Abstract
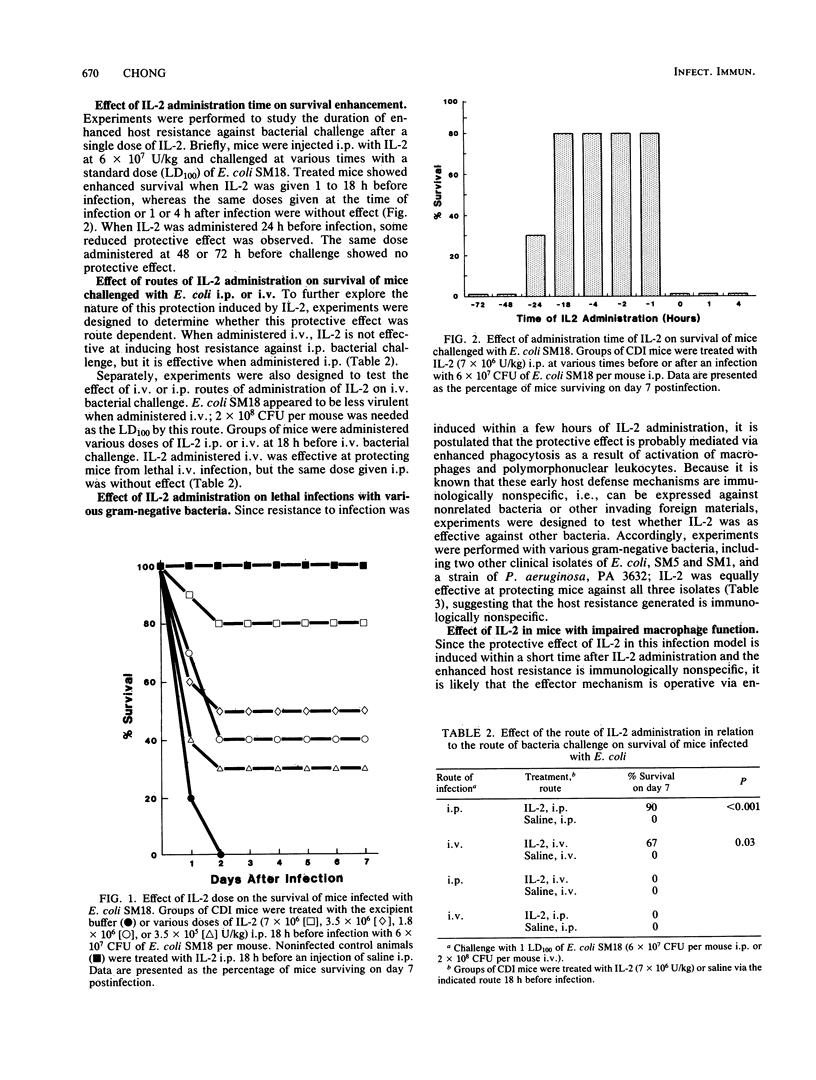
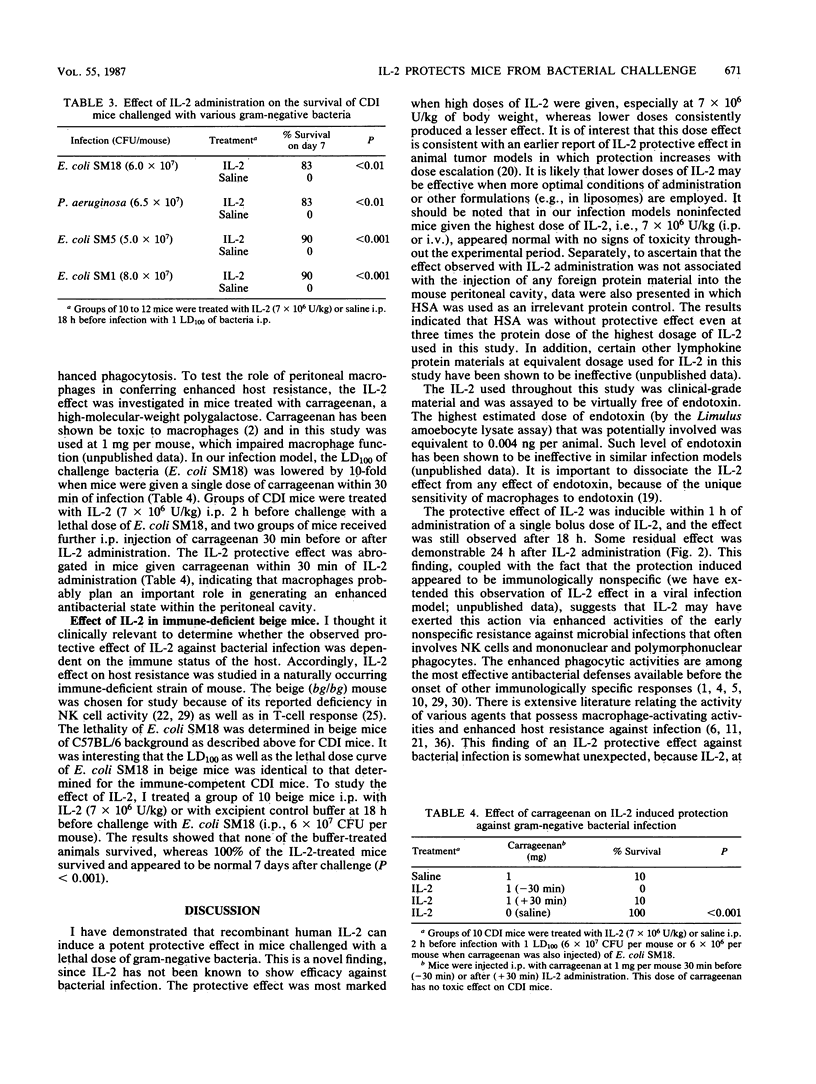
Prophylactic administration of recombinant human interleukin-2 (IL-2) in mice enhanced survival and produced complete recovery from an otherwise lethal acute bacterial infection. IL-2 was administered as a single intraperitoneal or intravenous bolus dose to CDI mice 18 h before challenge with a lethal dose of a clinical isolate of Escherichia coli type O2 (minimal 100% lethal dose, 6 X 10(7) CFU per mouse). At IL-2 dosages of 7 X 10(6) U/kg, 90% of treated CDI mice survived as compared to 0% for the excipient buffer control animals (P less than 0.001). This protective effect was also demonstrable in immune-deficient beige mice. The IL-2 effect was dose dependent; protection was consistently observed in mice pretreated with IL-2 at doses ranging from 1.8 X 10(6) to 7 X 10(6) U/kg. However, at 3.5 X 10(5) U/kg the protective effect was more variable. The route of administration of IL-2 was shown to play an important role; when IL-2 and challenge bacteria were given by the same route (either intravenously or intraperitoneally), protection was readily observable, but when IL-2 and challenge bacteria were given by different routes, little or no protective effect was observed. The protective effect was fully inducible as early as 1 h after IL-2 administration and was effective against various strains of gram-negative bacteria, indicating that the probable mode of action represents control of the establishment of infection by increased activity of the nonspecific host defense mechanisms. The IL-2 effect was abrogated by the administration of carrageenan, suggesting a possible role of macrophages. These data demonstrate that IL-2 may be a potentially useful adjunct for the prophylaxis of bacterial infections in both clinical and veterinary medicine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Macrophage activation and nonspecific immunity. Int Rev Exp Pathol. 1978;18:303–346. [PubMed] [Google Scholar]
- COHN Z. A. Determinants of infection in the peritoneal cavity. I. Response to and fate of Staphylococcus aureus and Staphylococcus albus in the mouse. Yale J Biol Med. 1962 Aug;35:12–28. [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Schwartz H. J., Graham R. C., Jr Spectrum and possible mechanism of carrageenan cytotoxicity. Am J Pathol. 1971 Aug;64(2):387–404. [PMC free article] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Interleukin 2 enhances specific and nonspecific immune responses in experimental Chagas' disease. Infect Immun. 1985 Nov;50(2):354–357. doi: 10.1128/iai.50.2.354-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Floyd S. A. In vitro kinetics of phagocytosis and intracellular killing of gonococci by peritoneal macrophages from mice deficient in complement component 5. Infect Immun. 1982 Apr;36(1):363–370. doi: 10.1128/iai.36.1.363-370.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue J. H., Rosenberg S. A. The fate of interleukin-2 after in vivo administration. J Immunol. 1983 May;130(5):2203–2208. [PubMed] [Google Scholar]
- Donohue J. H., Rosenstein M., Chang A. E., Lotze M. T., Robb R. J., Rosenberg S. A. The systemic administration of purified interleukin 2 enhances the ability of sensitized murine lymphocytes to cure a disseminated syngeneic lymphoma. J Immunol. 1984 Apr;132(4):2123–2128. [PubMed] [Google Scholar]
- Doyle M. V., Lee M. T., Fong S. Comparison of the biological activities of human recombinant interleukin-2(125) and native interleukin-2. J Biol Response Mod. 1985 Feb;4(1):96–109. [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Knight N. B., Humphrey E. W., Simmons R. L. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985 Aug;49(2):257–264. doi: 10.1128/iai.49.2.257-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser-Smith E. B., Waters R. V., Matthews T. R. Correlation between in vivo anti-Pseudomonas and anti-Candida activities and clearance of carbon by the reticuloendothelial system for various muramyl dipeptide analogs, using normal and immunosuppressed mice. Infect Immun. 1982 Jan;35(1):105–110. doi: 10.1128/iai.35.1.105-110.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Seitz M., Kramer W., Till G., Resch K. The effects of phagocytosis, dextran sulfate, and cell damage on PGE1 sensitivity and PGE1 production of macrophages. J Immunol. 1978 Apr;120(4):1187–1194. [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Holter W., Grunow R., Stockinger H., Knapp W. Recombinant interferon-gamma induces interleukin 2 receptors on human peripheral blood monocytes. J Immunol. 1986 Mar 15;136(6):2171–2175. [PubMed] [Google Scholar]
- Kawase I., Brooks C. G., Kuribayashi K., Olabuenaga S., Newman W., Gillis S., Henney C. S. Interleukin 2 induces gamma-interferon production: participation of macrophages and NK-like cells. J Immunol. 1983 Jul;131(1):288–292. [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Morahan P. S., Dempsey W. L., Volkman A., Connor J. Antimicrobial activity of various immunomodulators: independence from normal levels of circulating monocytes and natural killer cells. Infect Immun. 1986 Jan;51(1):87–93. doi: 10.1128/iai.51.1.87-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985 Jul;135(1):646–652. [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L. Enhancement of the neonate's nonspecific immunity to Klebsiella infection by muramyl dipeptide, a synthetic immunoadjuvant. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3395–3399. doi: 10.1073/pnas.75.7.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. Adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. Important Adv Oncol. 1986:55–91. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Hamilton E. B., Benninger A. H., Marafino B. J., Jr In vivo antiviral activity of recombinant murine gamma interferon. J Interferon Res. 1985 Spring;5(2):339–345. doi: 10.1089/jir.1985.5.339. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Tomita T., Blumenstock E., Kanegasaki S. Phagocytic and chemiluminescent responses of mouse peritoneal macrophages to living and killed Salmonella typhimurium and other bacteria. Infect Immun. 1981 Jun;32(3):1242–1248. doi: 10.1128/iai.32.3.1242-1248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Weigent D. A., Stanton G. J., Johnson H. M. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect Immun. 1983 Sep;41(3):992–997. doi: 10.1128/iai.41.3.992-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Dallman M. J., Fathman C. G. Administration of recombinant interleukin 2 in vivo induces a polyclonal IgM response. J Exp Med. 1986 Jun 1;163(6):1607–1612. doi: 10.1084/jem.163.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Browder I. W., Di Luzio N. R. Immunotherapeutic modification of Escherichia coli--induced experimental peritonitis and bacteremia by glucan. Surgery. 1983 Mar;93(3):448–454. [PubMed] [Google Scholar]
- Yamamoto J. K., Farrar W. L., Johnson H. M. Interleukin 2 regulation of mitogen induction of immune interferon (IFN gamma) in spleen cells and thymocytes. Cell Immunol. 1982 Jan 15;66(2):333–341. doi: 10.1016/0008-8749(82)90183-6. [DOI] [PubMed] [Google Scholar]



