Abstract
The antigenic characteristics of the lipooligosaccharide (LOS) of Haemophilus influenzae type b (Hib) were examined in strains obtained over an extended period of time. These Hib strains were isolated from patients with systemic Hib disease in Dallas, Tex., over a 20-year period and in New York City between 1941 and 1956. The antigenic characteristics of the LOS of these Hib strains were examined by using a set of four murine monoclonal antibodies directed against epitopes present in the oligosaccharide portion of the LOS molecule. The same basic set of LOS antigenic determinants that is expressed by recent Hib isolates was also found to be present in this collection of Hib strains spanning a 40-year period. Some variation with time was detected in the distribution of the systemic disease isolates among four Hib LOS antigenic groups; however, only 2 of 188 Hib isolates failed to react with a set of two LOS-specific monoclonal antibodies. Therefore, little variation has occurred among Hib strains with regard to the LOS epitopes defined by these monoclonal antibodies over a considerable period of time.
Full text
PDF

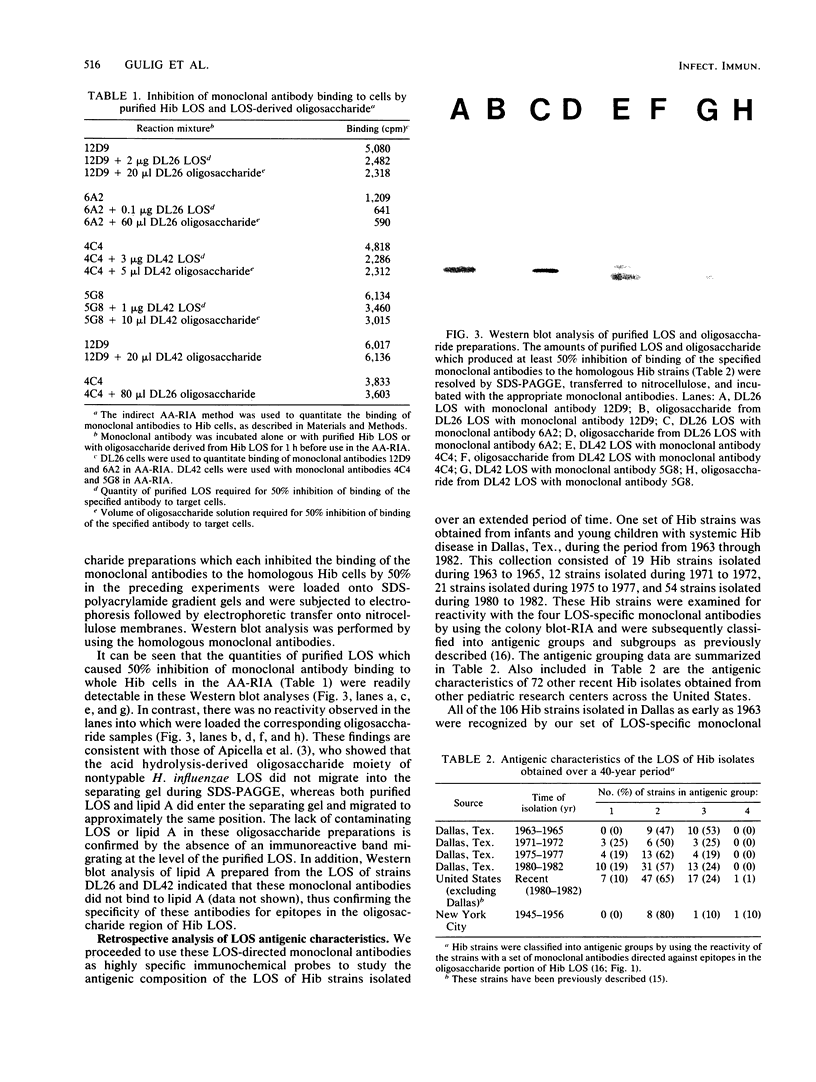




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Dudas K. C., Campagnari A., Rice P., Mylotte J. M., Murphy T. F. Antigenic heterogeneity of lipid A of Haemophilus influenzae. Infect Immun. 1985 Oct;50(1):9–14. doi: 10.1128/iai.50.1.9-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Cochi S. L., Broome C. V. Vaccine prevention of Haemophilus influenzae type b disease: past, present and future. Pediatr Infect Dis. 1986 Jan-Feb;5(1):12–19. doi: 10.1097/00006454-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Erwin A. L., Kenny G. E. Haemophilus influenzae type b isolates show antigenic variation in a major outer membrane protein. Infect Immun. 1984 Nov;46(2):570–577. doi: 10.1128/iai.46.2.570-577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Insel R. A. Characterization of lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1978 Dec;138(6):719–730. doi: 10.1093/infdis/138.6.719. [DOI] [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Ferrieri P. Susceptibility of phenotypic variants of Haemophilus influenzae type b to serum bactericidal activity: relation to surface lipopolysaccharide. J Infect Dis. 1986 Feb;153(2):223–231. doi: 10.1093/infdis/153.2.223. [DOI] [PubMed] [Google Scholar]
- Gotoff S. P. On the surface of Haemophilus influenzae. J Infect Dis. 1981 May;143(5):747–748. doi: 10.1093/infdis/143.5.747. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Holmans P. L., Hansen E. J. Immunogenic proteins in cell-free culture supernatants of Haemophilus influenzae type b. Infect Immun. 1984 Apr;44(1):41–48. doi: 10.1128/iai.44.1.41-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973 Feb;78(2):259–269. doi: 10.7326/0003-4819-78-2-259. [DOI] [PubMed] [Google Scholar]
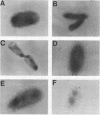
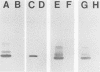
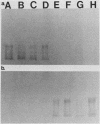
- Hampton C. M., Barenkamp S. J., Granoff D. M. Comparison of outer membrane protein subtypes of Haemophilus influenzae type b isolates from healthy children in the general population and from diseased patients. J Clin Microbiol. 1983 Sep;18(3):596–600. doi: 10.1128/jcm.18.3.596-600.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Sparling P. F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. L., Loftus T. A., Hansen E. J. Cloning and surface expression in Escherichia coli of a structural gene encoding a surface protein of Haemophilus influenzae type b. Infect Immun. 1985 Oct;50(1):236–242. doi: 10.1128/iai.50.1.236-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Anderson P. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J Infect Dis. 1985 May;151(5):869–877. doi: 10.1093/infdis/151.5.869. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Pichichero M. E. Lipopolysaccharide subtypes of Haemophilus influenzae type b from an outbreak of invasive disease. J Clin Microbiol. 1984 Aug;20(2):145–150. doi: 10.1128/jcm.20.2.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R. Immunoblot method for identifying surface components, determining their cross-reactivity, and investigating cell topology: results with Haemophilus influenzae type b. Anal Biochem. 1984 Nov 15;143(1):196–204. doi: 10.1016/0003-2697(84)90576-1. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen J. H., Tauber I., Leeuwenberg A. D., Beckers P. J., Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977 Jul;136(1):43–49. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide composition of three strains of Haemophilus influenzae. Can J Microbiol. 1984 Sep;30(9):1184–1187. doi: 10.1139/m84-185. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. R., Rossi A. A. Molecular cloning of DNA coding for outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1986 Jun;52(3):812–817. doi: 10.1128/iai.52.3.812-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Smith D. H., Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978 Oct;138(4):427–436. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Rubin L. G., Connelly C. J., Inzana T. J., Moxon E. R. Alteration of the cell wall of Haemophilus influenzae type b by transformation with cloned DNA: association with attenuated virulence. J Infect Dis. 1985 Sep;152(3):485–492. doi: 10.1093/infdis/152.3.485. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis. 1983 Sep;148(3):385–394. doi: 10.1093/infdis/148.3.385. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]