Abstract
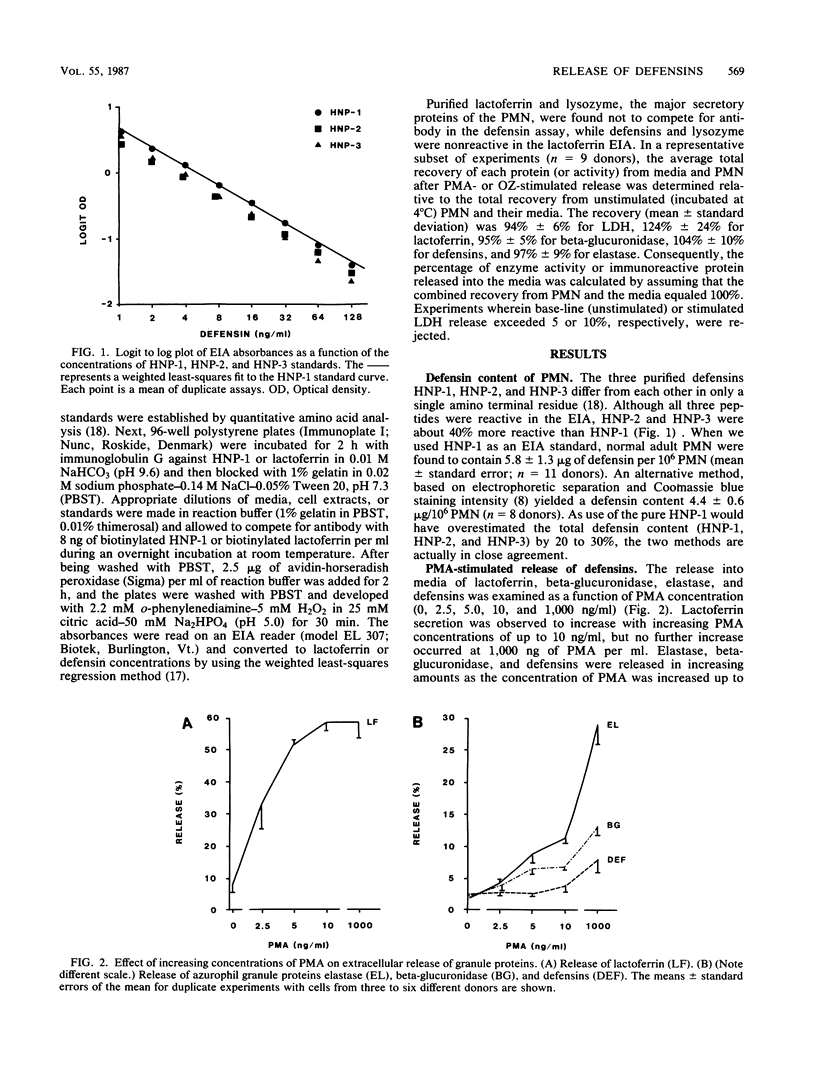
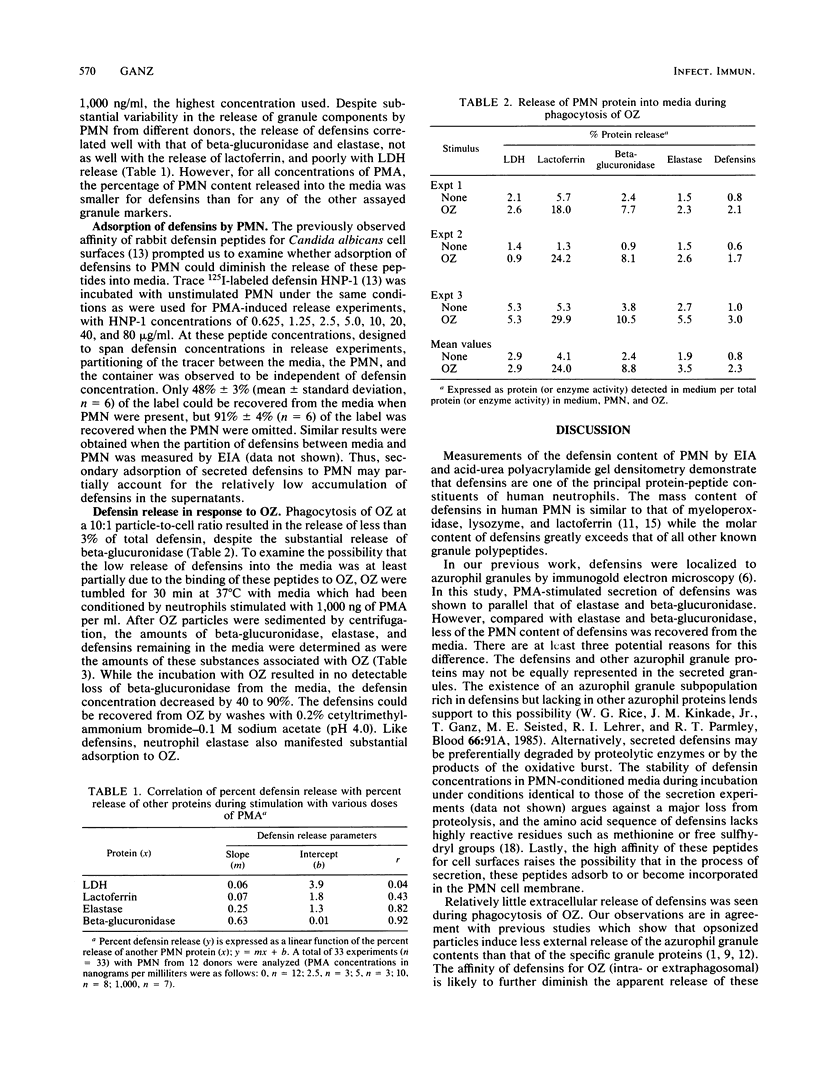
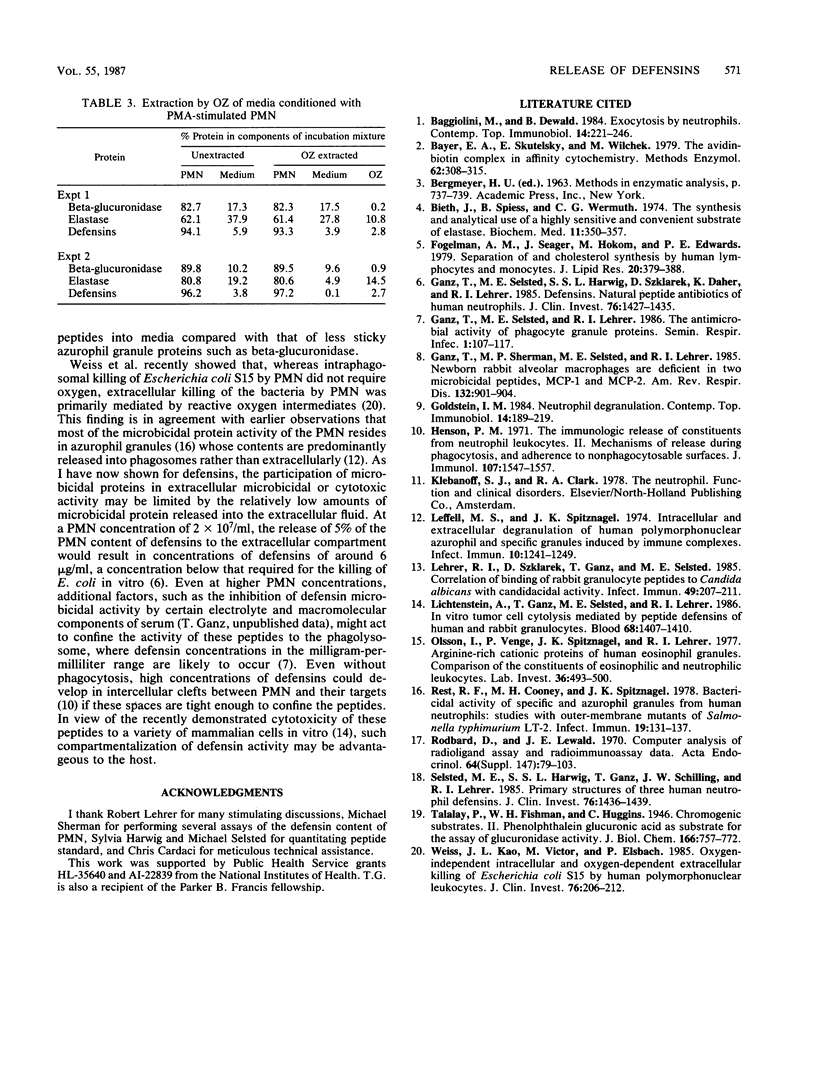
Human polymorphonuclear leukocytes (PMN) contain three antimicrobial and cytotoxic peptides which belong to a family of mammalian granulocyte peptides named defensins. To determine their potential availability for extracellular microbicidal or cytotoxic events, we quantified the extracellular release of defensins after stimulation of human PMN with phorbol myristate acetate and opsonized zymosan. As determined by enzyme immunoassay and confirmed by polyacrylamide gel electrophoresis and densitometry, 10(6) human PMN contained 4 to 5 micrograms of defensins. After stimulation with a high concentration of phorbol myristate acetate (1 microgram/ml), about 8% of PMN defensins were found in the media. Release of defensins correlated best with the release of azurophil granule marker beta-glucuronidase or elastase and poorly with the release of either the specific granule marker lactoferrin or cytoplasmic lactate dehydrogenase. Phagocytosis of opsonized zymosan resulted in the extracellular release of less than 3% of PMN defensins. The factors responsible for less release of defensins into media relative to the release of other azurophil granule proteins may include heterogeneity of azurophil granules and the affinity of defensins for cellular surfaces and opsonized particles. In vivo, defensins are most likely to reach effective microbicidal or cytotoxic concentrations in PMN-rich exudates (pus), in confined environments of the phagolysosomes, or in intercellular clefts between PMN and their targets.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B. Exocytosis by neutrophils. Contemp Top Immunobiol. 1984;14:221–246. doi: 10.1007/978-1-4757-4862-8_8. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Skutelsky E., Wilchek M. The avidin-biotin complex in affinity cytochemistry. Methods Enzymol. 1979;62:308–315. doi: 10.1016/0076-6879(79)62235-8. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Antimicrobial activity of phagocyte granule proteins. Semin Respir Infect. 1986 Jun;1(2):107–117. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Sherman M. P., Selsted M. E., Lehrer R. I. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985 Oct;132(4):901–904. doi: 10.1164/arrd.1985.132.4.901. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M. Neutrophil degranulation. Contemp Top Immunobiol. 1984;14:189–219. doi: 10.1007/978-1-4757-4862-8_7. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Intracellular and extracellular degranulation of human polymorphonuclear azurophil and specific granules induced by immune complexes. Infect Immun. 1974 Dec;10(6):1241–1249. doi: 10.1128/iai.10.6.1241-1249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Ganz T., Selsted M. E. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985 Jul;49(1):207–211. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein A., Ganz T., Selsted M. E., Lehrer R. I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986 Dec;68(6):1407–1410. [PubMed] [Google Scholar]
- Olsson I., Venge P., Spitznagel J. K., Lehrer R. I. Arginine-rich cationic proteins of human eosinophil granules: comparison of the constituents of eosinophilic and neutrophilic leukocytes. Lab Invest. 1977 May;36(5):493–500. [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Bactericidal activity of specific and azurophil granules from human neutrophils: studies with outer-membrane mutants of Salmonella typhimurium LT-2. Infect Immun. 1978 Jan;19(1):131–137. doi: 10.1128/iai.19.1.131-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Lewald J. E. Computer analysis of radioligand assay and radioimmunoassay data. Acta Endocrinol Suppl (Copenh) 1970;147:79–103. doi: 10.1530/acta.0.065s079. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Kao L., Victor M., Elsbach P. Oxygen-independent intracellular and oxygen-dependent extracellular killing of Escherichia coli S15 by human polymorphonuclear leukocytes. J Clin Invest. 1985 Jul;76(1):206–212. doi: 10.1172/JCI111947. [DOI] [PMC free article] [PubMed] [Google Scholar]



