Abstract
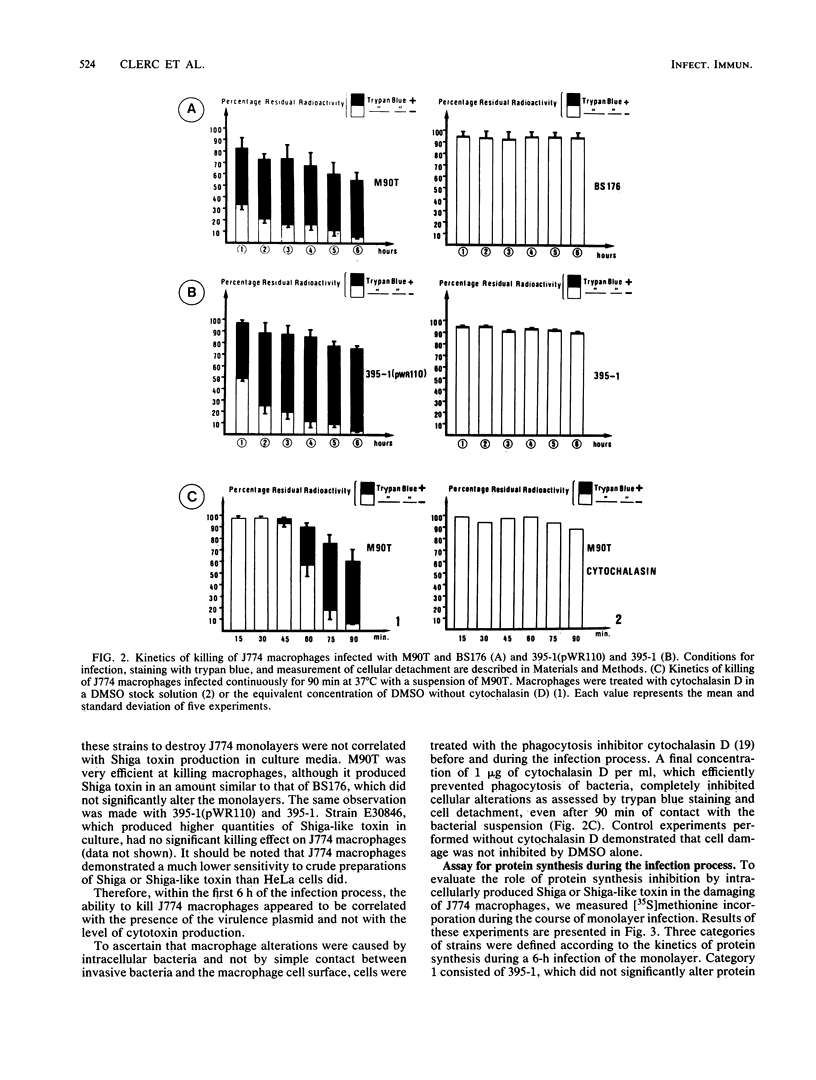
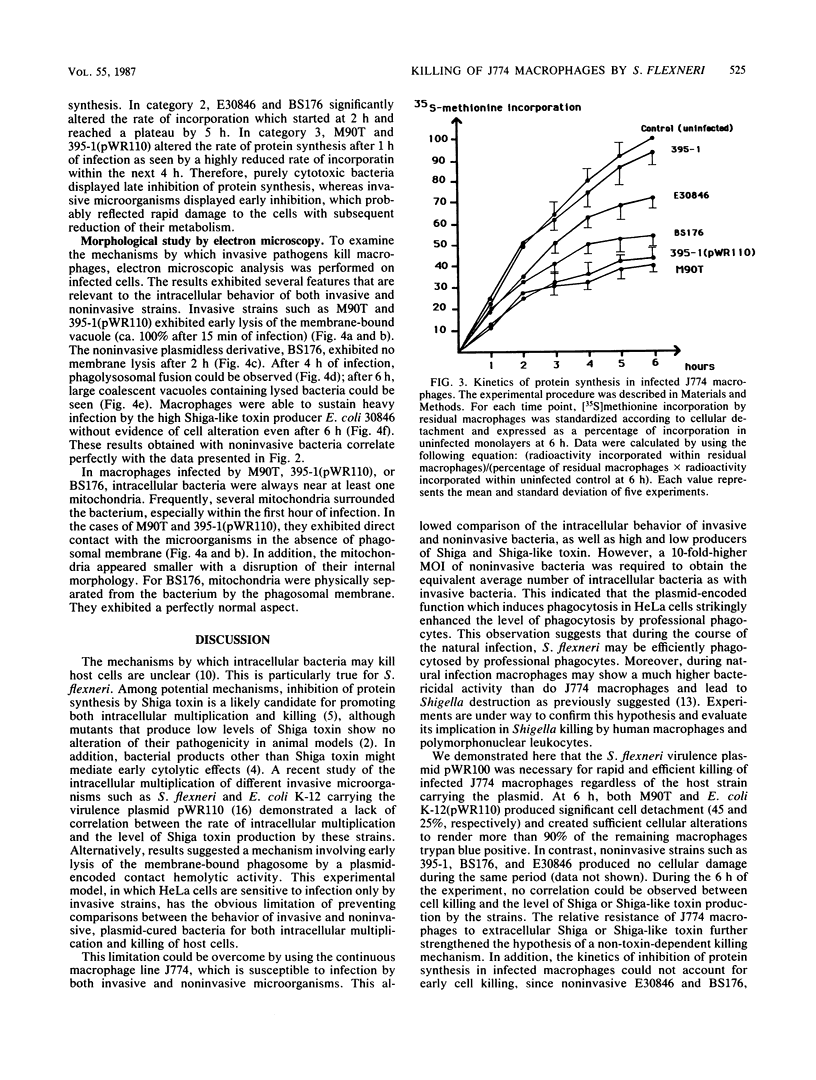
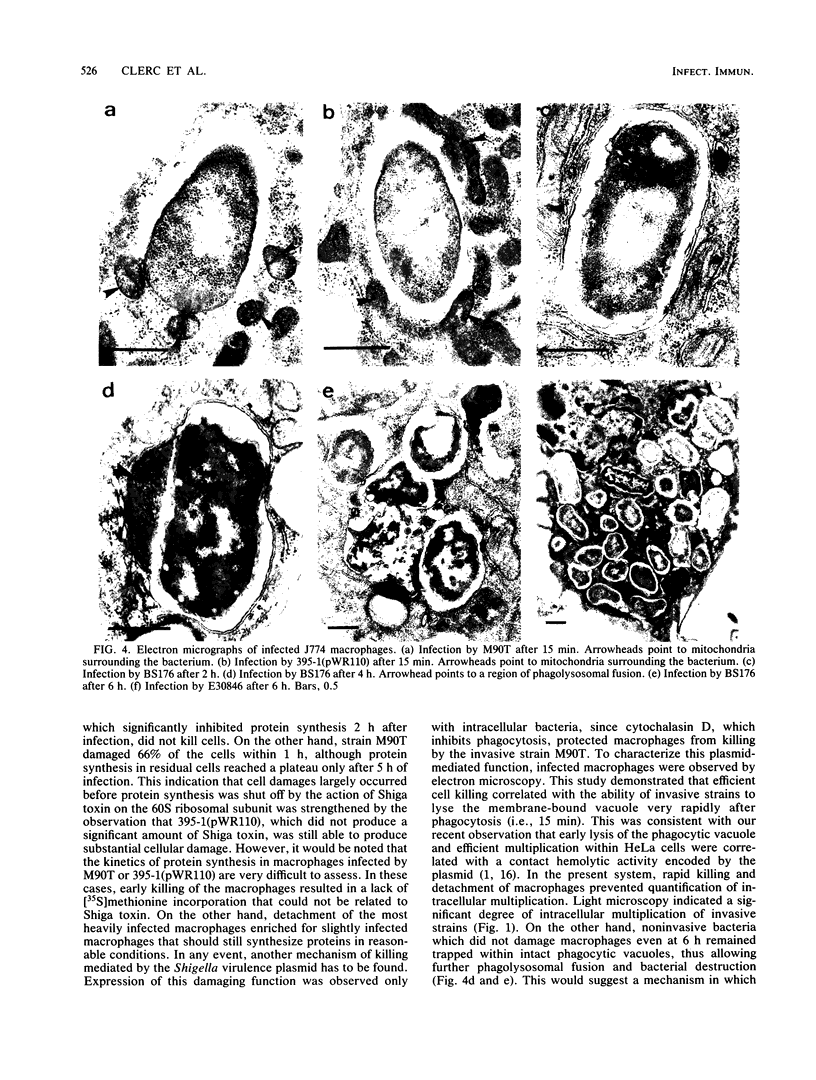
In Shigella flexneri a 220-kilobase plasmid encodes the ability to invade nonprofessional phagocytes by a mechanism similar to phagocytosis. In this report, the continuous macrophage cell line J774 was used to study the intracellular fate of both invasive and noninvasive strains. pWR100, the virulence plasmid of S. flexneri serotype 5, mediated very efficient and rapid killing of J774 macrophages, as measured by cellular detachment and uptake of trypan blue. For this to occur, the bacteria had to be within the cells, since the macrophages were protected by cytochalasin D. A battery of strains differing in their levels of Shiga toxin production showed that inhibition of protein synthesis by Shiga toxin, as measured by [35S]methionine incorporation into infected macrophages, was not required for early killing of cells. Damage to J774 macrophages rather correlated with the ability of invasive bacteria to rapidly and efficiently lyse the membrane of the phagocytic vacuole. The role of the release of bacteria within the cytosol for subsequent expression of cytotoxic activity is discussed, and mitochondria are proposed as a potential target for this activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clerc P., Baudry B., Sansonetti P. J. Plasmid-mediated contact haemolytic activity in Shigella species: correlation with penetration into HeLa cells. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):267–278. doi: 10.1016/s0769-2609(86)80033-3. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Cytotoxicity of Shigella dysenteriae 1 for cultured mammalian cells. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2485–2490. doi: 10.1093/ajcn/33.11.2485. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. M., Lior H., Bezanson G. S. Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet. 1983 Jan 1;1(8314-5):76–76. doi: 10.1016/s0140-6736(83)91616-1. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Fischer D. G., Koren H. S. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J774.1. J Immunol. 1977 Dec;119(6):2060–2066. [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]