Abstract
A germline TP53 R337H mutation is present in childhood adrenocortical tumors (ACT) from southern Brazil. Other genetic alterations are also frequently found in these tumors. This study was designed to assess whether alterations of the 11p15 region exist in childhood ACT, accounting for IGF2 overexpression in these tumours, and how they are related to clinical outcome. Tumor DNA of 12 children with ACT (4 adenomas and 8 carcinomas) and from the blood of their parents was analyzed. All patients showed 11p15 LOH in the tumor. In contrast to the single case of paternal LOH, IGF2 was overexpressed in tumors with maternal allele loss. Our data show that 11p15 LOH is a widespread finding in childhood ACT not related with malignancy, contrarily to adult ACT. Alterations in the expression of other genes in the same region (e.g. CDKN1C) may contribute to ACT tumorigenesis.
Keywords: adrenal cortex, cancer, loss of heterozygosity
Introduction
Adrenocortical tumors (ACT) are a rare neoplasm that can be found either in the spectrum of genetically determined syndromes (Beckwith-Wiedemann, Li-Fraumeni, Carney's complex, MEN type I, McCune-Albright) or isolated, in a familiar or sporadic fashion [see 1 for review]. In children they most commonly present with virilization, due to the production of androgenic steroids, which may be associated with Cushing syndrome, while signs of increased mineralocorticoid production are very rare [2]. Survival rate at 5 years in children with ACT is 54 to 75% [2, 3]. Despite easy diagnosis of the tumor and a similar pattern of gene expression profiles [4, 5], differences between childhood and adult ACTs exist at various levels, including clinical manifestations and hormone secretion profile. Accurate prognostic predictions beyond the parameters of tumor size, weight and mitotic activity [2, 3] are more difficult for childhood than for adult ACT.
The predominant androgenic hormone profile is common to the adrenal cortex fetal zone and childhood ACT. Because of that, childhood ACT is thought to originate from the adrenal fetal zone, a part of the human adrenal cortex transiently present during development, which regresses immediately after birth due to massive programmed cell death [6, 7]. The possibility of abnormal fetal zone cell survival followed by proliferation is likely to occur whenever TP53 is non-functional, which is the case in more than 50% of the cases of sporadic ACT or linked to the Li-Fraumeni syndrome [8, 9]. In southern Brazil, where the incidence of childhood ACT is about ten to fifteen times higher than in the rest of the world, almost all tumors present the germline TP53 R337H mutation, with loss of the wild type allele in most of the cases [10]. This mutation may predispose carriers to reduced TP53 function in conditions of elevated temperature and pH [11]. In addition, other factors may play an important role in the pathogenesis of ACT, including an increased copy number and expression of transcription factor Steroidogenic Factor 1 (SF-1) [12–14], loss of the putative protective role of the alpha inhibin gene (INHA) [15] and increased levels of the IGF2 growth factor [5, 7]. In particular, very high expression of the IGF2 transcript and protein has been found in nearly all childhood ACT studied [5, 7]. IGF2 is a growth factor abundantly expressed by the human fetal adrenal which triggers fetal adrenocortical cell proliferation and steroidogenesis in a fashion regulated by ACTH [6]. The IGF2 gene on chromosome 11p15 is expressed in an imprinted fashion from the paternal allele, under the control of a differentially methylated region (DMR1) or imprinting centre 1 (IC1) lying between IGF2 and the more telomeric H19 gene. IGF2 and H19 expression is regulated by a common set of enhancers located 3' of H19 [see 16 for review]. In normal conditions, the IC1 is methylated on the paternal allele, thereby hindering the accessibility of the zinc finger protein CTCF, whose binding on the maternal allele blocks the access of the IGF2 promoter to the downstream enhancers and inhibits its expression. Another differentially methylated region (DMR2) or imprinting centre 2 (IC2) exists in 11p15 in a location close to IC1, that sets the conditions for expression of the KCNQ1 and CDKN1C genes from the maternal allele and the antisense KCNQ1OT1 transcript from the paternal allele. Importantly, a defect of imprinting of the 11p15 region causes the Beckwith–Wiedemann syndrome, which manifests as a syndrome of somatic overgrowth, congenital malformations and tumor (including adrenocortical cancer) predisposition [16]. Previous studies in adult ACT have shown that IGF2 gene overexpression and abnormalities in the 11p15 region are present frequently in carcinomas but only rarely in adenomas [17], while a single study in pediatric ACT found increased IGF2 levels and loss of heterozygosity (LOH) at 11p15 in all 4 patients analyzed [7].
Our previous gene expression profiling study of childhood ACT revealed that, on top of very high IGF2 mRNA levels, these tumors express significantly reduced levels of the KCNQ1 and CDKN1C transcripts compared to the normal adrenal cortex [5]. These data suggest that alterations in the 11p15 imprinted region exist in childhood ACT positive for the R337H TP53 mutation and prompted us to analyze this genomic region and to correlate our findings to clinical and pathological data.
Patients and Methods
Patients
Clinical data of the 12 childhood ACT patients included in the study for 11p15 LOH are shown in Table 1. In all cases, one of the parents or legal representatives signed an informed consent form approved by the Ethics Committee of the Hospital de Clínicas of the Federal University of Paraná.
Table 1.
Clinical and pathological data of patients.
| Patien | Age at diagnosis (months) | Histolog y | Gender | Clinical type | Staging (initial) | Recurrence | Outcome and follow-up | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | Adenoma | F | V+C | - | No | Alive w/o disease 59 m | - |
| 2 | 21 | ACC | M | V | I | No | Alive w/o disease 87 m | - |
| 3 | 39 | ACC | F | V | III | No | Deceased 52 m | Adrenal insufficiency |
| 4 | 18 | ACC | M | V | III | Local/Lymp h nodes | Deceased 29 m | Dead of disease progression |
| 5 | 52 | ACC | F | V+C | II | Lung | Deceased 63 m | Adrenal insufficiency |
| 6 | 110 | ACC | M | V+C | II | Lung | Deceased 132 m | Dead of disease progression |
| 7 | 9 | Adenoma | F | V+C | - | No | Alive w/o disease 78 m | - |
| 8 | 59 | ACC | F | V | II | Local/Liver/Lung | Deceased 89 m | Dead of disease progression |
| 9 | 11 | Adenoma | F | V | - | No | Alive w/o disease 49 m | - |
| 10 | 26 | ACC | F | V | I | No | Alive w/o disease 80 m | - |
| 11 | prenatal | Adenoma | F | NF | - | No | Alive w/o disease 49 m | - |
| 12 | 152 | ACC | F | C+Hypert | IV | Lung | Deceased 189 m | Adrenal + renal insufficiency |
ACC, adrenocortical carcinoma; V, virilizing; C, Cushing's syndrome
DNA extraction
For peripheral blood DNA extraction, frozen samples (500 µl whole blood) were thawed and washed three times by centrifugation in red blood cell lysis buffer (1.6 M sucrose, 5% Triton X-100, 25 mM MgCl2). Lymphocyte pellets were resuspended in 380 µl of proteinase K buffer (75 mM NaCl, 24 mM EDTA, 1% SDS, 40 µg/mL proteinase K) and incubated at 60°C for 30 minutes. Samples were allowed to chill at room temperature, then 110 µl of 5.5M NaCl were added and debris was removed by centrifugation. DNA was precipitated with 2 volumes of absolute ethanol, pelleted by centrifugation and washed with 75% ethanol. The DNA pellet was dried at room temperature and resuspended in 50 µl of ultrapure water.
For tumor DNA extraction, samples (25–30 □g) were incubated with proteinase K and DNA was purified according to the above-mentioned protocol, omitting the lysis step.
TP53 mutation analysis
The presence of the TP53 R337H mutation was evaluated by PCR-RFLP analysis, as previously described [10].
Loss of heterozygosity analysis
The minisatellite markers D11S922, D11S4046, Tyrosine Hydroxylase (TH) and D11S1318 (Fig. 1A) were analyzed by single-strand chain polymorphism (SSCP) as follows. Purified DNA was amplified with Biotaq DNA polymerase RED (Bioline, London, UK) in a 25-µl reaction containing 200 µM dNTPs, 1 mM Mg++ (1.5 mM Mg++ for D11S1318), 20 pmoles 32P-labeled forward primer, 20 pmoles reverse primer, and 2 U Taq polymerase. Primers sequences are shown in Table 2. For the D11S4046 and TH minisatellites, amplification was performed by initial denaturation (95°C, 2 min), followed by 28 cycles of denaturation (94°C for 45 seconds), annealing (60°C for 45 seconds) and extension (72°C for 45 seconds), and a final elongation step (72°C for 5 minutes). For D11S922 and D11S1318, annealing temperature was 58°C; all other parameters were the same. Two microliters of PCR reaction were mixed with 7 µl of loading buffer (95% formamide, 20 mM EDTA, 10 mM NaOH, 0.025% bromophenol blue, 0.025% xylene cyanol) and loaded onto a 6% polyacrylamide – 8 M urea gel (6% acrylamide / bis-acrylamide 29:1, 8 M urea, 1x TBE, 0,07% ammonium persulfate, 0,05% TEMED). Gels were run at 35 W for 3 hours, dried in a gel-dryer, and exposed at room temperature overnight to autoradiograph film (Kodak, Milan, Italy).
Figure 1.
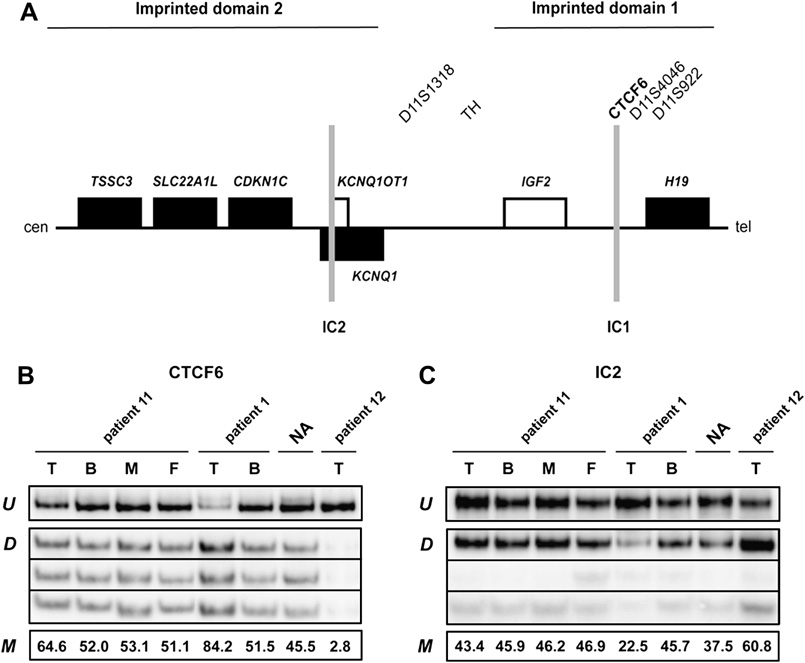
Structure of the imprinted region in chromosome 11p15 harboring the IGF2 gene and methylation analysis of patients 1, 11 and 12. A, Diagram showing the position of imprinted domains 1 and 2. Genes expressed normally only from the paternal allele are shown with white rectangles and genes expressed only from the maternal allele are indicated with black rectangles. The two imprinting centres (IC1 and IC2) are shown in grey. Position of minisatellite markers used for LOH analysis and sites studied for methylation (bold) are indicated. cen, centromere; tel, telomere. B, methylation analysis at CTCF6 (IC1) and C, IC2 sites of 11p15. Genomic DNA samples from tumor (T) and blood (B) of patient 11 and her parents (M, mother; F, father), from tumor (T) and blood (B) of patient 1, from normal adrenal (NA) and from tumor (T) of patient 12 were treated with bisulfite, PCR amplified and then digested with restriction enzymes. U: undigested (unmethylated) DNA. D: digested (methylated) DNA. M: methylated fraction (measured by densitometry and expressed as percent of undigested). The different intensity of the three digested bands in IC2 reflects different fragment length.
Table 2.
Sequences of primers used for LOH analysis.
| Name | Sequence |
|---|---|
| D11S922F | GGGGCATCTTTGGCTA |
| D11S922R | TCCGGTTTGGTTCAGG |
| D11S4046F | ACTCCAGCCTGGGAAAC |
| D11S4046R | TGATAGACACACCCATTGC |
| HUMTH01F | GTGGGCTGAAAAGCTCCCGATTAT |
| HUMTH01R | GTGATTCCCATTGGCCTGTTCCTC |
| D11S1318F | CCCGTATGGCAACAGG |
| D11S1318R | TGTGCATGTNCATGAGTG |
| CTCF6-For | GAGTTTGGGGGTTTTTGTATAGTAT |
| CTCF6-Rev | CTTAAATCCCAAACCATAACACT |
| IC2-For | GTTATTTTATATTTAGTTAGTGTTTTATG |
| IC2-Rev | TCTTACTAAAAAACTCCCTAAAAATC |
Methylation analysis
One microgram of genomic DNA was modified by bisulfite treatment with the EpiTect Bisulfite Kit (Qiagen, Milan, Italy) as by vendor's instructions. CTCF6 and IC2 genomic regions were amplified by PCR (primers sequences are shown in Table 2) and subjected to BstUI digestion, as previously described [18, 19]. Results were analyzed and quantified by phosphoimaging.
Results
Patients' data are shown in Table 1. Four tumors were histologically classified as adenomas and eight as carcinomas. Ten patients presented with virilization that was associated with Cushing's signs and elevated cortisol levels in four cases, one tumor was a non-functioning adenoma (patient 11) and another patient (patient 12) had Cushing's signs and elevated cortisol levels associated with hypertension and high aldosterone levels. Remarkably, in this latter patient, a 12-year-old child, the adrenocortical tumor was a relapse of a previous tumor diagnosed and excised 8 years before.
A diagram of the 11p15 region harboring the IGF2 gene and the position of markers used for analysis is shown in Figure 1A. Of the twelve patients examined, ten showed strong LOH at 11p15 at all informative loci (Table 3). In most of the cases where the parental origin of the lost allele was identifiable (8/12), this was the maternal one. This finding correlated with IGF2 overexpression data in ACT [5]. One case (patient 11) had weak LOH (with loss of the maternal allele; Table 3). Methylation analysis in this tumor sample (Fig. 1B, C) showed slight increased (64.6) and decreased (43.4) methylation at CTCF6 and IC2 sites, respectively. In one single case (patient 12), the LOH analysis was inconclusive due to the lack of peripheral blood and maternal samples (results indicated either a complete loss of the paternal allele, or homozygosity at all loci; Table 3). Methylation analysis (Figure 1B, C) showed very strong hypomethylation at the sixth CTCF binding site (2.8% methylation) and hypermethylation at IC2 (60.8% methylation) in tumor DNA of patient 12, indicative of the potential loss or aberrant methylation of the paternal allele. In agreement with this finding, expression data [5] showed no IGF2 overexpression in this tumor.
Table 3.
Summary of the results of LOH and methylation analyses.
| Microsatellite analysis results | IGF2 expression data | Methylation status | |||||
|---|---|---|---|---|---|---|---|
| Patient | D11S922 | D11S4046 | TH | D11S1318 | CTCF6-IC1 | IC2 | |
| 1 | LOH | N. I. | Maternal LOH | Maternal LOH | OVER | 84% | 23% |
| 2 | LOH | N. I. | Maternal LOH | Maternal LOH | OVER | N.D. | N.D. |
| 3 | Maternal LOH | Maternal LOH | Maternal LOH | Maternal LOH | N.A. | N.D. | N.D. |
| 4 | LOH | Maternal LOH | LOH | Maternal LOH | OVER | N.D. | N.D. |
| 5 | LOH | Maternal LOH | LOH | Maternal LOH | N.A. | N.D. | N.D. |
| 6 | LOH | Maternal LOH | Maternal LOH | N. I. | OVER | N.D. | N.D. |
| 7 | LOH | LOH | LOH | LOH | OVER | N.D. | N.D. |
| 8 | Maternal LOH | Maternal LOH | Maternal LOH | Maternal LOH | OVER | N.D. | N.D. |
| 9 | LOH | LOH | N. I. | LOH | N.A. | N.D. | N.D. |
| 10 | INC | N. I. | LOH | N. I. | N.A. | N.D. | N.D. |
| 11 | INC | Weak maternal LOH | INC | Weak maternal LOH | N.A. | 65% | 43% |
| 12 | Probable paternal LOH | INC | Probable paternal LOH | INC | NO OVER | 3% | 61% |
Maternal LOH, loss of heterozygosity with absence of the maternal allele; N.I., not informative; INC, inconclusive; OVER, IGF2 overexpression; NO OVER, no IGF2 overexpression; N.A., not available; N.D., not done.
Discussion
In the present analysis of 12 childhood ACT (4 adenomas and 8 carcinomas) patients presenting with the R337H TP53 germline mutation and TP53 LOH in the tumor, all of them showed LOH for microsatellite markers located in 11p15 at both sides of the IGF2 gene. In 5 cases with IGF2 LOH with loss of the maternal allele where IGF2 levels were measured in our previous study [5], they were invariably highly increased compared to normal adrenal tissue (Table 3). This is linked to the differential methylation status of the paternal vs. maternal allele. Interestingly, patient 12 retained only the maternal allele in her adrenocortical carcinoma, which was consistent with very low IGF2 mRNA and protein levels present in the same sample (this sample is denominated ACC6 in the study of West et al. [5]). From these data, it can be concluded that LOH at 11p15 is a widespread finding in childhood ACT which can account for the high IGF2 levels expressed in these tumors and which is not correlated with clinical outcome, differently from adult ACT [17]. These data are consistent with a previous study that showed that IGF2 transgenic mice with elevated postnatal IGF2 serum levels presented adrenal hyperplasia associated with enhanced steroidogenesis but not tumors, presumably due to a direct mitogenic effect of IGF2 on adrenocortical cells, [20]. While additional studies on a larger cohort of patients are required to substantiate the impact of our results on childhood ACT prognosis, they confirm and extend the previous findings of Wilkin et al. on a series of only 4 cases, only one of which presented a germline TP53 mutation [7]. The very high incidence of 11p15 LOH in our series could be linked to genomic instability in these ACT determined by the TP53 mutation, and possibly influenced by exposure to yet unknown environmental factors. While our study only focused on markers surrounding the IGF2 gene, it is very likely that a relatively large genomic region in 11p15 has a paternal-only origin in childhood ACT, as shown by the decreased levels of KCNQ1 and CDKN1C, which are normally expressed from the maternal allele, in the tumors [5]. It is then possible that altered expression of other genes in 11p15 may synergize with elevated IGF2 levels to confer growth advantage to adrenocortical cell clones presenting 11p15 LOH. In particular, the role of a diminished expression of the Cdk inhibitor p57Kip2 encoded by the CDKN1C gene appears important, since mutations in this gene are associated with the Beckwith-Wiedemann syndrome [21, 22] and mice lacking p57Kip2 have adrenocortical hyperplasia together with other signs present in humans with Beckwith-Wiedemann syndrome [23]. We suggest that tumorigenesis in childhood ACT positive for the R337H TP53 mutation is a result of multiple events triggered by defective apoptosis and genomic instability produced by the mutation. A cascade of genetic alterations may then ensue with varying incidence [11p15 LOH, amplification and overexpression of transcription factor SF-1 [12, 13], growth factor and growth factor receptor overexpression [5], inhibin alpha gene mutations [15] and possibly other factors] that are involved in adenoma formation, but not in malignancy. This latter may result from the intervention of further, yet unknown genetic lesions which modify the tumor pattern of gene expression producing a characteristic signature [5]. Each one of these genetic alterations represents then a possible target for new drugs that will hopefully complement cytotoxic treatments in use today in patients with extended disease [24].
Acknowledgements
This study was supported by grants from CNRS (PICS), Association Recherche sur le Cancer (3142), ARCUS Brésil project, contrat d’interface INSERM-CHU de Nice and Fondation Recherche Médicale to E.L.; MIUR PRIN 2005 and Associazione Italiana Ricerca sul Cancro to A.R.; NIH CA63230 to G.P.Z.
Abbreviations
- ACT
adrenocortical tumors
- LOH
loss of heterozygosity
- DMR
differentially methylated region
- IC
imprinting centre
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koch CA, Pacak K, Chrousos GP. The molecular pathogenesis of hereditary and sporadic adrenocortical and adrenomedullary tumors. J Clin Endocrin Metab. 2004;87:5367–5384. doi: 10.1210/jc.2002-021069. [DOI] [PubMed] [Google Scholar]
- 2.Michalkiewicz E, Sandrini R, Figueiredo B, Miranda EC, Caran E, Oliveira-Filho AG, Marques R, Pianovski MA, Lacerda L, Cristofani LM, Jenkins J, Rodriguez-Galindo C, Ribeiro RC. Clinical and outcome characteristics of children with adrenocortical tumors. An analysis of 254 cases from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 3.Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003;27:867–881. doi: 10.1097/00000478-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West AN, Neale GA, Pounds S, Figueredo BC, Rodriguez-Galindo C, Pianovski MA, Oliveira Filho AG, Malkin D, Lalli E, Ribeiro R, Zambetti GP. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 6.Mesiano S, Jaffe RP. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 7.Wilkin F, Gagné N, Paquette J, Oligny LL, Deal C. Pediatric adrenocortical tumors: molecular events leading to insulin-like growth factor II gene overexpression. J Clin Endocrin Metab. 2000;85:2048–2056. doi: 10.1210/jcem.85.5.6589. [DOI] [PubMed] [Google Scholar]
- 8.Wagner J, Portwine C, Rabin K, Leclerc JM, Narod SA, Malkin D. High frequency of germiline p53 mutations in childhood adrenocortical cancer. J Natl Cancer Inst. 1994;86:1707–1710. doi: 10.1093/jnci/86.22.1707. [DOI] [PubMed] [Google Scholar]
- 9.Varley JM, McGown G, Thorncroft M, James LA, Margison GP, Forster G, Evans DG, Harris M, Kelsey AM, Birch JM. Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, DeLacerda L, Rabin M, Cadwell C, Sampaio G, Cat I, Stratakis CA, Sandrini R. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci USA. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiammarino EL, Lee AS, Cadwell C, Zhang W, Bothner B, Ribeiro RC, Zambetti G, Kriwacki RW. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol. 2002;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo BC, Cavalli LR, Pianovski MAD, Lalli E, Sandrini R, Ribeiro RC, Zambetti G, DeLacerda L, Rodrigues GA, Haddad BR. Amplification of the Steroidogenic Factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- 13.Pianovski MAD, Cavalli LR, Figueiredo BC, Santos SCL, Doghman M, Ribeiro RC, Oliveira AG, Michalkiewicz E, Rodrigues GA, Zambetti G, Haddad BR, Lalli E. SF-1 overexpression in childhood adrenocortical tumors. Eur J Cancer. 2006;42:1040–1043. doi: 10.1016/j.ejca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Doghman M, Karpova T, Rodrigues GA, Arhatte M, DeMoura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. Increased Steroidogenic Factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 15.Longui CA, Lemos-Marini SH, Figueiredo B, Mendonca BB, Castro M, Liberatore R, Jr, Watanabe C, Lancellotti CL, Rocha MN, Melo MB, Monte O, Calliari LEP, Guerra G, Jr, Baptista MTM, Sbragia-Neto L, Latronico AC, Moreira A, Tardelli AM, Nigri A, Taymans SE, Stratakis CA. Inhibin α-subunit (INHA) gene and locus changes in paediatric adrenocortical tumors from TP53 R337H mutation heterozygote carriers. J Med Genet. 2004;41:354–359. doi: 10.1136/jmg.2004.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weksberg R, Smith AC, Squire J, Sadowski P. Beckwith-Wiedemann syndrome demonstrates a role for epigenetic control of normal development. Hum Mol Genet. 2003;12:R61–R68. doi: 10.1093/hmg/ddg067. [DOI] [PubMed] [Google Scholar]
- 17.Gicquel C, Raffin-Sanson ML, Gaston V, Bertagna X, Plouin PF, Schlumberger M, Louvel A, Luton JP, Le Bouc Y. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab. 1997;82:2559–2565. doi: 10.1210/jcem.82.8.4170. [DOI] [PubMed] [Google Scholar]
- 18.Sparago A, Cerrato F, Vernucci M, Ferrero GB, Silengo MC, Riccio A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet. 2004;36:958–960. doi: 10.1038/ng1410. [DOI] [PubMed] [Google Scholar]
- 19.Sparago A, Russo S, Cerrato F, Ferraiuolo S, Castorina P, Selicorni A, Schwienbacher C, Negrini M, Ferrero GB, Silengo MC, Anichini C, Larizza L, Riccio A. Mechanisms causing imprinting defects in familial Beckwith-Wiedemann syndrome with Wilms' tumor. Hum Mol Genet. 2007;16:254–264. doi: 10.1093/hmg/ddl448. [DOI] [PubMed] [Google Scholar]
- 20.Weber MM, Fottner C, Schmidt P, Brodowski KM, Gittner K, Lahm H, Engelhardt D, Wolf E. Postnatal overexpression of IGF-II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–1543. doi: 10.1210/endo.140.4.6660. [DOI] [PubMed] [Google Scholar]
- 21.Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, Nakayama M, Niikawa N, Mukai T. An imprinted gene p57(KIP2) is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- 22.O'Keefe D, Dao D, Zhao L, Sanderson R, Warburton D, Weiss L, Anyane-Yeboa K, Tycko B. Coding mutations in p57(KIP2) are present in some cases of Beckwith-Wiedemann syndrome but are rare or absent in Wilms tumors. Am J Hum Genet. 1997;61:295–303. doi: 10.1086/514854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Liégeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57(KIP2) indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 24.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]