Abstract
The role of T helper (Th) 1 and Th2 polarization in acute graft-versus-host-disease (GVHD) is unclear. We investigated the role of Th2 cytokine secretion by utilizing donor T cells that cannot make IL-4, IL-5, IL-9 and IL-13 from quadruple cytokine deficient (Quad-KO) animals in a well-characterized BALB/c → C57BL/6 model of allogeneic bone marrow transplant (BMT). B6 recipients of BALB/c Quad-KO T cells demonstrated greater clinical severity, target organ damage and mortality from GVHD than recipients of BALB/c wild type (WT) T cells. When compared with donor T cells that are deficient in STAT6 signaling or the signature Th2 cytokine, IL-4, Quad-KO T cells demonstrated greater GVHD mortality. Mechanistic studies demonstrated that Quad-KO T cells demonstrated enhanced T cell proliferation than WT T cells when stimulated with either allogeneic APCs or with non-specific stimuli such as anti-CD3 mAb. Quad-KO T cells also secreted greater amounts of Th1 cytokines and IL-17 compared to WT T cells. Deficiency of Th2 cytokines, however, did not alter the allo-specific cytotoxic responses (CTL), the numbers of immuno-regulatory CD4+CD25+ Foxp3+ T cells (Tregs) or their suppressive functions. Our data thus unequivocally demonstrate that deficiency of the four classical Th2 cytokine enhances T cell proliferative responses & aggravates GVHD.
Keywords: Th2 cytokine, Th1 response, Bone marrow transplantation, Graft-versus-host disease
Introduction
Naïve CD4+ T cells upon activation by antigens differentiate into cytokine secreting effector/helper T (Th) cells. Based on the pattern of cytokine secretion CD4+ effector T cell responses have been classically divided into Th1, Th2 and now Th17 responses. Th1 cells promote primarily cell-mediated responses while Th2 cells promote humoral immune responses [1, 2]. Th1 cells produce interferon (IFN)-γ and interleukin 2 (IL-2) and the conventional Th2 cells secrete IL-4, IL-5, IL-9 and IL-13. The immunological milieu, especially the cytokine environment, is critical for inducing either Th1 or Th2 responses. Th1 differentiation is initiated by signaling through TCR and the activation of signal transducer and activator of transcription (STAT) 4. Several cytokines such as IL-12, IL-18 and others activate STAT4 and differentiate naïve CD4+ T cells into IFN-γ producing Th1 cells. Th2 differentiation is initiated by signaling through TCR and STAT6 mediated signaling [3]. However, it has been demonstrated that under certain conditions Th2 polarization can also occur in the absence of STAT6 [4]. IL-4 is the primary inducer of Th2 polarization. Signals from TCR and IL-4 receptor promote naïve CD4+ T cell differentiation into Th2 cells. However, IL-4 and IL-13 share a common receptor, IL-4Rα, and impaired development of Th2 cells and type-2 responses have been described both with isolated or combined IL-4 and IL-13 deficiency indicating that IL-4 and IL-13 have overlapping roles in the induction of type-2 responses and Th2 cell polarization [5, 6]. Secretion of IL-5 and IL-9 instead or in addition to IL-4 and IL-13 is also considered to be Th2 response. Therefore, even though IL-4 is considered to be the signature Th2 cytokine, other Th2 cytokines such as IL-5, IL-9 and IL-13 also have both and unique and overlapping functions [5, 7–9]. T helper responses now include a third major subset, Th17 [10]. A combination of TGF-β plus IL-6 and the transcription factors STAT3 and ROR-γT are essential for initial differentiation of Th17, which plays an important role in several immune mediated disease processes, but their role in acute GVHD is yet to be explored.
Graft versus host disease (GVHD) is the major complication of allogeneic bone marrow transplantation (BMT). GVHD pathophysiology is complex, but is critically dependent on the recognition of host MHC and / or minor histocompatibility antigens on host APCs by the naïve donor T cells and the subsequent differentiation of donor T cells primarily into Th1 cells [10]. The role of Th1/Th2 cell balance in pathophysiology of GVHD is unclear. For example, several studies have demonstrated that polarization towards Th2 regulates GVHD. Few others have, by contrast, shown that Th1 polarization can also mitigate GVHD [11]. Deficiency of the signature Th2 cytokine IL-4 in donor T cells caused reduction in the severity of GVHD [12]. In addition, absence of STAT6 in donor T cells caused distinct target organ damage and GVHD related mortality, albeit at a slightly slower rate, when compared with WT donor T cells [13]. However these studies did not evaluate the impact of complete absence of all Th2 cytokines because both IL-4 and STAT6 deficient animals can secrete other Th2 cytokines under certain conditions [4]. Therefore, to determine the impact of complete absence of all Th2 cytokines, we explored the effect of combined quadruple (IL-4, IL-5, IL-9 and IL-13) Th2 cytokine deficiency (Quad-KO) in donor T cells on GVHD in a well characterized MHC mismatched BALB/c→B6 model of allogeneic BMT.
Materials and Methods
Mice
Female C57BL/6 (B6, H-2b), BALB/c (H-2d) mice were purchased from the Jackson Laboratories (Bar Harbor, Maine). BALB/c-background IL-4 deficient (IL-4KO, H-2d) and BALB/c-background STAT6 deficient (STAT6KO, H-2d) mice were purchased from the Jackson Laboratories. BALB/c-background quadruple type 2 cytokine deficient (Quad-KO, H-2d) mouse was described previously [14]. Briefly, multiple rounds of gene targeting in embryonic stem (ES) cells were performed to generate mouse lines containing combinations of disruptions in the il-4/il-5/il-13 gene cluster. Animals derived from ES cells targeted at multiple alleles were then interbred with IL-9-deficient animals to complete the disruption of up to four Th2 cytokines because the genes encoding the Th2 cytokines il-4, il-5, and il-13 all map to a gene cluster mouse chromosome 11 while il-9 is mapped to mouse chromosome 13 [14].
Bone marrow transplantation
Splenic T cells from BALB/c or Quad-KO donors were enriched by AutoMACS using anti-CD90 microbeads (purity of ≥ 90%). Bone marrow T cells from wild type BALB/c were also depleted by AutoMACS. Recipient B6 mice were irradiated (137Cs source) with 11 Gy total body irradiation (TBI) and injected with 4 × 106 T cells from either allogeneic wild type (WT), Quad-KO, IL-4KO or STAT6KO BALB/c donors along with 5 × 106 T cell-depleted bone marrow cells (TCD-BM) from WT BALB/c donors. BALB/c mice injected with BALB/c TCD-BM served as syngeneic controls. Mice were housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated water for the first 3 weeks after BMT and filtered water thereafter. Survival was monitored daily, clinical GVHD was assessed weekly and detailed histopathologic analyses of liver and intestine were performed as described previously [16]. All animal studies were performed per the institutional IACUC guidelines.
Flow cytometric analysis
Flow cytometric analysis was performed using FITC-, PE, or APC-conjugated monoclonal antibodies (mAbs) to mouse CD4, CD8, H2Dd (BD Pharmingen, San Jose, CA), CD25 and Foxp3 (eBioscience, San Diego, CA). Cells were stained, analyzed on a FACSVantage SE (Becton Dickinson, San Jose, CA).
Enzyme-linked immunosorbent assay (ELISA)
ELISAs for TNF-α, IL-2, IFN-γ (BD Pharmingen), IL-17 (R&D Systems, Minneapolis, MN) and Limulus Amebocyte Lysate (LAL) assay (Bio Whittaker, Walkersville, MD) for lipopolysaccaride (LPS) were performed as described previously and according to manufacturers protocol [16].
In vitro proliferation assay
For measurement of allogeneic responses, BALB/c or Quad-KO T cells were magnetically separated from the spleen by AutoMACS using CD90-microbeads and 2 × 105 of these were incubated with 2 × 104 of B6 bone marrow-derived dendritic cells for 48h, 72h and 96h. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells during the last 12 h of culture was measured. For measurement of non-specific TCR stimulation induced responses, 1 × 105 CD90+ T cells or CD4+ T cells were stimulated with soluble anti-CD3e (clone 145-2C11, eBioscience, San Diego, CA) (1 µg/ml) for 48h, 72h and 96h. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells during the last 6 h of culture was measured.
Cell-mediated cytotoxicity assay
Spleen cells (3 × 106) from naïve BALB/c or Quad-KO mice were cultured with irradiated (137Cs source) B6 spleen cells (5 × 106) at 37°C in a 7.5% CO2 atmosphere for 5 days and used as effector cells after making CD8+ T cell number the same. In some experiments, 14 days after BMT the splenocytes from the recipient animals were harvested and normalized for donor CD8+ T cells and then utilized directly as effector cells. P815 (H-2d for syngeneic) and EL4 (H-2b for allogeneic) were labeled by incubating 2 × 106 cells with 2 MBq of Na2 51CrO4 (PerkinElmer Life, Boston, MA) for 1.5 h at 37°C in a 5% CO2 atmosphere and used as target cells. After washing 2.5 × 103 labeled targets were resuspended and the 51Cr-release assay was performed. These preparations were added to triplicate wells at varying effector-to-target ratios and incubated for 4 h. Maximal and background release was determined by the addition of Triton-X 100 (Sigma) or media alone to targets, respectively. 51Cr activity in supernatants taken 4 hours later was determined in an autogamma counter (Packard, Meridian, CT).
In vitro suppression assay
CD4+CD25− and CD4+CD25+ T cells were isolated from spleen cells from wild type or Quad-KO animals using MACS system. Briefly, spleen cells were incubated with biotin conjugated anti-CD8, CD11b, CD45R(B220), DX5 and Ter-119. CD4+ cells were negatively selected by anti-biotin microbeads. CD4+CD25− and CD4+CD25+ T cells were separated using PE-conjugated anti-CD25 and anti-PE microbeads. The purity of each type of cells was > 85%. CD4+CD25+ T cells were serially diluted from 1 × 104 to 1250 cells/well and incubated with 1 × 104 CD4+CD25− T cells and 5 × 104 irradiated (137Cs source) syngeneic BALB/c spleen cells in the presence of 5 µg/ml anti-CD3e mAb (clone 145-2C-11, eBioscience, San Diego, CA) for 72h. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells was measured during the last 6 h of culture.
Histology
Formalin-preserved small and large bowel were embedded in paraffin, cut into 5-µm thick sections, and stained with haematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (C. Liu). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD [17]. Briefly, for small intestine: villous blunting, crypt regeneration, loss of enterocyte brush border, luminal sloughing of cellular debri, crypt cell apoptosis, crypt destruction, and lamina propria lymphocytic infiltrate; for colon: crypt regeneration, surface colonocytes, colonocyte vacuolization, surface colonocyte attenuation, crypt cell apoptosis, crypt destruction, and lamina propria lymphocytic infiltrate. The scoring system denoted 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen. After scoring the codes were broken and data compiled.
Statistical analysis
The Mann Whitney-U test was used for the statistical analysis of in vitro data while the Wilcoxon rank test was used to analyze survival data. P< 0.05 was considered statistically significant.
Results and Discussion
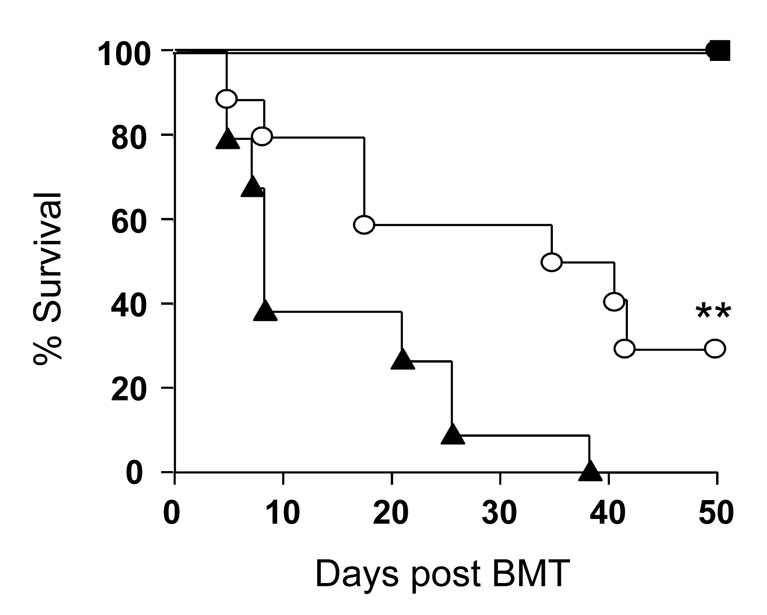
The role of Th1 / Th2 polarization of donor T cells in the patho-physiology of acute GVHD is not fully understood. To evaluate the role of Th2 cytokines from donor T cells in the severity of acute GVHD, we utilized T cells from quadruple IL-4, IL-5, IL-9 and IL-13 deficient (Quad-KO) donors in a well characterized MHC mismatched BALB/c→B6 experimental model of GVHD. Recipient B6 mice were lethally irradiated and injected with 4 × 106 T cells from allogeneic WT or Quad-KO BALB/c donors along with 5 × 106 T cell-depleted (TCD) BM from WT BALB/c donors. BALB/c animals that received BM from WT and T cells from Quad-KO donors served as syngeneic controls. As shown in Figure 1, B6 animals that received Quad-KO T cells demonstrated significantly greater GVHD mortality than B6 animals that received allogeneic T cells from WT BALB/c animals (P< 0.01). All of the syngeneic B6 animals irradiated with 11 Gy and injected with B6 BM and T cells as well as the syngeneic BALB/c animals that received Quad-KO donor T cells survived demonstrating lack of toxicity from either conditioning or homeostatic expansion of Quad-KO T cells (Fig. 1).
Figure 1. Severe acute GVHD induced by Quad-KO T cells.
Recipient C57BL/6 were irradiated (11Gy) and injected 4 × 106 wild type BALB/c CD90+ T cells (○ n= 10) or Quad-KO CD90+ T cells (▲, n= 10) along with 5 × 106 wild type BALB/c TCD-BM or syngeneic B6 (n= 5) BM and T cells . Recipient BALB/c (n= 6) animals were irradiated (8 Gy) and injected 4 × 106 wild type BALB/c CD90+ T cells (●) along with 5 × 106 wild type BALB/c TCD-BM. Survival was monitored daily. ** ○ vs ▲, P<0.01. Data shown are from 1 of 2 experiments with similar results.
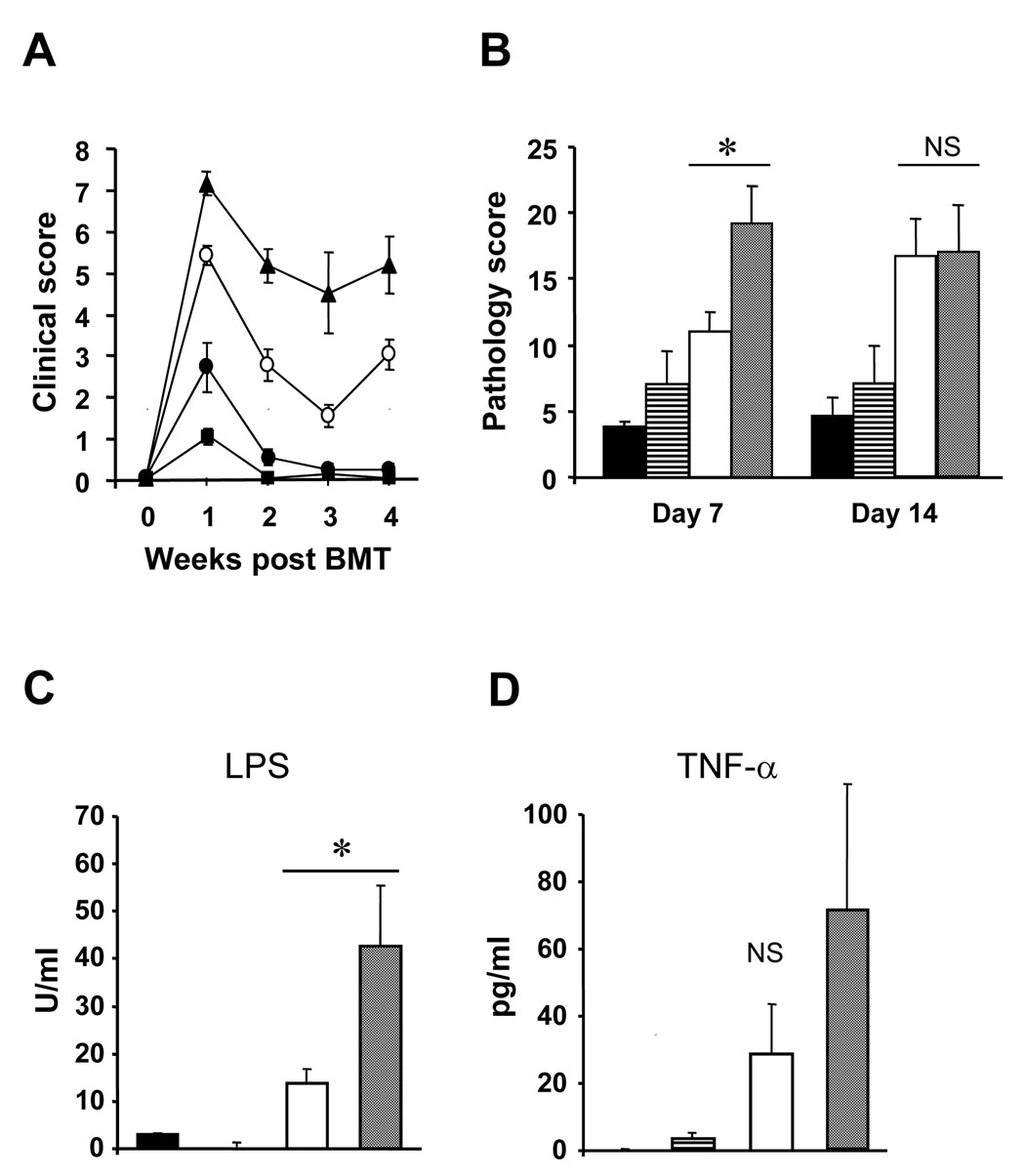
In addition to survival, we also analyzed all other parameters that indicate the severity and specificity of acute GVHD in this model, namely: clinical score, target organ histopathology and serological analyses (LPS and TNF-α) [18, 19]. As shown in Figure 2A, consistent with increased mortality, the B6 animals that received allogeneic Quad-KO T cells demonstrated significantly greater clinical severity than either the syngeneic B6 or syngeneic BALB/c animals. The increase in GVHD in the allogeneic recipients of Quad-KO mice when compared to animals that received cells from WT allogeneic donors was also reflected target organ histopathology on day 7 (Fig 2B). This analysis was performed on day +7 as earlier studies with similar MHC mismatched experimental BMT models have demonstrated that differences in target organ damage correlated well with GVHD specific damage while conditioning related damage was minimal at this time point [16, 18]. Additional analyses were performed on day +14 to determine whether the greater severity in histopathology persisted. As shown in Figure 2B, although all of the allogeneic animals continued to show greater damage than syngeneic controls the target organ histopathology of animals that received allogeneic cells from both the WT and Quad KO was similar. We next measured serum levels of LPS and TNF-α on day +7 and found that their levels were higher in the allogeneic B6 mice that received cells from Quad KO donors when compared with animals that were injected with allogeneic WT T cells (Figure 2C and 2D). Collectively these data demonstrate that complete deficiency of the four classical Th2 cytokines causes more severe GVHD as determined by survival, clinical severity that correlated with greater histo-pathological damage and serological parameters early after BMT.
Figure 2. Pathology and serological marker were higher in the hosts of Quad-KO T cells.
Recipient C57BL/6 were irradiated (11Gy) and injected 4 × 106 wild type BALB/c CD90+ T cells (○) or Quad-KO CD90+ T cells (▲) along with 5 × 106 wild type BALB/c TCD-BM. Recipient BALB/c were irradiated (8 Gy) and injected 4 × 106 wild type BALB/c CD90+ T cells (●) along with 5 × 106 wild type BALB/c TCD-BM. Recipient C57BL/6 were irradiated (11 Gy) and injected 4 × 106 C57BL/6 CD90+ T cells (■) along with 5 × 106 C57BL/6 TCD-BM. Clinical GVHD was assessed weekly (A) as described in Materials and Methods. Pathology of GVHD target gastrointestinal systems of the hosts on day 7 and 14 after BMT were evaluated as described in Materials and Methods (B). Day 7 serum LPS (C) and TNF-α (D) concentration were measured by Amebocyte Lysate (LAL) assay and ELISA respectively. The results from BALB/c→BALB/c (black bars), C57BL/6→C57BL/6 (striped bars), BALB/c→B57BL/6 (white bars) and Quad-KO→C57BL/6 (gray bars) BMT mouse samples are shown as the same column patterns in B, C and D. Data shows mean ± SEM. * P<0.05, NS: not significant
STAT6 is critical for both IL-4, the signature Th2 cytokine, and IL-13 signaling and plays in Th2 polarization. Although Th2 polarization of naïve donor T cells has been shown to reduce GVHD, when compared to WT donor T cells, IL-4 deficient donor T cells caused less severe GVHD, while STAT6 deficient (near but not complete absence of Th2 cytokine secretion) donor T cells caused similar GVHD, but in distinct target organs. By contrast, we observed that Quad-KO T cells caused greater GVHD. We therefore next compared the ability of different and variable Th2 cytokine deficiency in donor T cells in causing GVHD. To this end, to rule out the potential strain related variability, we simultaneously compared the severity of GVHD in the absence of IL-4 or STAT6 or all four Th2 cytokine in the same BMT model.
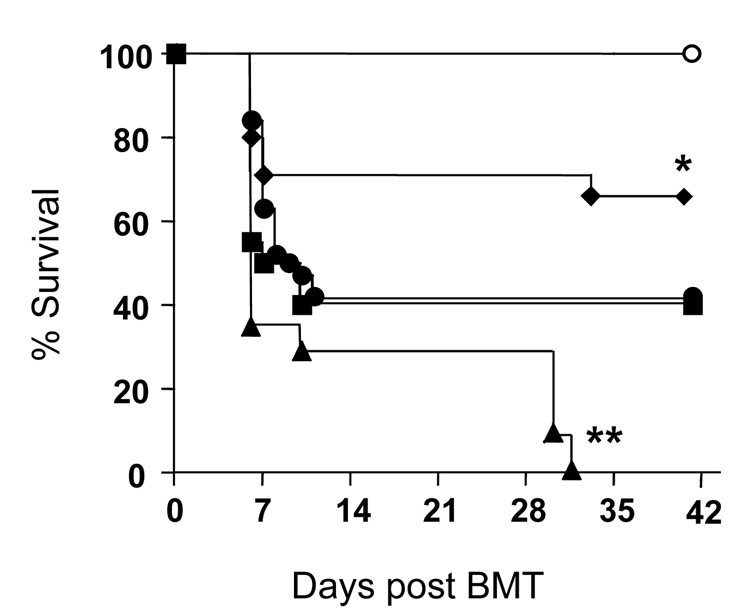
Lethally irradiated B6 animals received TCD BM from WT BALB/c donors along with T cells from either the WT or IL-4KO or STAT6KO or Quad-KO BALB/c donors. As shown in Figure 3, consistent with earlier data, 100% of the B6 animals that received Quad-KO T cells were dead by the end of the observation period. By contrast, animals that received T cells from either the WT or IL-4KO or STAT6KO demonstrated significantly less mortality. Furthermore, in accordance with previous reports [13, 14], survival of the animals receiving IL-4KO T cells was better, while that of the recipients receiving STAT6KO T cells was comparable to that of received WT T cells (67% (14/21) or 40% (8/20), respectively, Fig. 3). These data show that in contrast to variable effect of partial Th2 deficiency of donor T cells (as in IL-4KO and STAT6KO), complete absence of all Th2 cytokines aggravates GVHD.
Figure 3. Combined Th2 cytokine deficiency cause greater GVHD mortality.
Recipients C57BL/6 were irradiated (11Gy) and injected with 4 × 106 T cells from C57BL/6 (○, n =9), wild type BALB/c (●, n = 12), BALB/c-background IL-4KO (◆, n =21), STAT6KO (■, n =20) and Quad-KO (▲, n =11) along with 5 × 106 background-matched wild type TCD-BM and survival was monitored daily. Data are combined from 2 similar experiments. ▲. vs ●,■,◆,** P<0.03; ●. vs ◆,* P<0.05;●. vs ■, P= NS.
We next evaluated the potential mechanisms for the induction of greater severity of acute GVHD by the absence of all Th2 cytokines. Because donor T cell functions are critical for GVHD, we hypothesized that the complete absence of Th2 cytokines would enhance the T cell functional capabilities. To this end we systematically evaluated several T cell functional parameters such as donor T cell proliferation, cytokine secretion, cytotoxicity (CTL) and regulatory T cell functions.
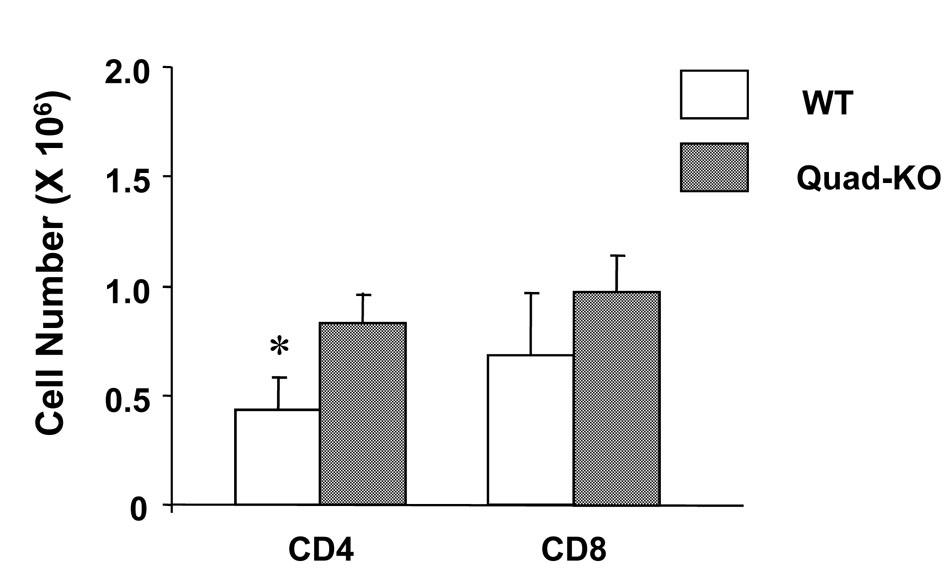
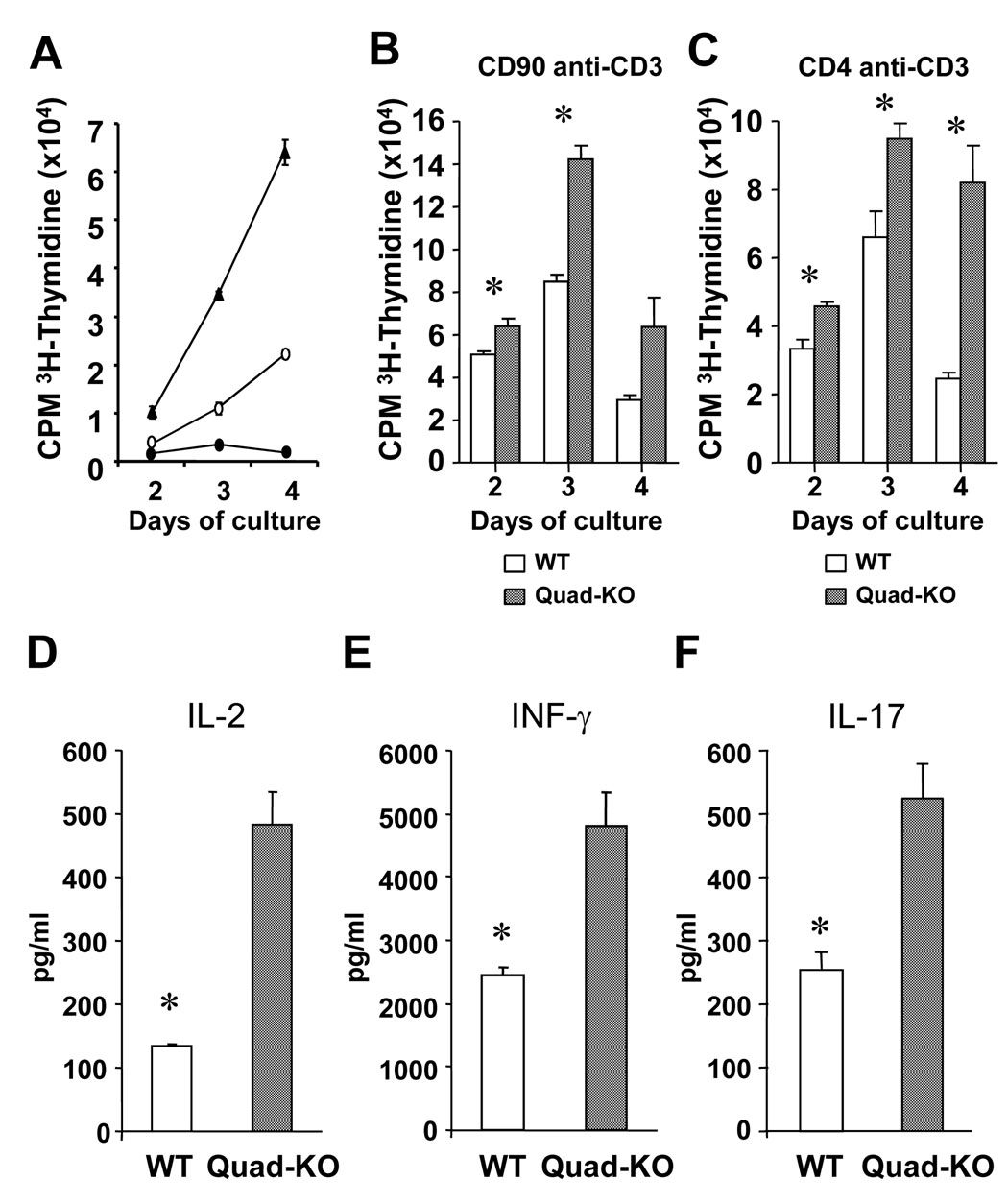
We first determined whether the Quad-KO T cells demonstrated greater proliferation, in vivo, after allogeneic BMT by harvesting the splenocytes from the recipient animals. As shown in Figure 4, Quad-KO T cells demonstrated significantly greater T cell expansion when compared to the expansion of donor T cells from the B6 animals that received T cells from WT donors. We next analyzed whether the greater expansion of Quad-KO T cells is specific allo-stimulation or is a consequence of T cell intrinsic proliferative effect when compared to WT T cells. Quad-KO T cells showed significantly greater proliferation than WT T cells when stimulated in an MLR with allogeneic B6 BM derived dendritic cells (Fig. 5A). The increase in proliferation was also, as expected, associated with greater Th1 cytokine secretion, IFN-γ, IL-2 and also IL-17 (Fig. 5D and 5E, 5F).
Figure 4. Donor T cell expansion in vivo.
On day 7 after BMT, spleen cells harvested from the recipients received wild type or Quad-KO T cells, and staining with mAbs against donor-maker (H-2Dd) and CD4 or CD8. The cells were analyzed by FACSVantage SE and the numbers of donor-derived CD4 or CD8 in the host spleen (n = 3–4 mice/ group) were calculated. The difference of the number of donor CD4+ cells was statistically significant. Data shows mean ± SEM. * P<0.05.
Figure 5. In vitro proliferation and cytokine production of Quad-KO T cells.
CD90+ T cells from Quad-KO (▲), wild type BALB/c (○) and C57BL/6 (●) mice were cultivated with C57BL/6 bone marrow derived DCs induced by GM-CSF. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells was measured during the last 12 h of indicated culture days (A). CD90+ T cells from Quad-KO and wild type BALB/c mice were cultivated in the presence of soluble anti-CD3e mAb (1 µg/ml) for 48h. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells was measured during the last 6 h (B). CD4+ T cells from Quad-KO and wild type BALB/c mice were cultivated in the presence of soluble anti-CD3e mAb (1 µg/ml) for 48h. Incorporation of 3H-thymidine (1 µCi/well) by proliferating cells was measured during the last 6 h (C). Culture supernatants from C57BL/6 DCs and wild type or Quad-KO T cells were harvested and measured the concentration of IL-2 (D), IFN-γ (E) and IL-17 (F). Data are from one of three similar experiments and shown as mean ± SEM. * P<0.05
Next, given greater in vivo proliferation of Quad KO CD4+ when compared to WT CD4+, we analyzed whether this was due to intrinsic differences in proliferation or due to differences in the frequency of alloreactive T cell precursors. Consistent with the difference in proliferation from in vivo or in vitro allo-stimulation, as shown in Figure 5B, there was a significant difference in proliferation of the Quad-KO and the WT CD90+ T cells CD4+ T cells upon non-specific TCR by anti-CD3 mAb. These data suggest that there might be cell intrinsic proliferative differences between the Quad-KO T cells and WT CD4+T cells. While the exact mechanisms for this remain unclear, it is likely that the differences in response to allo-stimulation are unlikely to be secondary to greater allo-precursor frequency. However, it is possible that the proliferative differences observed in vivo might have been additionally enhanced by the presence of greater tertiary signals from the cytokines such as TNF-α and IFN-γ.
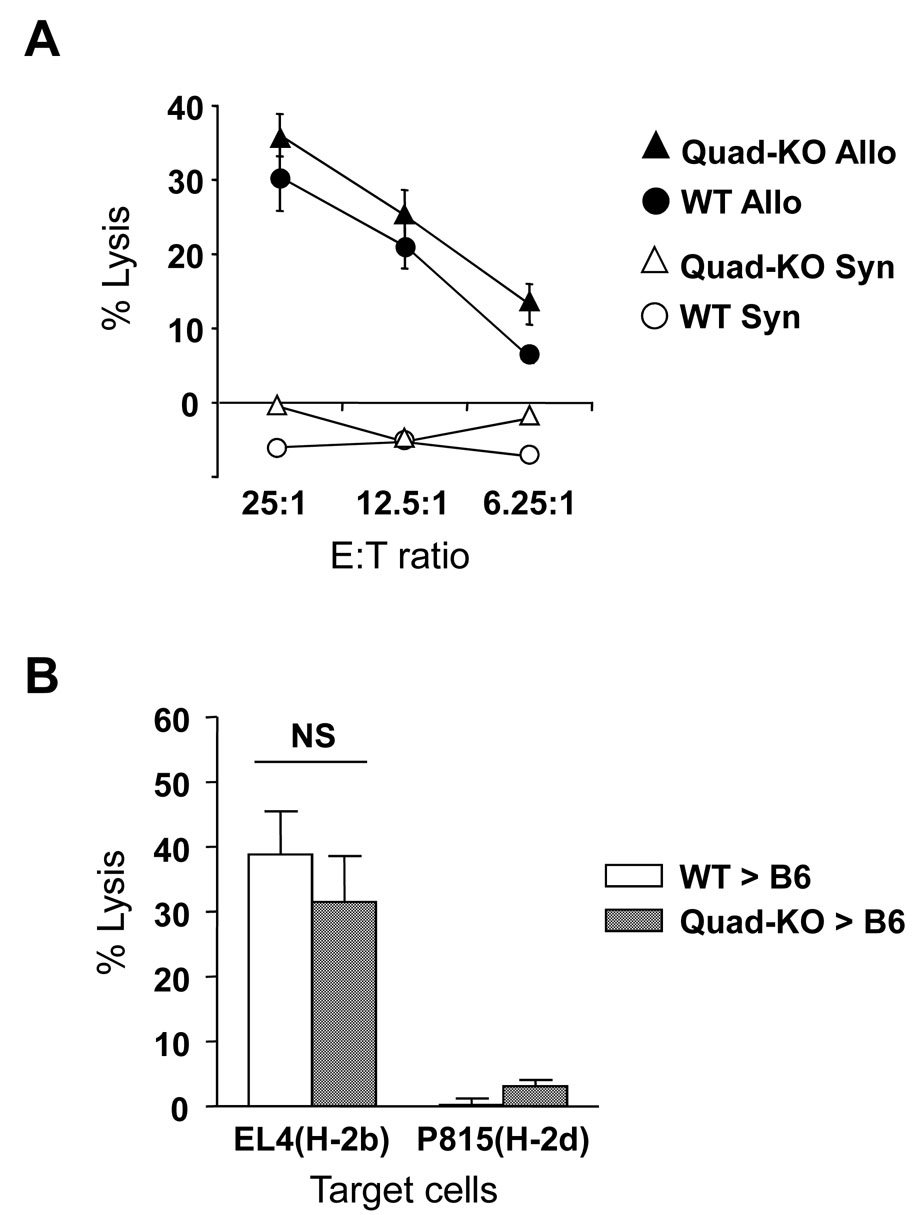
Cytotoxicity (CTL) is a critical function of T cells, and donor T cell CTL plays an important role in the severity of GVHD [20]. We therefore, next compared the allo-specific cytotoxicity of the CD8+ T cells from the Quad-KO and WT animals after in vitro stimulation and normalization for CD8+ T cells as in Materials and Methods. As shown in Figure 6A, there were no significant per cell differences in the allo-specific cytotoxicity of CD8+ T cells from the Quad-KO CD8+ T cells when compared to CD8+ cells from the WT animals. Given greater proliferation of Quad KO T cells than WT CD4+ T cells after allo-BMT, we next determined whether overall CD8+ CTL was altered in the presence of CD4+ T cells after in vivo allo-stimulation. To this end, splenocytes were harvested on day +14 after BMT from the allogeneic recipients that received T cells from Quad KO and WT T cells. The cells were normalized for all donor T cells and evaluated for allo-specific cytotoxicity against EL-4 targets as in Materials and Methods. As shown in Figure 6B, no significant differences were observed between in overall CTL killing by the CD8+ T cells from the Quad KO and WT donors after in vivo stimulation in the presence of CD4+ T cells. Together, these data suggest that greater severity of acute GVHD caused by Quad-KO T cells is likely due to greater proliferation CD4+ T cell expansion and the related enhanced cytokine storm but not due to enhanced CD8+ CTL function.
Figure 6. Allo-specific cytotoxic activities of Quad-KO T cells.
(A) Spleen cells from wild type or Quad-KO mice were incubated with allogeneic C57BL/6 spleen cells for 5 days. The cells were stained with PE-anti-CD8 mAb and analyzed the percentage of CD8+ cells. After making CD8+ T cell number the same, cells were incubated with 51Cr-labeled EL4 (H-2b for allogeneic) and P815 (H-2d for syngeneic) for 1.5 h at 37°C in a 5% CO2 atmosphere at indicated ratio. Specific lysis of Quad-KO against allogeneic (▲), BALB/c against allogeneic (●), Quad-KO against syngeneic (open triangle) and BALB/c against syngeneic (○) are shown. Data shows mean ± SEM. P= NS
(B) Spleen cells were harvested on day +14 after BMT from allogeneic recipients of T cells wild type (open bar) or Quad-KO (solid bar) mice. The cells were normalized after staining for PE-anti-CD3 mAb and FITC CD45.1 cells. They were then utilized in a CTL assay against 51Cr-labeled EL4 (H-2b for allogeneic) and P815 (H-2d for syngeneic) targets. Data shows mean ± SEM. Open bar vs solid bar, P= NS
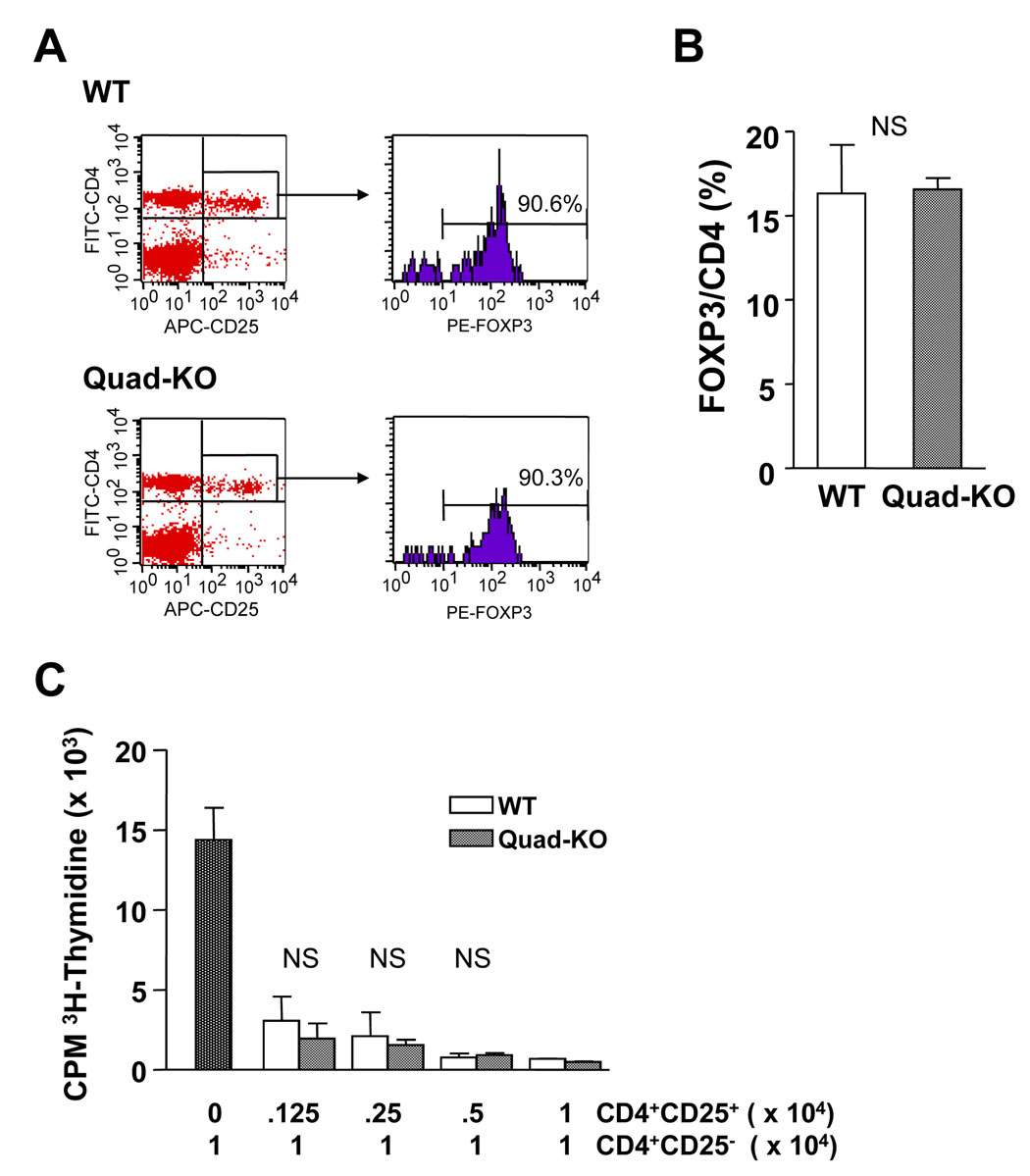
Because recent data show that the naturally arising CD4+CD25+ donor T cells (Tregs) can down regulate GVHD [21], we next tested whether the greater severity of GVHD caused by Quad-KO T cells might be due to a reduction in either the numbers or the function of the CD4+CD25+Foxp3+ natural regulatory T cells. We found no significant differences between the Quad-KO and WT animals in the numbers of Tregs (Figure 7A and B). Moreover, the Tregs from Quad-KO animals demonstrated equivalent suppression of the naïve WT CD4+CD25− proliferation (Figure 7C). Conversely, we also explored whether naïve Quad-KO T cells were equally amenable to suppression by the naturally arising Tregs. WT Tregs caused equivalent suppression of naïve Quad-KO and WT T cells (data not shown). Thus, these data demonstrate that (a) the four Th2 cytokines are not essential for either the development or the function of naturally arising Tregs and (b) that the Quad-KO naïve T cells exhibit no inherent differences in their ability to be suppressed by Tregs. Recent data show that IL-9 has an important regulatory role in allograft rejection model [22], and therefore it is possible that the Quad-KO T cell recipients show aggravated GVHD as a consequence of the absence of the inhibitory effect of IL-9. It remains to be determined whether the absence of IL-5 or IL-9 or IL-13 alone would aggravate GVHD.
Figure 7. Suppressive activities of Quad-KO CD4+CD25+ T cells.
(A) Splenocytes were harvested from Quad KO (n= 7, gray bar) and WT (n= 7, (black bar) animals and then analyzed for the percent of CD4+25+Foxp3+ T cells. Black bar vs gray bar, P= NS.
Suppression activities of CD4+CD25+ T cells isolated from (B) wild type BALB/c (WT) or (c) Quad-KO animals (Quad-KO) against CD4+CD25− T cells isolated from wild type BALB/c animals were measured. CD4+CD25+ T cells isolated from WT or Quad-KO animals were serially diluted from 1 × 104 to 1250 and cultivated with 104 CD4+CD25− T cells isolated from WT animals in the presence of 5 × 104 irradiated WT BALB/c spleen cells and 5 µg/ml of anti-CD3e mAb for 72h. Incorporation of 3H-thymidine (1µCi/well) by proliferating cells was measured during the last 6 h of culture. Data shows mean ± SEM. P= NS.
T helper responses have now grown to include a third major subset, Th17, in addition to the Th1 and Th2 subsets. A combination of TGF-β plus IL-6 and the transcription factors STAT3 and ROR-γT were recently described to be essential for initial differentiation of Th17 cells while IL-23 and IL-21 for the later stabilization and amplification of the Th17 cell subsets. They have recently emerged as a critical subset that plays an essential role in the protection against certain extra-cellular pathogens and also against certain self-antigens that lead to the development of inflammation and severe autoimmunity [23]. But the role of IL-17 in pathophysiology of GVHD is not known. We evaluated whether Quad-KO T cells show greater Th17 polarization than the WT T cells. We measured the concentration of IL-17 in the sera collected from the recipients 7 days after BMT and culture supernatant of MLR. The concentrations of IL-17 on day 7 between the recipients of Quad-KO and WT T cells were not significantly different (data not shown), but the amount of IL-17 in the MLR supernatant of Quad-KO T cells against B6 was significantly higher than the that of WT BALB/c T cells (Fig. 5E). These data suggest that the absence of Th2 cytokines might lead to an enhanced Th17 polarization. Future studies will determine whether IL-17 is critical for the exacerbation of GVHD caused by the absence of Th2 cytokines.
Together our data clearly demonstrate that complete absence of Th2 cytokine secretion by donor T cells enhances GVHD mortality and that this aggravation is associated with greater expansion and cytokine secretion but not due to alteration in either the CTL or regulatory functions of the donor T cells. Thus preservation of a full repertoire of Th2 cytokines might help mitigate GVHD.
Acknowledgments
Funding sources
Supported by Grants from National Institutes of Health K08 AI052863-01 (P.R.), P.R. is the recipient of the Alaina J. Enlow Scholar Award from Amy Strelzer Manasevit – National Marrow Donor Program and the Clinical Faculty Development Award from the American Society of Transplantation.
Abbreviations
- BMT
bone marrow transplantation
- GVHD
graft-versus- host disease
- Th1
T helper 1
- Th2
T helper 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Ann Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 4.Alexander L Dent, Jane Hu-Li, William E Paul, Louis M Staudt. T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc Natl Acad Sci. 1998;95:13823–13828. doi: 10.1073/pnas.95.23.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9(3):423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189(10):1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang W, Löhning M, Gao Z, Assenmaher M, Ranganath S, Radbruch A, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12(1):27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 8.Kopf M, Brombacher F, Hodgikin PD, Ramsay AJ, Milbourne EA, Dai WJ, et al. IL- 5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4(1):15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 9.Townsend MJ, Fallon PG, Matthews DJ, Smith P, Jolin HE, McKenzie AN. IL-9- deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13(4):573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 10.Steinman L. A brief history oh Th17, the first major revision in the Th1/Th2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara JL, Cooke KR, Teshima T. The Pathophysiology of Graft-vs.-Host Disease. In: Ferrara JL, Cooke KR, Deeg HJ, editors. Graft-vs.-Host Disease. New York, NY: Marcel Dekker; 2005. pp. 1–34. [Google Scholar]
- 12.Sykes M, Szot GL, Nguyen PL, Pearson DA. Interleukin-12 inhibits murine graft-versus-host disease. Blood. 1997;86(6):2429–2438. [PubMed] [Google Scholar]
- 13.Murphy WJ, Welniak LA, Taub DD, Witrout RH, Taylor PA, Vallera DA, et al. Differential effects of the absence on interferon-γ and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102(9):1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105(9):1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9 and IL-13. Immunity. 2002;17(1):7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 16.Reddy P, Teshima T, Hildebrandt G, Williams DL, Liu C, Cooke KR, et al. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101(7):2877–2885. doi: 10.1182/blood-2002-08-2566. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P, Teshima T, Kukuruga M, Ordemann R, Liu C, Lowler K, et al. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001;194(10):1433–1440. doi: 10.1084/jem.194.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damages and inflammatory cytokines. Blood. 1997;90(8):3204–3213. [PubMed] [Google Scholar]
- 19.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmmonte J, Jr, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 20.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. [PubMed] [Google Scholar]
- 21.Nguyen V, Zeiser R, Negrin RS. Role of naturally arising regulatory T cells in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:995–1009. doi: 10.1016/j.bbmt.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell triology. Curr Opin Immunol. 2007;19(6):652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]