Abstract
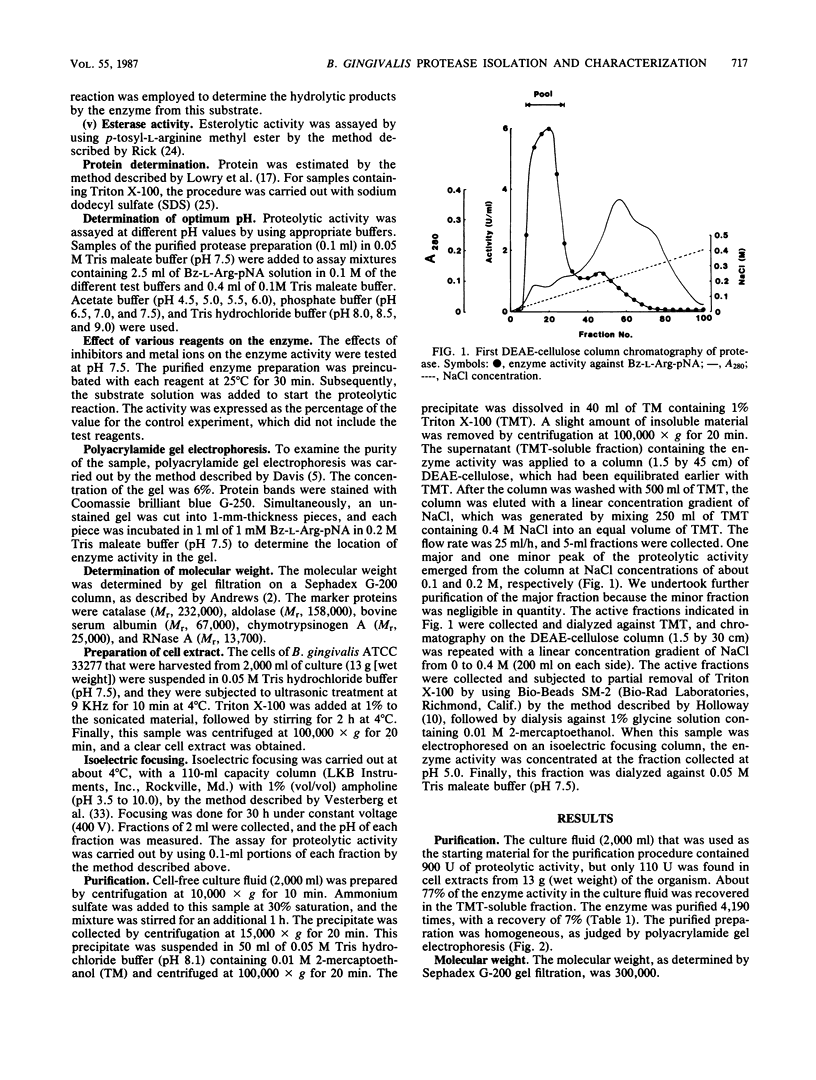
A protease was purified from Bacteroides gingivalis ATCC 33277 culture fluid by sequential procedures including ammonium sulfate precipitation, ion-exchange chromatography, and isoelectric focusing. The enzyme was active against benzoyl-L-arginine-p-nitroanilide, carbobenzoxy-L-phenylalanyl-L-valyl-L-arginine-p-nitroanilide azoalbumin, azocasein, azocoll, and p-tosyl-L-arginine methyl ester. The molecular weight of the enzyme was about 300,000 as determined by gel filtration. Its isoelectric point was 5.0. The maximum activity was found at pH 7.5, and the optimum temperature for activity was between 40 and 45 degrees C. The apparent Km value for benzoyl-L-arginine-p-nitroanilide was 2 mM. The enzyme was inhibited by sulfhydryl group-blocking reagents, tosyl-L-lysine chloromethyl ketone, and EDTA. Soybean trypsin inhibitor and diisopropylfluorophosphate were not inhibitory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko Y., Hayakawa M., Murai S., Takiguchi H. Glycylprolyl dipeptidylaminopeptidase from Bacteroides gingivalis. J Dent Res. 1985 Feb;64(2):106–111. doi: 10.1177/00220345850640020201. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn T. H. The protease liberated from Bacteroides amylophilus strain H18 by mechanical disintegration. J Gen Microbiol. 1968 Aug;53(1):37–51. doi: 10.1099/00221287-53-1-37. [DOI] [PubMed] [Google Scholar]
- Béchet J. J., Gardiennet M. C., Yon J. Etude de l'inhibition de l'activité estérasique de la trypsine par les dérivés acides et amides des esters spécifiques. Biochim Biophys Acta. 1966 Jul 6;122(1):101–115. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Hazlewood G. P., Jones G. A., Mangan J. L. Hydrolysis of leaf Fraction 1 protein by the proteolytic rumen bacterium Bacteroides ruminicola R8/4. J Gen Microbiol. 1981 Apr;123(2):223–232. doi: 10.1099/00221287-123-2-223. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. Rapid identification of Bacteroides gingivalis. J Clin Microbiol. 1982 Feb;15(2):345–346. doi: 10.1128/jcm.15.2.345-346.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Loesche W. J., Paunio K. U., Woolfolk M. P., Hockett R. N. Collagenolytic activity of dental plaque associated with periodontal pathology. Infect Immun. 1974 Feb;9(2):329–336. doi: 10.1128/iai.9.2.329-336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- NAGAI Y., SAKAKIBARA S., NODA H., AKABORI S. Hydrolysis of synthetic peptides by collagenase. Biochim Biophys Acta. 1960 Jan 29;37:567–569. doi: 10.1016/0006-3002(60)90531-x. [DOI] [PubMed] [Google Scholar]
- Nakata H., Ishii S. I. Substrate activation in the trypsin-catalyzed hydrolysis of benzoyl-L-arginine p-nitroanilide. Biochem Biophys Res Commun. 1970 Oct 23;41(2):393–400. doi: 10.1016/0006-291x(70)90517-6. [DOI] [PubMed] [Google Scholar]
- Nakata H., Yoshida N., Narahashi Y., Ishii S. Substrate activation and substrate inhibition of trypsin-like enzymes from three strains of Streptomyces species. J Biochem. 1972 Jun;71(6):1085–1088. doi: 10.1093/oxfordjournals.jbchem.a129859. [DOI] [PubMed] [Google Scholar]
- Oronsky A. L., Perper R. J., Schroder H. C. Phagocytic release and activation of human leukocyte procollagenase. Nature. 1973 Dec 14;246(5433):417–419. doi: 10.1038/246417a0. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Toda K., Otsuka M., Ishikawa Y., Sato M., Yamamoto Y., Nakamura R. Thiol-dependent collagenolytic activity in culture media of Bacteroides gingivalis. J Periodontal Res. 1984 Jul;19(4):372–381. doi: 10.1111/j.1600-0765.1984.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Raeste A. M. Activation of latent collagenase of human leukocytes and gingival fluid by bacterial plaque. J Dent Res. 1978 Jul-Aug;57(7-8):844–851. doi: 10.1177/00220345780570071401. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., Kippuw N., de Graaff J. Enzymatic characterization of oral and non-oral black-pigmented Bacteroides species. Antonie Van Leeuwenhoek. 1986;52(2):163–171. doi: 10.1007/BF00429320. [DOI] [PubMed] [Google Scholar]



