Abstract
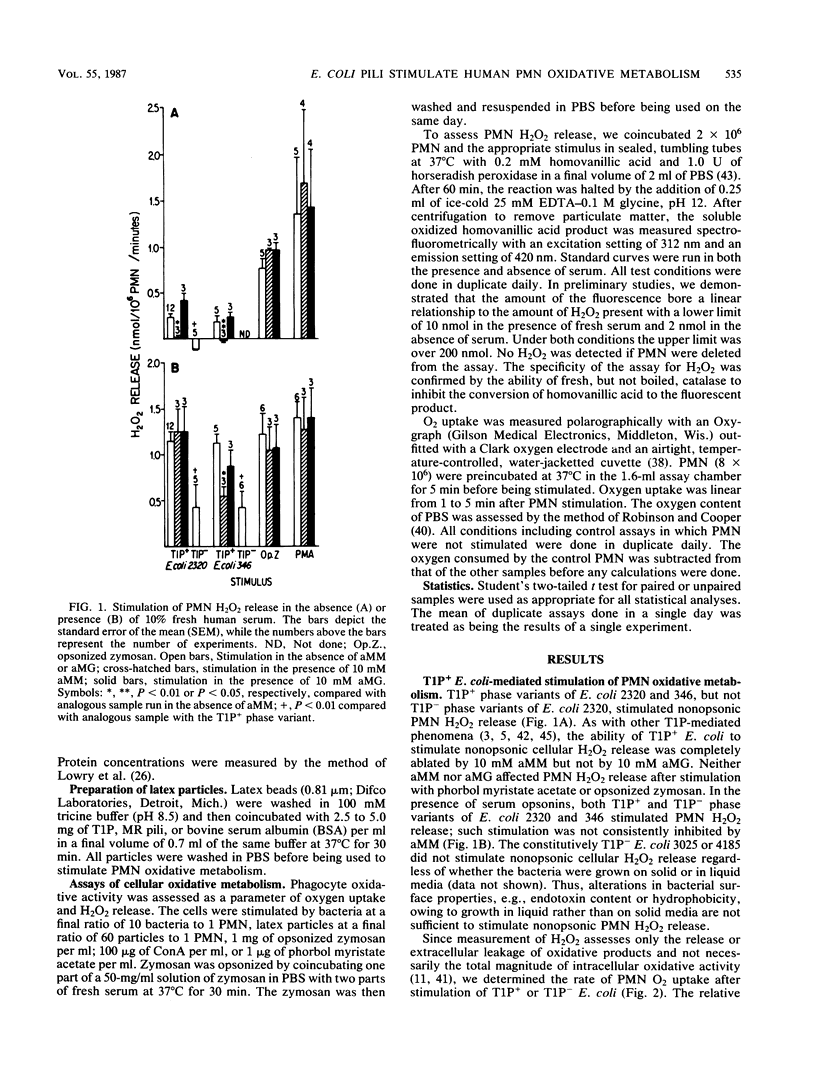
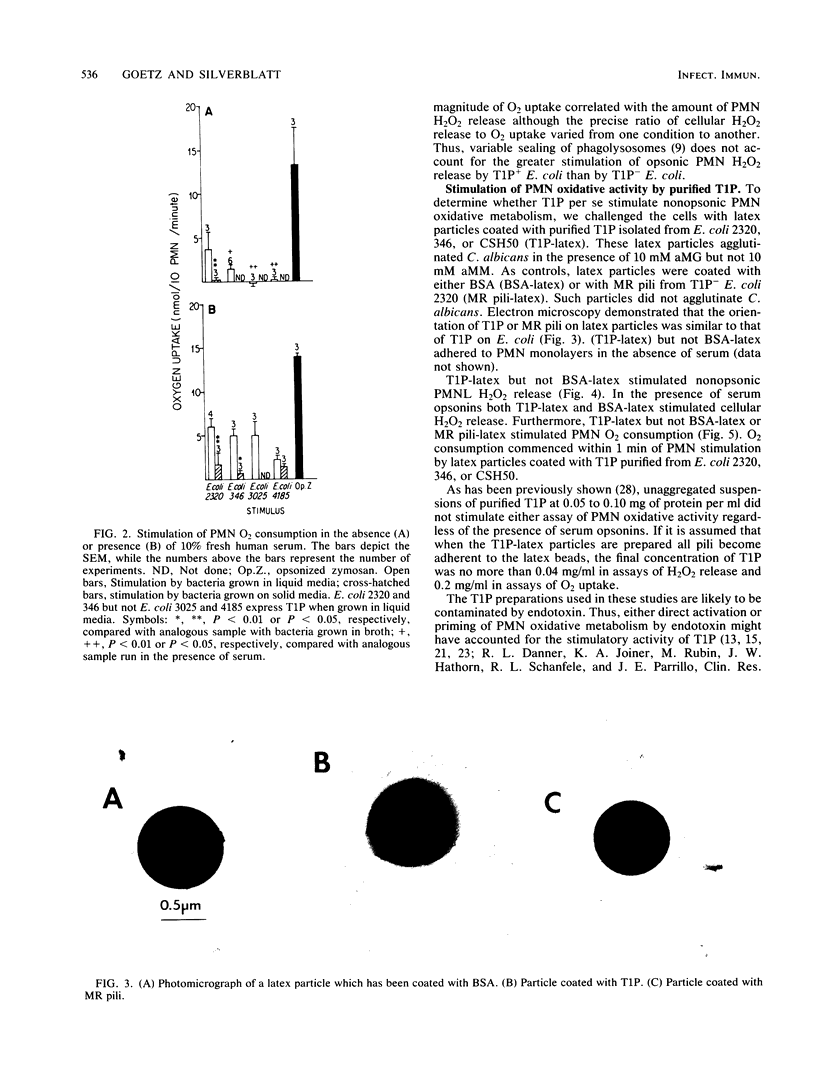
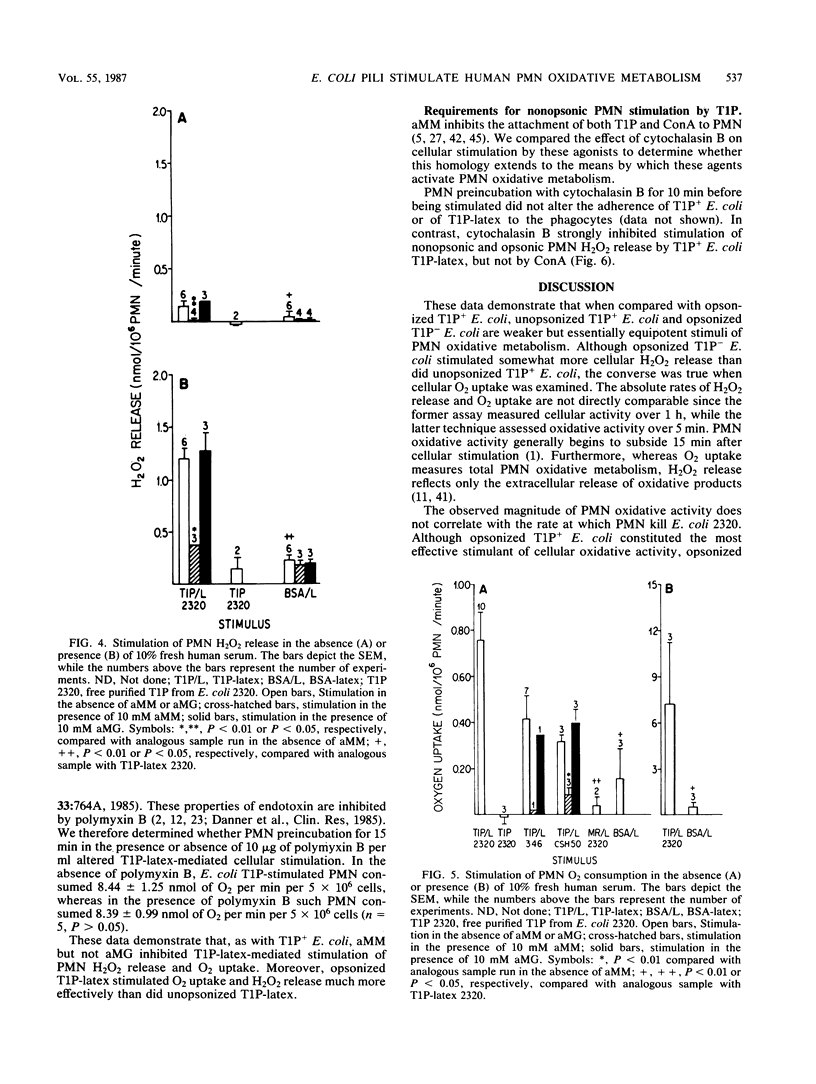
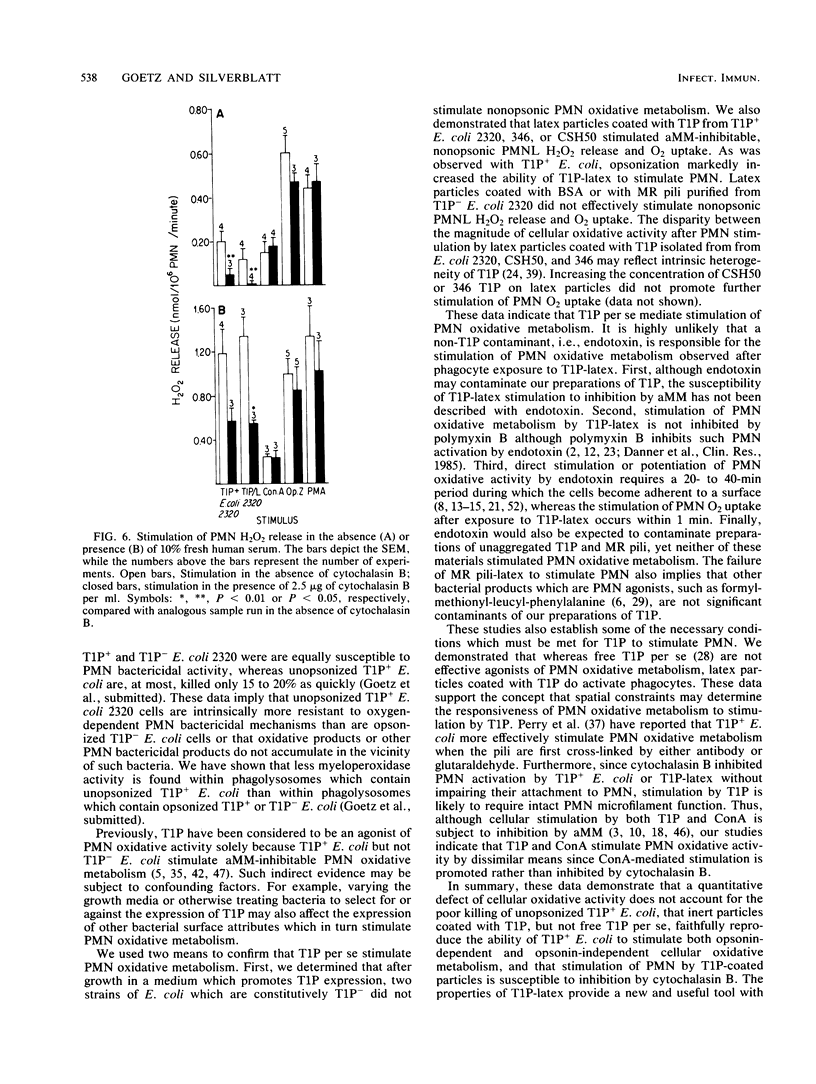
We compared the degree to which Escherichia coli phase variants which do (T1P+ E. coli) or do not (T1P- E. coli) express type 1 pili (T1P) stimulate human polymorphonuclear leukocyte (PMN) oxidative activity. Unopsonized T1P+ E. coli stimulated the release of 0.20 to 0.24 nmol of H2O2 per 10(6) PMN per min and the consumption of 1.4 to 4.0 nmol of O2 per 10(6) PMN per min; no measurable PMN oxidative activity was stimulated by unopsonized T1P- E. coli. In the presence of serum opsonins, T1P+ E. coli stimulated the release of 1.12 to 1.16 nmol of H2O2 per 10(6) PMN per min and the consumption of 5.0 to 6.0 nmol of O2 per 10(6) PMN per min, whereas T1P- E. coli stimulated the release of 0.42 to 0.43 nmol of H2O2 per 10(6) PMN per min and the consumption of 0.6 to 2.0 nmol of O2 per 10(6) PMN per min. Although unaggregated T1P did not stimulate PMN, latex beads coated with T1P (T1P-latex) stimulated alpha-methylmannoside-inhibitable, opsonin-independent PMN oxidative activity. The activity stimulated by either T1P+ E. coli or T1P-latex was susceptible to inhibition by cytochalasin B. Latex particles coated with bovine serum albumin or mannose-resistant pili did not stimulate PMN. These data indicate that T1P+ E. coli stimulate PMN oxidative metabolism more effectively than do T1P- E. coli and that a similar PMN oxidative response follows cellular stimulation by either unopsonized T1P+ or opsonized T1P- E. coli. Furthermore, T1P-latex faithfully mimics the ability of T1P+ E. coli to stimulate PMN oxidative metabolism. Such particles may be useful in further analyses of cellular responses to T1P+ E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannatyne R. M., Harnett N. M., Lee K. Y., Biggar W. D. Inhibition of the biologic effects of endotoxin on neutrophils by polymyxin B sulfate. J Infect Dis. 1977 Oct;136(4):469–474. doi: 10.1093/infdis/136.4.469. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Yoder M., Bonsib S., Schmidt M., Ho P., Jersild R., Baehner R. L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med. 1979 Mar;93(3):506–514. [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Heterogeneity of human neutrophil phagolysosomes: functional consequences for candidacidal activity. Blood. 1984 Jul;64(1):147–151. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Wilson M. K., Newburger P. E. Con-A-stimulated superoxide production by granulocytes: reversible activation of NADPH oxidase. Blood. 1982 Nov;60(5):1188–1194. [PubMed] [Google Scholar]
- Cohen M. S., Metcalf J. A., Root R. K. Regulation of oxygen metabolism in human granulocytes: relationship between stimulus binding and oxidative response using plant lectins as probes. Blood. 1980 Jun;55(6):1003–1010. [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Sieber O. E., Jr, Ratajczak H., Bennett B. B. Modification of human neutrophil response to endotoxin with polymyxin B sulfate. J Infect Dis. 1974 Oct;130(4):384–387. doi: 10.1093/infdis/130.4.384. [DOI] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Granulocyte activation by endotoxin. II. Role of granulocyte adherence, aggregation, and effect of cytochalasin B, and comparison with formylated chemotactic peptide-induced stimulation. J Immunol. 1983 Feb;130(2):863–868. [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Dodd D. C., Eisenstein B. I. Antigenic quantitation of type 1 fimbriae on the surface of Escherichia coli cells by an enzyme-linked immunosorbent inhibition assay. Infect Immun. 1982 Nov;38(2):764–773. doi: 10.1128/iai.38.2.764-773.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander R. M., Thomas V. L., Forland M. Mannose-resistant hemagglutination and P receptor recognition of uropathogenic Escherichia coli isolated from adult patients. J Infect Dis. 1985 Mar;151(3):508–513. doi: 10.1093/infdis/151.3.508. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive interaction of Escherichia coli with human peripheral leukocytes in vitro. Infect Immun. 1979 Nov;26(2):520–527. doi: 10.1128/iai.26.2.520-527.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive stimulation of human leukocyte chemiluminescence by Escherichia coli. Infect Immun. 1979 Dec;26(3):1014–1019. doi: 10.1128/iai.26.3.1014-1019.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Normark S., Falkow S., Schoolnik G. K. Mannose-sensitive and Gal-Gal binding Escherichia coli pili from recombinant strains. Chemical, functional, and serological properties. J Exp Med. 1983 Nov 1;158(5):1713–1719. doi: 10.1084/jem.158.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ohman L., Hed J., Stendahl O. Interaction between human polymorphonuclear leukocytes and two different strains of type 1 fimbriae-bearing Escherichia coli. J Infect Dis. 1982 Dec;146(6):751–757. doi: 10.1093/infdis/146.6.751. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Ofek I., Silverblatt F. J. Enhancement of mannose-mediated stimulation of human granulocytes by type 1 fimbriae aggregated with antibodies on Escherichia coli surfaces. Infect Immun. 1983 Mar;39(3):1334–1345. doi: 10.1128/iai.39.3.1334-1345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rene P., Dinolfo M., Silverblatt F. J. Serum and urogenital antibody responses to Escherichia coli pili in cystitis. Infect Immun. 1982 Nov;38(2):542–547. doi: 10.1128/iai.38.2.542-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottini G., Cian F., Soranzo M. R., Albrigo R., Patriarca P. Evidence for the involvement of human polymorphonuclear leucocyte mannose-like receptors in the phagocytosis of Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):307–312. doi: 10.1016/0014-5793(79)80636-5. [DOI] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström T., Ohman L. The effect of monoclonal antibodies against Escherichia coli type 1 pili and capsular polysaccharides on the interaction between bacteria and human granulocytes. Scand J Immunol. 1984 Oct;20(4):299–305. doi: 10.1111/j.1365-3083.1984.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R., Silverblatt F. J. Antibacterial mechanisms of antibody to mannose-sensitive pili of Escherichia coli. J Infect Dis. 1983 May;147(5):882–889. doi: 10.1093/infdis/147.5.882. [DOI] [PubMed] [Google Scholar]
- Weitberg A. B., Weitzman S. A., Destrempes M., Latt S. A., Stossel T. P. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Yamada O., Moldow C. F., Sacks T., Craddock P. R., Boogaerts M. A., Jacob H. S. Deleterious effects of endotoxin on cultured endothelial cells: an in vitro model of vascular injury. Inflammation. 1981 Jun;5(2):115–126. doi: 10.1007/BF00914201. [DOI] [PubMed] [Google Scholar]




