R. Deployment of tensegrity structures. Int. J. Solids and Structures. 2003;40:4637–4657. [Google Scholar]
- Sultan C, Stamenovic D, Ingber DE. A Computational Tensegrity Model Predicts Dynamic Rheological Behaviors in Living Cells. Annals of Biomedical Engineering. 2004;32:520–530. doi: 10.1023/b:abme.0000019171.26711.37. [DOI] [PubMed] [Google Scholar]
- Tibert AG, Pellegrino S. Review of Form-Finding Methods for Tensegrity Structures. International Journal of Space Structures. 2003;18:209–223. [Google Scholar]
- Vera C, Skelton R, Bossens F, Sung LA. 3-D Nanomechanics of an Erythrocyte Junctional Complex in Equibiaxial and Anisotropic Deformations. Annals of Biomedical Engineering. 2005;33:1387–1404. doi: 10.1007/s10439-005-4698-y. [DOI] [PubMed] [Google Scholar]
- Volokh KY, Vilnay O, Belsky M. Tensegrity architecture explains linear stiffening and predicts softening of living cells. Journal of biomechanics. 2000;33:1543–1549. doi: 10.1016/s0021-9290(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction Across the cell surface and through the cy toskeleton. Science. 1993;206:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


