Abstract
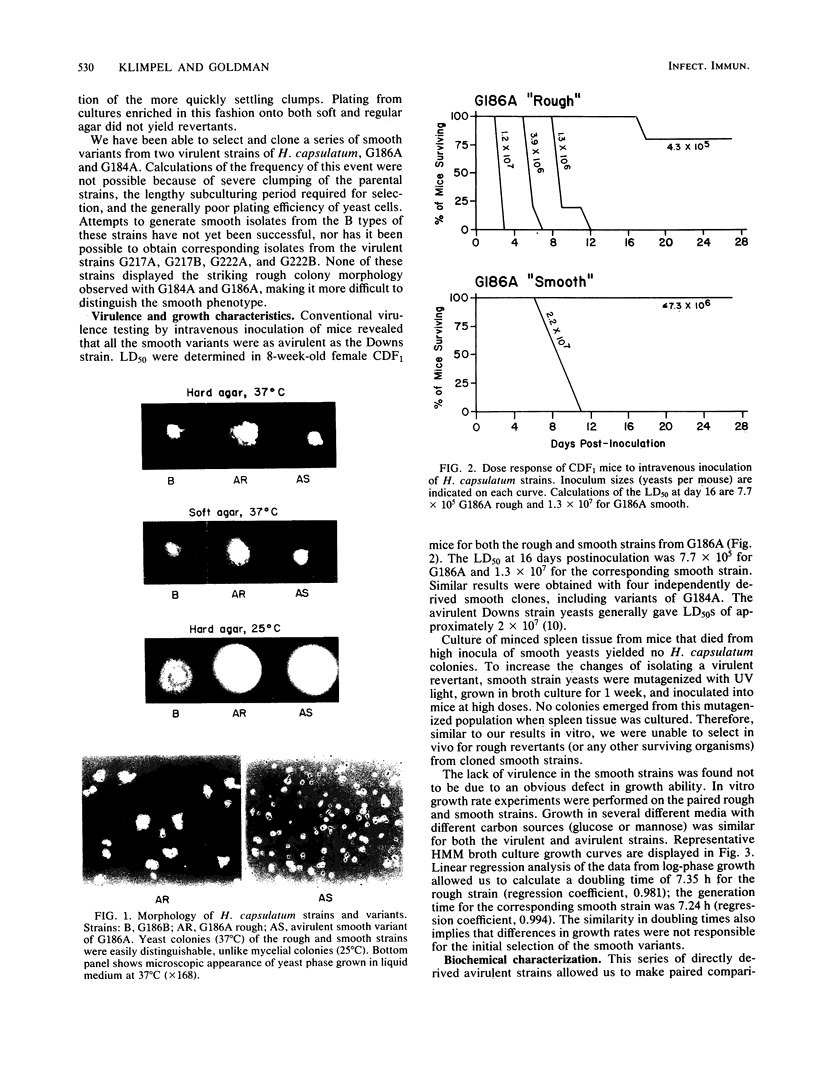
A selection procedure was developed which allowed us to isolate spontaneous isogenic avirulent clones from virulent strains of Histoplasma capsulatum. The avirulent yeasts had a unique phenotype: they did not aggregate like the parental strains but grew as dispersed budded and unbudded single cells in liquid medium. On solid medium, the avirulent variant strains grew as smooth-textured colonies, whereas the virulent parental strains grew as rough convoluted colonies. Virulence testing in mice demonstrated that the smooth variants gave 50% lethal dose values similar to those of the avirulent Downs strain. Growth curves for the paired rough and smooth strains were similar. Furthermore, they had the same protein profiles when crude cell fractions were separated on one-dimensional polyacrylamide gels or when whole-cell extracts were separated by two-dimensional gel electrophoresis. Electrophoresis of culture supernatants, however, revealed a difference in a released low-molecular-weight peptide that may be related to virulence. In addition to their usefulness in comparative virulence studies, these avirulent strains should prove valuable for H. capsulatum genetic experiments because of the unique ability of these yeasts to grow without clumping.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. L., Berry R. W., Telser A. A sodium dodecyl sulfate--polyacrylamide gel electrophoresis system that separates peptides and proteins in the molecular weight range of 2500 to 90,000. Anal Biochem. 1983 Jul 15;132(2):365–375. doi: 10.1016/0003-2697(83)90022-2. [DOI] [PubMed] [Google Scholar]
- Daniels L. S., Berliner M. D., Campbell C. C. Varying virulence in rabbits infected with different filamentous types of Histoplasma capsulatum. J Bacteriol. 1968 Nov;96(5):1535–1539. doi: 10.1128/jb.96.5.1535-1539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann B., Imbault P., Weil J. H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal Biochem. 1973 Aug;54(2):454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- Gass M., Kobayashi G. S. Histoplasmosis. An illustrative case with unusual vaginal and joint involvement. Arch Dermatol. 1969 Dec;100(6):724–727. doi: 10.1001/archderm.100.6.724. [DOI] [PubMed] [Google Scholar]
- Goodwin R. A., Jr, Des Prez R. M. State of the art: histoplasmosis. Am Rev Respir Dis. 1978 May;117(5):929–956. doi: 10.1164/arrd.1978.117.5.929. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird W. J., Cavanaugh D. C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980 Apr;11(4):430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Sacco M., Maresca B., Schlessinger D., Painter A., Kobayashi G. S., Carratu L. Irreversible block of the mycelial-to-yeast phase transition of Histoplasma capsulatum. Science. 1986 Jan 31;231(4737):476–479. doi: 10.1126/science.3001938. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Otake S., Hirasawa M., Brown T. A., Kawabata Y., Kiyono H., Michalek S. M., McGhee J. R., Shiota T. Virulence of Streptococcus mutans: characterization of a serotype g antigen-defective mutants and its revertants. Infect Immun. 1981 Jan;31(1):151–159. doi: 10.1128/iai.31.1.151-159.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R. P., Berkhout F. J. Comparative pathogenicity of albino and brown types of Histoplasma capsulatum for mice. J Infect Dis. 1972 May;125(5):504–508. doi: 10.1093/infdis/125.5.504. [DOI] [PubMed] [Google Scholar]
- Vincent R. D., Goewert R., Goldman W. E., Kobayashi G. S., Lambowitz A. M., Medoff G. Classification of Histoplasma capsulatum isolates by restriction fragment polymorphisms. J Bacteriol. 1986 Mar;165(3):813–818. doi: 10.1128/jb.165.3.813-818.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]