Abstract
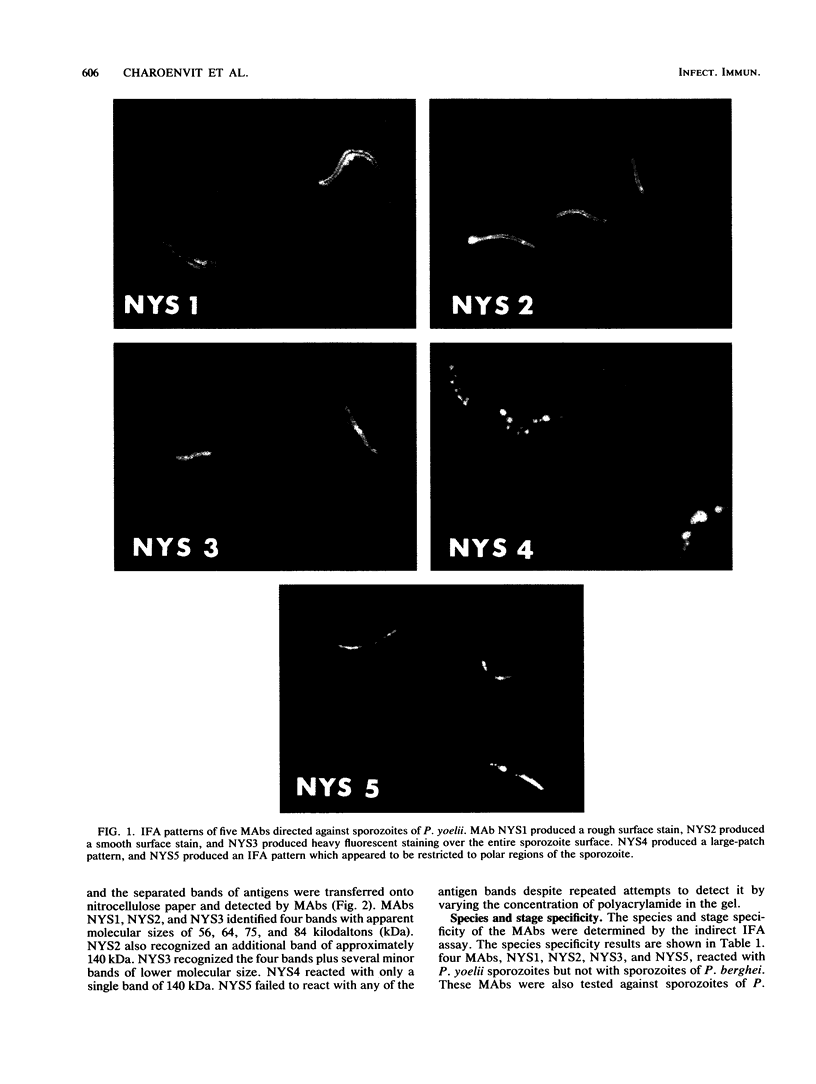
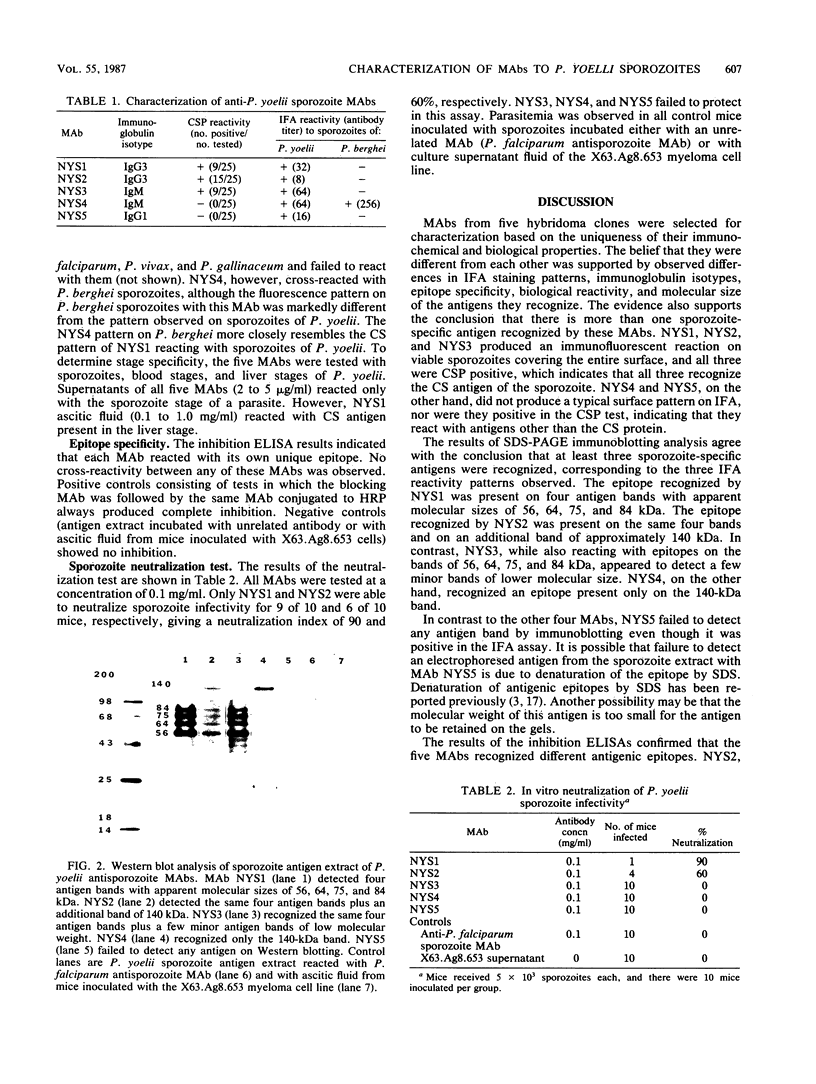
A battery of monoclonal antibodies against Plasmodium yoelii sporozoites was produced. Five of these (NYS1 through NYS5) were selected for characterization. All five were positive in the indirect immunofluorescent antibody test with P. yoelii sporozoites; however, each showed a different immunofluorescence pattern. Although NYS1 (immunoglobulin G3 [IgG3]), NYS2 (IgG3), and NYS3 (IgM) were positive in the circumsporozoite precipitation test, only NYS1 and NYS2 were able to neutralize sporozoite infectivity in mice. NYS4 (IgM) and NYS5 (IgG1) were not positive in the precipitation test and did not protect mice from sporozoite infection. All except NYS4 were species as well as stage specific. NYS4 cross-reacted with sporozoites of P. berghei. Electrophoretic immunoblotting analysis showed that these monoclonal antibodies detected sporozoite antigens of various molecular weights. Inhibition enzyme-linked immunosorbent assays indicated that each recognized a different antigenic epitope. The differences in their immunochemical and biological reactivity make them useful for screening a variety of P. yoelii antigens in recombinant DNA libraries. These antigens will be used in an animal model system for vaccine development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Bosworth J. M., Jr, Brimfield A. A., Naylor J. A., Hunter K. W., Jr Measurement of monoclonal antibody concentrations in hybridoma cultures: comparison of competitive inhibition and antigen capture enzyme immunoassays. J Immunol Methods. 1983 Sep 16;62(3):331–336. doi: 10.1016/0022-1759(83)90177-1. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cochrane A. H., Santoro F., Nussenzweig V., Gwadz R. W., Nussenzweig R. S. Monoclonal antibodies identify the protective antigens of sporozoites of Plasmodium knowlesi. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5651–5655. doi: 10.1073/pnas.79.18.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Campbell G. H., Leef M. F., Beaudoin R. L. Production of monoclonal antibodies by hybridomas sensitized to sporozoites of Plasmodium berghei. J Parasitol. 1982 Dec;68(6):1029–1033. [PubMed] [Google Scholar]
- Ellis J., Ozaki L. S., Gwadz R. W., Cochrane A. H., Nussenzweig V., Nussenzweig R. S., Godson G. N. Cloning and expression in E. coli of the malarial sporozoite surface antigen gene from Plasmodium knowlesi. Nature. 1983 Apr 7;302(5908):536–538. doi: 10.1038/302536a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Pacheco N. D., Strome C. P., Mitchell F., Bawden M. P., Beaudoin R. L. Rapid, large-scale isolation of Plasmodium berghei sporozoites from infected mosquitoes. J Parasitol. 1979 Jun;65(3):414–417. [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg J., Nussenzweig R., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil Med. 1969 Sep;134(10):1183–1190. [PubMed] [Google Scholar]
- Waehneldt T. V. Sodium dodecyl sulfate in protein chemistry. Biosystems. 1975 Mar;6(3):176–187. doi: 10.1016/0303-2647(75)90025-8. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Nussenzweig R. S., Potocnjak P., Nussenzweig V., Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]